Abstract
Post-stroke seizures present a global challenge, yet its frequency and factors associated with its incidence are poorly documented, particularly in the Middle East. Thus, this study aims to investigate post-stroke seizure frequency and stroke-associated factors among ischemic stroke patients in Saudi Arabia, addressing demographic, clinical, and comorbid aspects to improve prognosis, diagnosis, prevention, and management. A multicenter, cohort observational study included eligible ischemic stroke patients who were categorized into those who developed seizures after injury and those who did not. Additionally, the study assessed the association between post-stroke seizure and 12-month mortality, 12-month stroke recurrence, and the occurrence of hemorrhagic transformation (HT) within 30 days. The study involved 1235 ischemic stroke patients, in which 13.5% developed post-stroke seizures. Patients with post-stroke seizures had more extended hospital stays, higher intensive care unit (ICU) admission rates, and a higher prevalence of comorbidities. Factors independently associated with post-stroke seizures included previous stroke history (OR = 1.93; 1.35–2.75), ICU admission (OR = 1.7; 1.15–2.5), and depression (OR = 2.1; 1.38–3.30). Logistic regression revealed associations between post-stroke seizures and HT (OR = 2.61; 1.70-4.00), stroke recurrence (OR = 2.30; 1.58–3.36), and mortality (OR = 1.89; 1.33–2.68). However, after adjusting for covariates, post-stroke seizures were significantly associated with stroke recurrence only (aOR = 1.7; 1.11–2.63). Our study identifies notable associations and risk factors for post-stroke seizures in ischemic stroke patients. This underscores the importance of adopting a comprehensive approach to stroke care to enhance the prediction, prevention, and management of post-stroke seizures. Further research is warranted to validate these findings, enhance the understanding of post-stroke seizure mechanisms, and guide management strategies.
Keywords: Stroke, Post-stroke seizures, Right-side impairment, Stroke complications, Stroke mortality
Introduction
Stroke is a significant health burden, with an increased global incidence rate of 70% over the last three decades (Collaborators 2021). Stroke survivors, particularly older adults, are at a higher risk of developing spontaneous seizures (Lee et al. 2022). The reported incidence of post-stroke seizure varies widely between studies, ranging between 2% and 33% (Chang et al. 2022). Post-stroke seizures are associated with a severe decline in quality of life, longer hospitalization, and increased rates of morbidity and mortality (Huang et al. 2014). Therefore, early identification of seizure incidence and optimal management are essential to improve patients’ care and quality of life.
Currently, the factors associated with the incidence of post-stroke seizures are not well characterized. A previous systematic review of 37 studies exploring the risk factors, reported an association between post-stroke seizure and stroke severity, lesion size and location, cortical involvement, hemorrhagic transformation (HT), and atrial fibrillation (AF) (Feher et al. 2020). Except for those variables, studies exploring other potential risk factors reported inconsistent results. For instance, there is limited evidence linking co-morbidities, such as diabetes, hypertension, depression, dementia, and peripheral infection to post-stroke seizures (Galovic et al. 2021). Also, studies exploring the National Institutes of Health Stroke Scale (NIHSS) as a predictor of post-stroke seizure have mixed results (Labovitz et al. 2001; George et al. 2019). In addition, studies assessing the impact of seizure on clinical outcomes reported inconsistent findings, with some studies reporting seizure as a predictor for worse clinical outcomes, while other studies reported neutral findings (Feher et al. 2020).
Reasons for inconsistent results regarding predictors of post-stroke seizure incidence could be small sample size, inconsistent diagnostic criteria, timing of the seizure, length of stay, and acute vs. late post-stroke seizure (Beghi et al. 2011). Given the current increase in life expectancy and the expected increase in the number of stroke patients, identifying the ischemic stroke population at high risk is crucial. This study was conducted to characterize and identify stroke-related risk factors associated with post-stroke seizures in a large cohort of patients, including stroke signs and symptoms, complications, outcomes, and mortality. To the best of our knowledge, this study was the first in Saudi Arabia to investigate the association between the incidence of post-stroke seizures and various stroke signs and symptoms, including motor impairment, aphasia, and dysarthria. The main aim of this study was to assess incidence of post-stroke seizure and to explore the association between post-stroke seizure and stroke outcomes including 12-month recurrent stroke, HT, and 12-month mortality.
Methodology
Study design
A retrospective observational cohort study was conducted across two centers at King Abdulaziz Medical City (KAMC) in Jeddah and Riyadh, from the period of January 2016 to September 2022. KAMC functions as an academic referral tertiary care hospital, comprising 509 beds in Jeddah and 690 beds in Riyadh. Electronic medical records (BESTCare® 2.0) were used to screen eligible patients based on the inclusion and exclusion criteria.
