Abstract
1. The metabolism by the bovine lens of nine 14C-labelled l-amino acids was studied. These were: alanine, aspartate, glutamate, leucine, lysine, proline, serine, tyrosine and tryptophan. 2. All were taken up by the tissue and incorporated into protein. 3. Aspartate and glutamate, although poorly taken up, were readily metabolized to CO2. Radioactivity from glutamate was also found in glutathione, glutamine, proline and ophthalmic acid. Aspartate was converted into glutamate, glutathione, proline, alanine and lactate. 4. Alanine was largely converted into lactate, which was released into the medium, but incorporation of radioactivity into CO2, glutamate, glutathione, aspartate and lipids also occurred. 5. Radioactivity from leucine was detected in CO2, lipids, glutamate, glutathione, proline and glutamine. 6. Lysine was only slightly broken down by the bovine lens; radioactivity was observed in CO2, glutamate, glutathione, proline and two unidentified compounds. 7. Proline was metabolized to glutamate from which CO2, glutathione and glutamine were formed. Hydroxyproline in the capsule collagen was labelled. 8. Radioactivity from serine was found in CO2, lipids, glutathione, glycine, cystine, ATP, lactate and three unidentified compounds, one of which was probably taurine. 9. Neither tyrosine nor tryptophan were metabolized by the bovine lens. 10. The ability of the lens to metabolize amino acids was also shown by measurement of NH3 production: more NH3 was formed when glucose was absent from the incubation medium. 11. These experiments suggest that oxidation of amino acids is a source of energy for the lens.
Full text
PDF



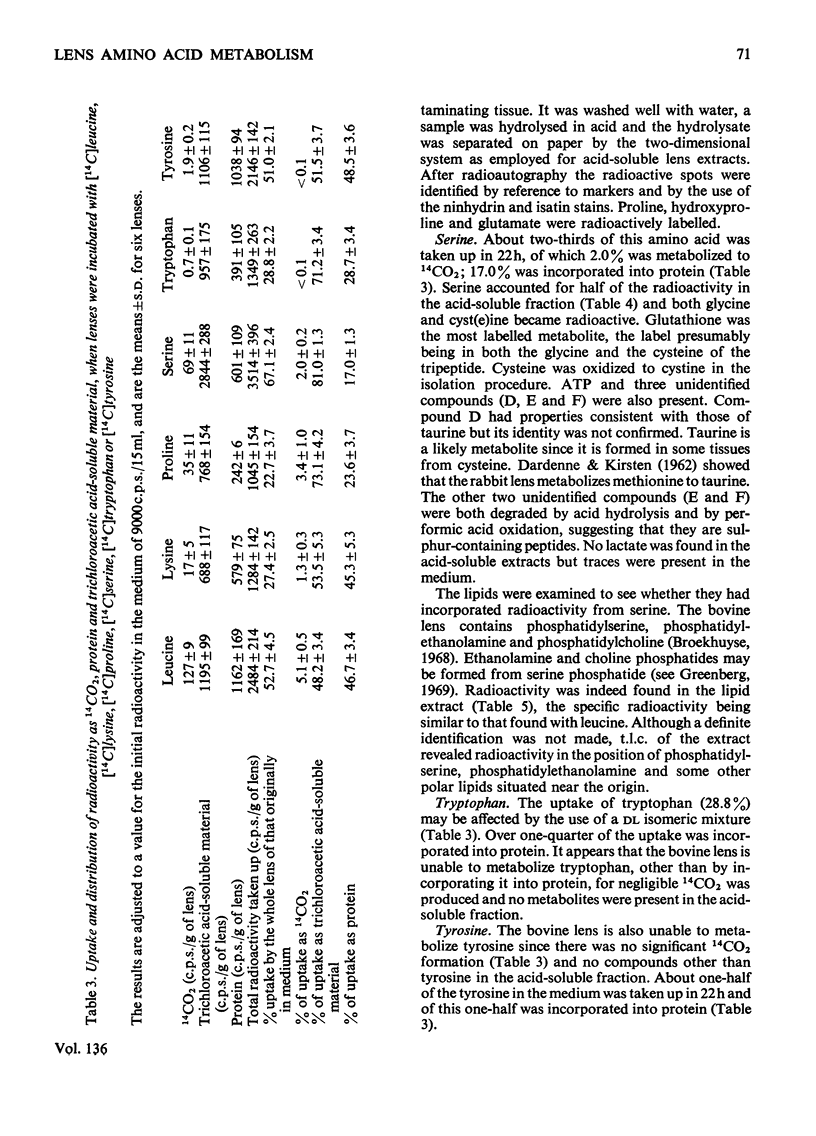
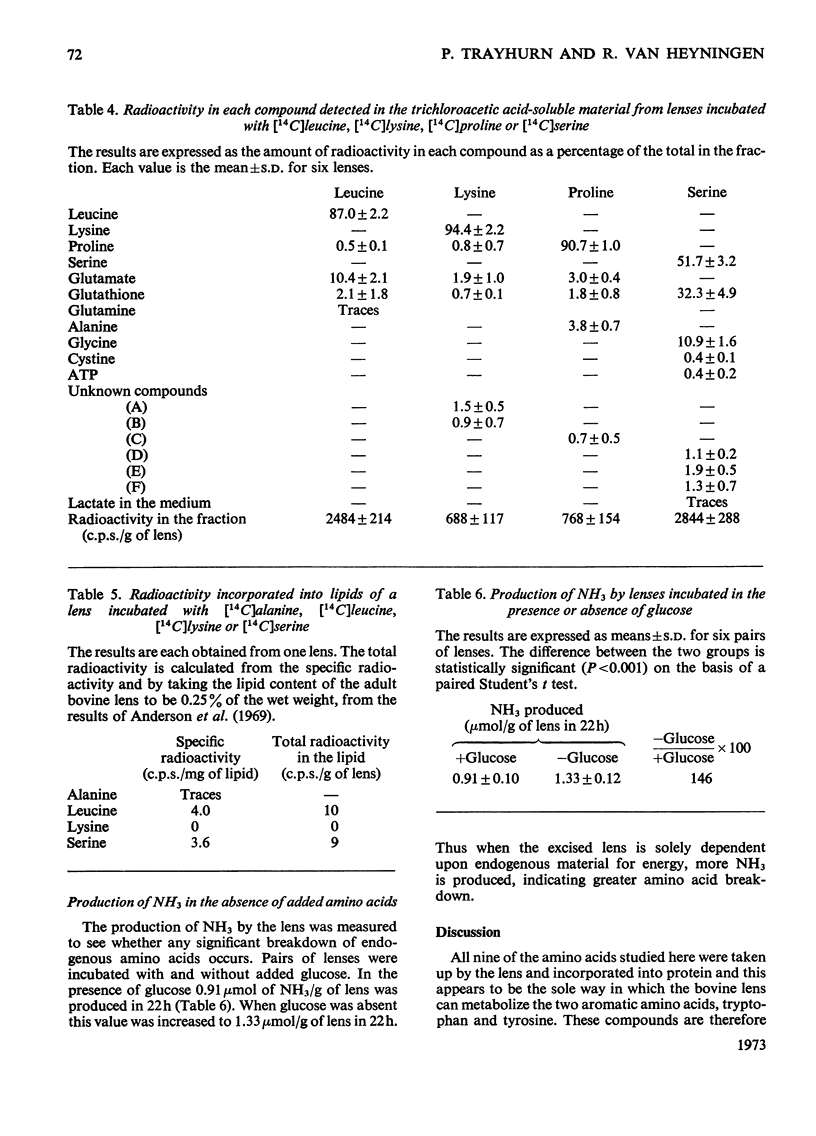



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMOORE J. E., BARTLEY W., VAN HEYNINGEN R. Distribution of sodium and potassium within cattle lens. Biochem J. 1959 May;72(1):126–133. doi: 10.1042/bj0720126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ATFIELD G. N., MORRIS C. J. Analytical separations by highvoltage paper electrophoresis. Amino acids in protein hydrolysates. Biochem J. 1961 Dec;81:606–614. doi: 10.1042/bj0810606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson R. E., Maude M. B., Feldman G. L. Lipids of ocular tissues. I. The phospholipids of mature rabbit and bovine lens. Biochim Biophys Acta. 1969 Oct 28;187(3):345–353. [PubMed] [Google Scholar]
- BELOFF-CHAIN A., CATANZARO R., CHAIN E. B., MASI I., POCCHIARI F., ROSSI C. The influence of insulin on carbohydrate metabolism in the isolated diaphragm muscle of normal and alloxan diabetic rats. Proc R Soc Lond B Biol Sci. 1955 May 17;143(913):481–503. doi: 10.1098/rspb.1955.0025. [DOI] [PubMed] [Google Scholar]
- Bradford H. F., Chain E. B., Cory H. T., Rose S. P. Glucose and amino acid metabolism in some invertebrate nervous systems. J Neurochem. 1969 Jun;16(3):969–978. doi: 10.1111/j.1471-4159.1969.tb08987.x. [DOI] [PubMed] [Google Scholar]
- Broekhuyse R. M. Phospholipids in tissues of the eye. I. Isolation, characterization and quantitative analysis by two-dimensional thin-layer chromatography of diacyl and vinyl-ether phospholipids. Biochim Biophys Acta. 1968 Mar 4;152(2):307–315. doi: 10.1016/0005-2760(68)90038-6. [DOI] [PubMed] [Google Scholar]
- CALAM D. H. Some new compounds in the lens. Exp Eye Res. 1962 Jun;1:436–442. doi: 10.1016/s0014-4835(62)80035-9. [DOI] [PubMed] [Google Scholar]
- CIUSA W., CRISTINI G., BARBIROLI G. THE TRANSMETHYLATION PROCESSES IN THE AQUEOUS HUMOUR AND IN THE LENS. Exp Eye Res. 1964 Jun;3:169–178. doi: 10.1016/s0014-4835(64)80032-4. [DOI] [PubMed] [Google Scholar]
- Calam D. H., Waley S. G. Amino acids and related compounds in the lens. Biochem J. 1964 Dec;93(3):526–532. doi: 10.1042/bj0930526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotlier E. Lysine transport and protein incorporation by the lens. Biochim Biophys Acta. 1971 Sep 14;241(3):798–806. doi: 10.1016/0005-2736(71)90007-1. [DOI] [PubMed] [Google Scholar]
- DARDENNE U., KIRSTEN G. Presence and metabolism of amino acids in young and old lenses. Exp Eye Res. 1962 Jun;1:415–421. doi: 10.1016/s0014-4835(62)80032-3. [DOI] [PubMed] [Google Scholar]
- Delcour J., Papaconstantinou J. Biochemistry of bovine lens proteins. IV. Synthesis and aggregation of -crystallin subunits in differentiating lens cells. J Biol Chem. 1972 May 25;247(10):3289–3295. [PubMed] [Google Scholar]