The King Abdullah International Medical Research Center (KAIMRC) Institutional Review Board approved the study in Riyadh, Saudi Arabia (study number: NRC24R/115/02). Given the observational nature of the study, informed consent was not deemed necessary for the patients, as we utilized de-identified retrospective data.
Study participants
Adults who were ≥ 18 years old and arrived at the emergency room within seven days of stroke symptoms or were admitted at the incident time with confirmed ischemic stroke diagnosis were included in this study (Alharbi et al. 2022). Computerized tomography (CT), magnetic resonance Imaging (MRI), or both were used to confirm the diagnosis of ischemic stroke.
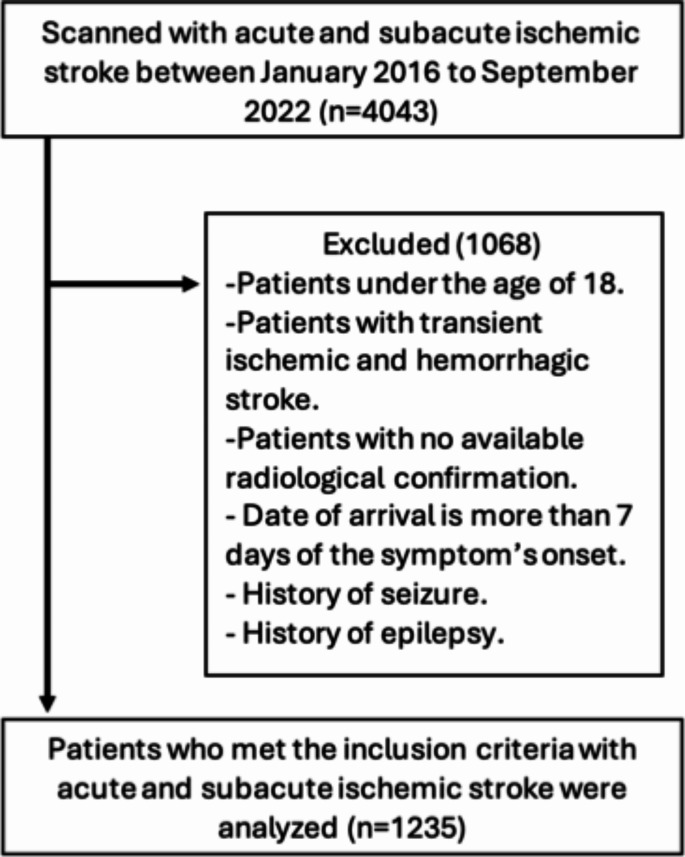
The patients in this study were classified based on the development of post-stroke seizures within a 12-month period. Those excluded from the study were individuals with a prior history of seizures or epilepsy prior to the stroke event, patients who experienced a transient ischemic attack or hemorrhagic stroke, as well as transferred patients who lacked admission data or those with complex cases for which data collection was not feasible, Fig. 1.
Fig. 1.
Flowchart of the included sample
Outcomes
The primary objective of the study was to estimate the incidence of post-stroke seizure. Additionally, the study assessed the association between post-stroke seizure and 12-month mortality, 12-month stroke recurrence, and the occurrence of HT within 30 days.
Data collection
The data collected included demographic data, stroke subtype according to The Trial of Org 10,172 in Acute Stroke Treatment (TOAST) classification of ischemic stroke: (1) large-artery atherosclerosis, (2) cardioembolism, (3) small-vessel occlusion, (4) stroke of other determined etiology, and (5) stroke of undetermined etiology (Amalia 2023), history of stroke (Alamri et al. 2024), initial stroke severity using NIHSS, administration of tissue plasminogen activator (tPA) or mechanical thrombectomy (MT), length of hospital stay, and intensive care unit (ICU) admission. In addition, data regarding history of comorbidities (hypertension, diabetes mellitus, dyslipidemia, AF, dementia, or depression), stroke signs and symptoms (motor impairment, aphasia, and dysarthria), and stroke complications (pneumonia, cerebral edema, deep vein thrombosis-pulmonary embolism (DVT-PE), impaired consciousness, HT, recurrent stroke, and mortality) were also collected.
Statistical analysis
This study aimed to assess various factors associated with post-stroke seizures and their potential impact on patient outcomes. Descriptive statistics for categorical data were generated using percentages and frequencies, while the mean and standard deviation or median and interquartile range were used for continuous data as appropriate. The differences in clinical and demographic variables based on post-stroke seizure status were evaluated using the chi-square test, t-test, and nonparametric statistics when appropriate. Subsequently, the relationship between post-stroke seizures and clinical and demographic variables was examined using multivariable binary logistic regression with the backward variable selection technique. Another multivariable binary logistic regression analysis was conducted to determine whether post-stroke seizures could predict post-stroke outcomes, such as death, stroke recurrence, and HT. The statistical analyses were performed using the SAS OnDemand for Academics software by SAS.
Results
Patient characteristics
A total of 1235 patients diagnosed with ischemic stroke were included in the current study. The patients had a mean age of 65.6 (± 12.6 years) and 61.7% were male. Table 1 presents the baseline and clinical characteristics of stroke patients where 13.5% of the patients developed post-stroke seizures. Most of the patients who developed seizures had either stroke subtype 1 or 5 (51% and 32%, respectively). Patients with seizures had twice more extended hospital stay (24.5 vs. 12.8 days, respectively; p < 0.0001), higher ICU admission rate (48.5% vs. 27.4%, respectively; p < 0.0001), and almost twice increase in the frequency of AF (17.4% vs. 9.6%, respectively; p = 0.0023), dementia (10.2% vs. 5.3%, respectively; p = 0.0143), and depression (22.2% vs. 11.4%, respectively; p = 0.0001) compared to those who did not have seizures. Details about patients’ characteristics are presented in Table 1.
Table 1.
Univariate analysis of patients’ characteristics by post-stroke seizure
| Characteristics | Total (N = 1235) |
Missing | Seizure | P | |
|---|---|---|---|---|---|
| Yes (N = 167) |
No (N = 1068) |
||||
| Age, mean ± SD | 65.5 ± 12.6 | 65.6 ± 13.7 | 65.5 ± 12.5 | 0.8668 | |
| Male, n (%) | 762 (61.7) | 0 | 101 (60.5) | 661 (61.9) | 0.7270 |
| Stroke subtype, n (%) | 140 | 0.0029 | |||
| 1 | 557 (50.9) | 80 (51.3) | 477 (50.8) | ||
| 2 | 275 (25.1) | 13 (8.3) | 58 (6.2) | ||
| 3 | 71 (6.5) | 24 (15.4) | 251 (26.7) | ||
| 4 | 28 (2.6) | 3 (1.9) | 25 (2.7) | ||
| 5 | 164 (15.0) | 36 (23.1) | 128 (13.6) | ||
| History of stroke, n (%) | 477 (39.3) | 21 | 91 (55.1) | 386 (36.8) | < 0.0001 |
| Initial NIHSS, median (IQR) | 6 (3–9) | 7 (5–12) | 6 (3–9) | < 0.0001 | |
| tPA therapy, n (%) | 118 (9.6) | 3 | 21 (12.6) | 97 (9.1) | 0.1569 |
| Mechanical thrombectomy, n (%) | 63 (5.1) | 2 | 15 (9.0) | 48 (4.5) | 0.0145 |
| Length of stay (days), mean ± SD | 14.4 ± 28.9 | 24.5 ± 41.4 | 12.8 ± 26.1 | < 0.0001 | |
| ICU admission, n (%) | 372 (30.2) | 5 | 81 (48.5) | 291 (27.4) | < 0.0001 |
| History of comorbidities, n (%) | |||||
| Hypertension | 1008 (81.7) | 1 | 144 (86.2) | 864 (81.0) | 0.1027 |
| Diabetes mellitus | 909 (73.7) | 1 | 129 (77.3) | 780 (73.1) | 0.2583 |
| Dyslipidemia | 556 (45.1) | 1 | 74 (44.3) | 482 (45.2) | 0.8351 |
| Atrial fibrillation | 131 (10.6) | 1 | 29 (17.4) | 102 (9.6) | 0.0023 |
| Dementia | 74 (6.0) | 1 | 17 (10.2) | 57 (5.3) | 0.0143 |
| Depression | 158 (12.8) | 2 | 37 (22.2) | 121 (11.4) | 0.0001 |
| Stroke signs and symptoms, n (%) | |||||
| LL motor impairment | 542 (44.4) | 15 | 81 (49.1) | 461 (43.7) | 0.1947 |
| LR motor impairment | 489 (40.2) | 19 | 79 (47.9) | 410 (39.0) | 0.0308 |
| UL motor impairment | 542 (44.3) | 12 | 80 (48.5) | 462 (43.7) | 0.2466 |
| UR motor impairment | 501 (41.1) | 17 | 85 (51.5) | 416 (39.5) | 0.0036 |
| Aphasia | 345 (28.1) | 6 | 63 (37.2) | 282 (26.6) | 0.0028 |
| Dysarthria | 671 (54.4) | 2 | 94 (56.3) | 577 (54.1) | 0.6023 |
| Stroke-related complications, n (%) | |||||
| Pneumonia | 275 (22.3) | 0 | 66 (39.5) | 209 (19.6) | < 0.0001 |
| Cerebral edema | 103 (8.4) | 1 | 29 (17.4) | 74 (6.9) | < 0.0001 |
| DVT-PE | 101 (8.2) | 0 | 29 (17.4) | 72 (6.7) | < 0.0001 |
| Impaired consciousness | 359 (29.1) | 2 | 80 (47.9) | 279 (26.17) | < 0.0001 |
BMI Body mass index, DVT-PE Deep vein thrombosis-pulmonary embolism, ICU Intensive care unit, IQR Interquartile range, LL lower left, LR lower right, NIHSS National Institutes of Health Stroke Scale, SD Standard deviation, tPA Tissue-type plasminogen activator, UL Upper left, UR Upper right
Stroke characteristics
The study analysis revealed that initial clinical stroke severity was associated with a higher risk of developing seizures (NIHSS = 8.8 ± 5.7 vs. 6.8 ± 5.4, respectively; p < 0.0001). Patients with impairments in their right upper and lower limbs had significantly higher incidence of seizures than those without right limb impairment (51.5% vs. 39.5%; p = 0.0036 and 47.9% vs. 39.0%, p = 0.03, respectively). Interestingly, patients with left limb impairment had no statistically significant difference in seizure incidence, Table 1.