- Griffiths M. H. The components of an alpha-glycerophosphate cycle and their relation to oxidative metabolism in the lens. Biochem J. 1966 Apr;99(1):12–21. doi: 10.1042/bj0990012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUGHES D. E., WILLIAMSON D. H. Removal of acid by trioctylamine from samples for microbiological assay. Biochem J. 1951 Apr;48(4):487–490. doi: 10.1042/bj0480487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hems R., Stubbs M., Krebs H. A. Restricted permeability of rat liver for glutamate and succinate. Biochem J. 1968 May;107(6):807–815. doi: 10.1042/bj1070807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KERN H. L. Accumulation of amino acids by calf lens. Invest Ophthalmol. 1962 Jun;1:368–376. [PubMed] [Google Scholar]
- KINOSHITA J. H., KERN H. L., MEROLA L. O. Factors affecting the cation transport of calf lens. Biochim Biophys Acta. 1961 Mar 4;47:458–466. doi: 10.1016/0006-3002(61)90541-8. [DOI] [PubMed] [Google Scholar]
- KINSEY V. E., REDDY D. V. Studies on the crystalline lens. X. Transport of amino acids. Invest Ophthalmol. 1963 Jun;2:229–236. [PubMed] [Google Scholar]
- Kaplan A. The determination of urea, ammonia, and urease. Methods Biochem Anal. 1969;17:311–324. doi: 10.1002/9780470110355.ch7. [DOI] [PubMed] [Google Scholar]
- MERRIAM F. C., KINSEY V. E. Studies on the crystalline lens. III. Incorporation of glycine and serine in the proteins of lenses cultured in vitro. AMA Arch Ophthalmol. 1950 Nov;44(5):651–658. [PubMed] [Google Scholar]
- MERRIAM F. C., KINSEY V. E. Studies on the crystalline lens: technic for in vitro culture of crystalline lenses and observations. Arch Ophthal. 1950 Jun;43(6):979–988. [PubMed] [Google Scholar]
- PIRIE A. Composition of ox lens capsule. Biochem J. 1951 Mar;48(3):368–371. doi: 10.1042/bj0480368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REDDY D. V., KINSEY V. E. Studies on the crystalline lens. IX. Quantitative analysis of free amino acids and related compounds. Invest Ophthalmol. 1962 Oct;1:635–641. [PubMed] [Google Scholar]
- Reddy D. V. Distribution of free amino acids and related compounds in ocular fluids, lens, and plasma of various mammalian species. Invest Ophthalmol. 1967 Oct;6(5):478–483. [PubMed] [Google Scholar]
- Reddy D. V., Klethi J., Kinsey V. E. Studies on the crystalline lens. XII. Turnover of glycine and glutamic acid in glutathione and ophthalmic acid in the rabbit. Invest Ophthalmol. 1966 Dec;5(6):594–600. [PubMed] [Google Scholar]