Patients who experienced seizures after suffering a stroke had a higher rate of complications compared to those who did not have seizures. These complications included pneumonia (39.5% vs. 19.6%; p = 0.00001), cerebral edema (17.4% vs. 6.9%; p = 0.00001), DVT-PE (17.4% vs. 6.7%; p = 0.00001), and loss of consciousness (47.9% vs. 26.17%; p = 0.00001).
Factors associated with post-stroke seizure
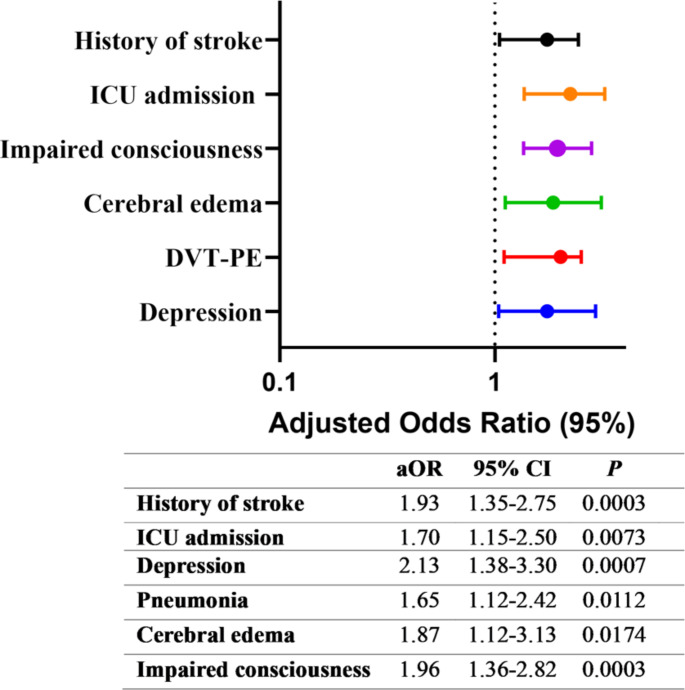
Individuals who had previously experienced a stroke were more likely to develop seizures than those who were experiencing their first-ever stroke (aOR = 1.93 [95% CI 1.35–2.75]; p = 0.0003). In addition, ICU admission was significantly correlated with seizure occurrence (aOR = 1.7 [95% CI 1.15–2.5]; p = 0.0073), Fig. 2. Similarly, patients who had depression were nearly twice as likely to develop seizures compared to those without depression (aOR = 2.1 [95% CI 1.38–3.30]; p = 0.0007), Fig. 2.
Fig. 2.
Factors associated with post-stroke seizure
Association between post-stroke seizure and stroke outcomes
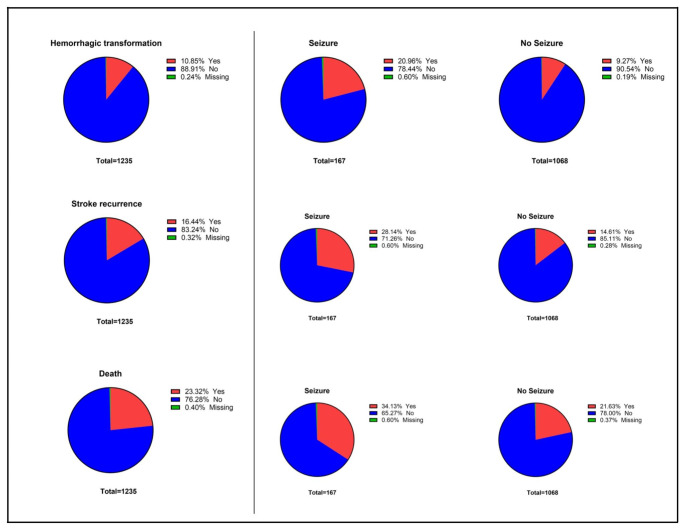
Figure 3 shows the mortality, recurrent stroke, and HT incidence rates among the study population and each group. Logistic regression analysis showed that post-stroke seizure was associated with HT (OR = 2.61 [95% CI 1.70–4.00]; P < 0.0001), stroke recurrence (OR = 2.30 [95% CI 1.58–3.36]; P < 0.0001), and mortality (OR = 1.89 [95% CI 1.33–2.68]; P = 0.0004), Table 2.
Fig. 3.
Post-stroke outcomes by seizure status
Table 2.
Association between post-stroke seizure and stroke outcome
| Unadjusted Model | Adjusted Model a | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | |
| Hemorrhagic transformation | 2.61 | 1.70–4.00 | < 0.0001 | 1.40 | 0.84–2.33 | 0.1992 |
| Stroke recurrence | 2.30 | 1.58–3.36 | < 0.0001 | 1.70 | 1.11–2.62 | 0.0151 |
| Death | 1.89 | 1.33–2.68 | 0.0004 | 0.93 | 0.59–1.38 | 0.6311 |
a The models were adjusted for a history of stroke, atrial fibrillation, dementia, depression, mechanical thrombectomy, ICU admission, pneumonia, cerebral edema, DVT-PE, impaired consciousness, motor impairment, and aphasia
However, following adjustment for covariates, logistic regression analysis found only stroke recurrence to be significantly associated with post-stroke seizure (aOR = 1.7 [95% CI 1.11–2.62]; p = 0.0151). Whereas the incidence of HT (aOR = 1.4 [95% CI 0.84–2.33]; p = 0.1992) and mortality (aOR = 0.93 [95% CI 0.59–1.38]; p = 0.6311) were not significantly associated with post-stroke seizures, Table 2.
Discussion
This retrospective cohort study investigated the incidence and burden of post-stroke seizures in Saudi Arabia. The average age of participants was 65.6 ± 12.6 years, and 61.7% were male. In the included sample, around 13.5% of patients with ischemic stroke developed post-stroke seizure. In previously published studies, the rate of post-stroke seizure in patients with ischemic stroke varied largely, ranging from 2.5 to 12% (Feher et al. 2020). This discrepancy could be explained by the heterogenicity in study design, mainly by differences in the inclusion period between stroke and seizure occurrence, ranging from 24 h to 30 days. The higher seizure incidence found in this study is anticipated as the period of inclusion between stroke and seizure was longer than in previous studies (12 months). Based on the TOAST classification (Amalia 2023), in the current study, around one-half of all stroke patients had subtype 1 (large vascular occlusion). This is consistent with a previous retrospective study reporting stroke subtype 1 to be the most prevalent, accounting for 59.6% of all stroke patients (Harris et al. 2018).
History of previous stroke was present in 39.9% of the sample; 55.1% of the seizure group, and 36.8% in the non-seizure group, Table 1. This study found an independent association between previous history of stroke and post-stroke seizure, Fig. 2. These findings align with a recent systematic review and meta-analysis, emphasizing a solid link between post-stroke seizure, heightened mortality, and severe disability in individuals with a prior history of stroke (Misra et al. 2023).
Predicting the outcome following a stroke is mainly influenced by stroke severity (Koton et al. 2022). The NIHSS score is the most frequently used scoring system worldwide to assess the severity of stroke (Amalia 2023). In the univariate model, this study reported that NIHSS score at admission was significantly associated with developing post-stroke seizures, however, this association was not significant in the multivariable model. These results are consistent with other reports indicating that a higher initial NIHSS score predicts post-stroke seizures (Alsaad et al. 2022; Zollner et al. 2020). ln addition, the present study showed stroke patients who encountered seizures exhibited a notably prolonged hospital stay and a higher rate of ICU admission compared to those who did not experience seizures. Upon using the multivariable regression model, ICU admission remained significantly associated with the occurrence of post-stroke seizure, Fig. 2. This is consistent with previous findings by Alsaad et al., in which patients with post-stroke seizure had more ICU admissions and longer length of hospital stay (Alsaad et al. 2022).
Moreover, the current report evaluated the influence of the thrombolysis and thrombectomy interventions. According to a research study conducted in the United States, there was a marked increase in post-stroke seizures in patients who underwent MT (Lekoubou et al. 2023). Approximately 1 in 20 acute ischemic stroke patients who underwent MT experienced seizures. The findings are consistent with this report which suggested that patients who underwent MT were more likely to develop post-stroke seizures, Table 1. This is in part owed to the larger vessels ischemic stroke is more severe in general (Tawil et al. 2016). Contrary to the former, thrombolysis with tPA is the preferred treatment for ischemic stroke only when administered within the critical first few hours after the onset of symptoms (Knecht et al. 2017). There was no statistically significant association between tPA therapy and the development of post-stroke seizures in the current study, Table 1. This finding could be of clinical significance in the management of ischemic stroke patients.