- Reddy V. N. Metabolism of glutathione in the lens. Exp Eye Res. 1971 May;11(3):310–328. doi: 10.1016/s0014-4835(71)80043-x. [DOI] [PubMed] [Google Scholar]
- Reddy V. N. Studies on intraocular transport of taurine. II. Accumulation in the rabbit lens. Invest Ophthalmol. 1970 Mar;9(3):206–219. [PubMed] [Google Scholar]
- SCHWERIN P., BESSMAN S. P., WAELSCH H. The uptake of glutamic acid and glutamine by brain and other tissues of the rat and mouse. J Biol Chem. 1950 May;184(1):37–44. [PubMed] [Google Scholar]
- Srivastava S. K., Beutler E. Cataract produced by tyrosinase and tyrosine systems in rabbitens in vitro. Biochem J. 1969 May;112(4):421–425. doi: 10.1042/bj1120421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trayhurn P., Van Heyningen R. Aerobic metabolism in the bovine lens. Exp Eye Res. 1971 Nov;12(3):315–327. doi: 10.1016/0014-4835(71)90156-4. [DOI] [PubMed] [Google Scholar]
- Trayhurn P., Van Heyningen R. The role of respiration in the energy metabolism of the bovine lens. Biochem J. 1972 Sep;129(2):507–509. doi: 10.1042/bj1290507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UDENFRIEND S., COOPER J. R. The enzymatic conversion of phenylalanine to tyrosine. J Biol Chem. 1952 Feb;194(2):503–511. [PubMed] [Google Scholar]
- Van Heyningen R. Assay of fluorescent glucosides in the human lens. Exp Eye Res. 1973 Jan 1;15(1):121–126. doi: 10.1016/0014-4835(73)90196-6. [DOI] [PubMed] [Google Scholar]
- Van Heyningen R. Fluorescent glucoside in the human lens. Nature. 1971 Apr 9;230(5293):393–394. doi: 10.1038/230393a0. [DOI] [PubMed] [Google Scholar]
- Van Heyningen R. The metabolism of glucose by the rabbit lens in the presence and absence of oxygen. Biochem J. 1965 Aug;96(2):419–431. doi: 10.1042/bj0960419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOLF G., BERGER C. R. The metabolism of hydroxyproline in the intact rat; incorporation of hydroxyproline into protein and urinary metabolites. J Biol Chem. 1958 Jan;230(1):231–240. [PubMed] [Google Scholar]
- Waley S. G. Metabolism of amino acids in the lens. Biochem J. 1964 Jun;91(3):576–583. doi: 10.1042/bj0910576. [DOI] [PMC free article] [PubMed] [Google Scholar]