The present study found an association between post-stroke seizure occurrence with aphasia and symptoms of right-side weakness, Table 1. It is well established that language areas are predominantly located in the left hemisphere, the dominant hemisphere, in 95–99% of right-handed individuals and roughly 70% of left-handed individuals (Kelly et al. 2004). Interestingly, motor impairments on the right upper and lower sides were significantly associated with a higher rate of seizures. A contrasting finding revealed that patients exhibiting unilateral weakness were less prone to early seizures following a stroke (Alsaad et al. 2022). However, there was no statistically significant disparity in the rate of seizure among patients with left-limb motor impairment in the present study. The specific location of the brain injury plays a pivotal role in determining the timing and duration of post-stroke seizures (Loscher et al. 2015). Further research is necessary to explore which basic and clinical characteristics make patients with right-side weakness/aphasia more susceptible to post-stroke seizures. It is also important to determine whether the location of the stroke and its severity affect the incidence of post-stroke seizures in this group of patients.
As presented in Table 1; Fig. 2, the post-stroke seizure group had higher odds of developing complications such as pneumonia, cerebral edema, DVT-PE, and impaired consciousness. Stroke-associated pneumonia is a prevalent complication of acute ischemic stroke (Jitpratoom and Boonyasiri 2024). In the present study, 22.3% of ischemic stroke patients developed pneumonia and it was independently associated with post-stroke seizure. This finding is in alignment with a previous multicenter study linking the correlation between post-stroke seizure to stroke-associated pneumonia infection (Huang et al. 2014). In addition, the present study found a statistically significant independent association between the incidence of cerebral edema with post-stroke seizures, Fig. 2. A multicentric cohort study from the United Kingdom revealed that cerebral edema could serve as an additional independent marker for identifying patients with high prevalence of acute seizures. Recent research has explored the role of blood-brain barrier (BBB) dysfunction in pathogenesis and the onset of seizures and epilepsy (Bernardino et al. 2024). One possible explanation of these findings is that cerebral edema may have disrupted the BBB further, leading to seizures in ischemic stroke patients.
DVT and PE were observed in 17.4% of ischemic stroke patients who experienced post-stroke seizures and only in 6.7% of those who were seizure-free. Researchers have firmly established that the occurrence of DVT in the lower extremities is a common complication of acute ischemic stroke (Han et al. 2023). DVT can lead to PE, a life-threatening condition that accounts for 13–25% of early deaths after a stroke (Kelly et al. 2004). Our results aligned with a study conducted in Saudi Arabia, which reported that patients who suffered from ischemic stroke developed PE, and a significant number of them encountered early seizures (Alsaad et al. 2022). According to a recent study, seizures are thought to be a consequence of ischemic hypoxic encephalopathy resulting from PE (Leong et al. 2022). However, our study failed to document DVT-PE independent association with the occurrence of post-stroke seizure.
In the current study, HT, stroke recurrence, and death were assessed to explore the association between post-stroke seizures and major adverse effects post-stroke. Two previous studies have reported an independent association between HT and post-stroke seizure incidence (Procaccianti et al. 2012; Brondani et al. 2019). In contrast, after adjusting for covariates, our study’s logistic regression analysis found no significant association between HT and post-stroke seizures. In addition to HT, post-stroke seizures have been linked to higher mortality rates (Lahti et al. 2021). Similarly, a study from Saudi Arabia by Alsaad et al., reported a higher mortality rate in patients with early seizures, with those experiencing early seizures having more than double the risk of in-hospital death compared to those without (Alsaad et al. 2022). However, in our study, logistic regression analysis did not show a significant association between post-stroke seizures and mortality rates after adjusting for covariates. This variation may be attributed to differences in study design, stroke severity, stroke subtypes, cortical involvement, and comorbidities across study populations.
Lastly, the current study reported stroke recurrence to be independently associated with post-stroke seizure. Similar to our study, results from a study conducted in Sweden reported stroke recurrence as an independent predictor of seizure incidence (Redfors et al. 2020). In contrast, another multicentric study conducted with cohorts from Portugal, Colombia, and the Netherlands indicated that experiencing a stroke recurrence does not increase the frequency of acute symptomatic seizures (Leal Rato et al. 2024). We anticipate that variations in demographics, genetics, and underlying health conditions among populations in different countries may influence the risk of seizures after stroke. Factors such as age, prevalence of vascular risk factors, and comorbidities can vary significantly.
One of the limitations of this report was the retrospective nature of the study. In addition, due to limitations in data collection, specific data related to type of seizure, the exact median time for seizure occurrence, and the differentiation between post-stroke seizure and post-stroke epilepsy was not documented. Future studies should aim to provide more comprehensive insights by using extensive and diverse cohorts that include a larger sample size, ethnicities, and geographic regions. Additionally, it is advisable to conduct long-term follow-up studies to examine the long-term effects of stroke on the incidence and recurrence of seizures. Moreover, it is important to evaluate whether a subset of the population, by categorizing patients based on variables such as seizure type and characteristics, stroke initial severity, and age, may influence the rate of post-stroke seizure. This approach will help in understanding the temporal relationships involved.
Conclusion
Our investigation into post-stroke seizures in patients with ischemic stroke revealed important associations and insights. Key risk factors for post-stroke seizures included a history of stroke and ICU admission. Moreover, MT was associated with post-stroke seizure, however it was not statistically significant in the multivariable model. Additionally, individuals with comorbidities such as AF, dementia, and depression showed a higher prevalence of seizures. Complications such as pneumonia, cerebral edema, DVT-PE were also significantly linked to post-stroke seizures. Notably, significant lower and upper right motor impairments, as well as aphasia, were observed among the stroke signs and symptoms in patients. Furthermore, a strong association was found between stroke recurrence and post-stroke seizures. This research underscores the necessity of a comprehensive approach to stroke care that considers both clinical factors and comorbid conditions to enhance the prediction, prevention, and management of post-stroke seizures.
Author contributions
E.A.R. Leading writing and editing the manuscript final review, supervising the writing, approving the manuscript, and submitting the study; Y.A. Conceptualization, formal analysis, review and approve the final manuscript; R.S.K. Writing and reviewing the manuscript, approval of final form of manuscript; J.I.K. Data curation, data cleaning, designing Fig. 1, review and accept the final form of the manuscript; A.E.A. Data curation, review and accept the final form of the manuscript; S.L. Data curation, review and accept the final form of the manuscript; R.A. Data curation, writing the discussion; K.A. Conceptualization, review the study and approve the final manuscript; F.F.A. Conceived the study, supervising study design and data collection, editing and approve the final form of the manuscript.
Funding
No fund was provided to conduct this study.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Alamri FF, Almarghalani DA, Alraddadi EA, Alharbi A, Algarni HS, Mulla OM et al (2024) The utility of serum glucose potassium ratio as a predictive factor for haemorrhagic transformation, stroke recurrence, and mortality among ischemic stroke patients. Saudi Pharm J 32(6):102082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alharbi AR, Alali AS, Samman Y, Alghamdi NA, Albaradie O, Almaghrabi M et al (2022) Vitamin D serum level predicts stroke clinical severity, functional independence, and disability-A retrospective cohort study. Front Neurosci 16:951283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsaad F, Alkeneetir N, Almatroudi M et al (2022) Early seizures in stroke - frequency, risk factors, and effect on patient outcomes in a tertiary center in Saudi Arabia. Neurosciences (Riyadh) 27(2):104–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amalia L (2023) Characteristic of acute ischemic stroke patients based on TOAST classification during COVID-19 pandemic era: a single centre study. Int J Gen Med 16:581–588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beghi E, D’Alessandro R, Beretta S et al (2011) Incidence and predictors of acute symptomatic seizures after stroke. Neurology 77(20):1785–1793 [DOI] [PubMed] [Google Scholar]
- Bernardino PN, Luo AS, Andrew PM et al (2024) Evidence implicating blood-brain barrier impairment in the pathogenesis of acquired epilepsy following acute organophosphate intoxication. J Pharmacol Exp Ther 388(2):301–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brondani R, de Almeida AG, Cherubini PA, Secchi TL, de Oliveira MA, Martins SCO et al (2019) Risk factors for epilepsy after thrombolysis for ischemic stroke: a cohort study. Front Neurol 10:1256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang RS, Leung WC, Vassallo M, Sykes L, Battersby Wood E, Kwan J (2022) Antiepileptic drugs for the primary and secondary prevention of seizures after stroke. Cochrane Database Syst Rev 2(2):CD005398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collaborators GBDS (2021) Global, regional, and national burden of stroke and its risk factors, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol 20(10):795–820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feher G, Gurdan Z, Gombos K et al (2020) Early seizures after ischemic stroke: focus on thrombolysis. CNS Spectr 25(1):101–113 [DOI] [PubMed] [Google Scholar]
- Galovic M, Ferreira-Atuesta C, Abraira L et al (2021) Seizures and epilepsy after stroke: epidemiology, biomarkers and management. Drugs Aging 38(4):285–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- George P, Punia V, Natteru PA, Hantus S, Newey C (2019) Predictors of seizure recurrence after acute symptomatic seizures in ischemic stroke patients. Neurosci J 2019:8183921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han L, Yang JM, Qian WY et al (2023) Risk factors for lower extremity deep vein thrombosis in acute stroke patients following endovascular thrombectomy: a retrospective cohort study. Front Neurol 14:1249365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris S, Sungkar S, Rasyid A, Kurniawan M, Mesiano T, Hidayat R (2018) TOAST subtypes of ischemic stroke and its risk factors: a hospital-based study at Cipto Mangunkusumo Hospital, Indonesia. Stroke Res Treat 2018:9589831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CW, Saposnik G, Fang J, Steven DA, Burneo JG (2014) Influence of seizures on stroke outcomes: a large multicenter study. Neurology 82(9):768–776 [DOI] [PubMed] [Google Scholar]
- Jitpratoom P, Boonyasiri A (2024) Factors associated with an increased risk of developing pneumonia during acute ischemic stroke hospitalization. PLoS One 19(1):e0296938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly J, Rudd A, Lewis RR, Coshall C, Moody A, Hunt BJ (2004) Venous thromboembolism after acute ischemic stroke: a prospective study using magnetic resonance direct thrombus imaging. Stroke 35(10):2320–2325 [DOI] [PubMed] [Google Scholar]
- Knecht T, Story J, Liu J, Davis W, Borlongan CV, Dela Pena IC (2017) Adjunctive therapy approaches for ischemic stroke: innovations to expand time window of treatment. Int J Mol Sci 18(12). 10.3390/ijms18122756 [DOI] [PMC free article] [PubMed]
- Koton S, Patole S, Carlson JM et al (2022) Methods for stroke severity assessment by chart review in the atherosclerosis risk in communities study. Sci Rep 12(1):12338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labovitz DL, Hauser WA, Sacco RL (2001) Prevalence and predictors of early seizure and status epilepticus after first stroke. Neurology 57(2):200–206 [DOI] [PubMed] [Google Scholar]
- Lahti AM, Huhtakangas J, Juvela S, Bode MK, Tetri S (2021) Increased mortality after post-stroke epilepsy following primary intracerebral hemorrhage. Epilepsy Res 172:106586 [DOI] [PubMed] [Google Scholar]
- Leal Rato M, Schön M, Zafra MP et al (2024) Acute symptomatic seizures in patients with recurrent ischemic stroke: a multicentric study. Epileptic Disord 26(6):787–796. 10.1002/epd2.20279 [DOI] [PubMed]
- Lee SH, Aw KL, Banik S, Myint PK (2022) Post-stroke seizure risk prediction models: a systematic review and meta-analysis. Epileptic Disord 24(2):302–314 [DOI] [PubMed] [Google Scholar]
- Lekoubou A, Colon YP, Bishu KG, Ngonde AT, Bonilha L, Ovbiagele B (2023) Prevalence and prognosis of seizures among patients undergoing mechanical thrombectomy for acute ischemic stroke: a look at pre-2015 aha/asa guidelines update regarding endovascular treatment. J Stroke Cerebrovasc Dis 32(5):107049 [DOI] [PubMed] [Google Scholar]
- Leong W, Zhang Y, Huang X et al (2022) Seizure as the clinical presentation of massive pulmonary embolism: case report and literature review. Front Med (Lausanne) 9:980847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loscher W, Hirsch LJ, Schmidt D (2015) The enigma of the latent period in the development of symptomatic acquired epilepsy - Traditional view versus new concepts. Epilepsy Behav 52(Pt A):78–92 [DOI] [PubMed] [Google Scholar]
- Misra S, Kasner SE, Dawson J et al (2023) Outcomes in patients with poststroke seizures: a systematic review and meta-analysis. JAMA Neurol 80(11):1155–1165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Procaccianti G, Zaniboni A, Rondelli F, Crisci M, Sacquegna T (2012) Seizures in acute stroke: incidence, risk factors and prognosis. Neuroepidemiology 39(1):45–50 [DOI] [PubMed] [Google Scholar]
- Redfors P, Holmegaard L, Pedersen A, Jern C, Malmgren K (2020) Long-term follow-up of post-stroke epilepsy after ischemic stroke: room for improved epilepsy treatment. Seizure 76:50–55 [DOI] [PubMed] [Google Scholar]
- Tawil SE, Cheripelli B, Huang X et al (2016) How many stroke patients might be eligible for mechanical thrombectomy? Eur Stroke J 1(4):264–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zollner JP, Misselwitz B, Kaps M et al (2020) National Institutes of Health Stroke Scale (NIHSS) on admission predicts acute symptomatic seizure risk in ischemic stroke: a population-based study involving 135,117 cases. Sci Rep 10(1):3779 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.