Abstract
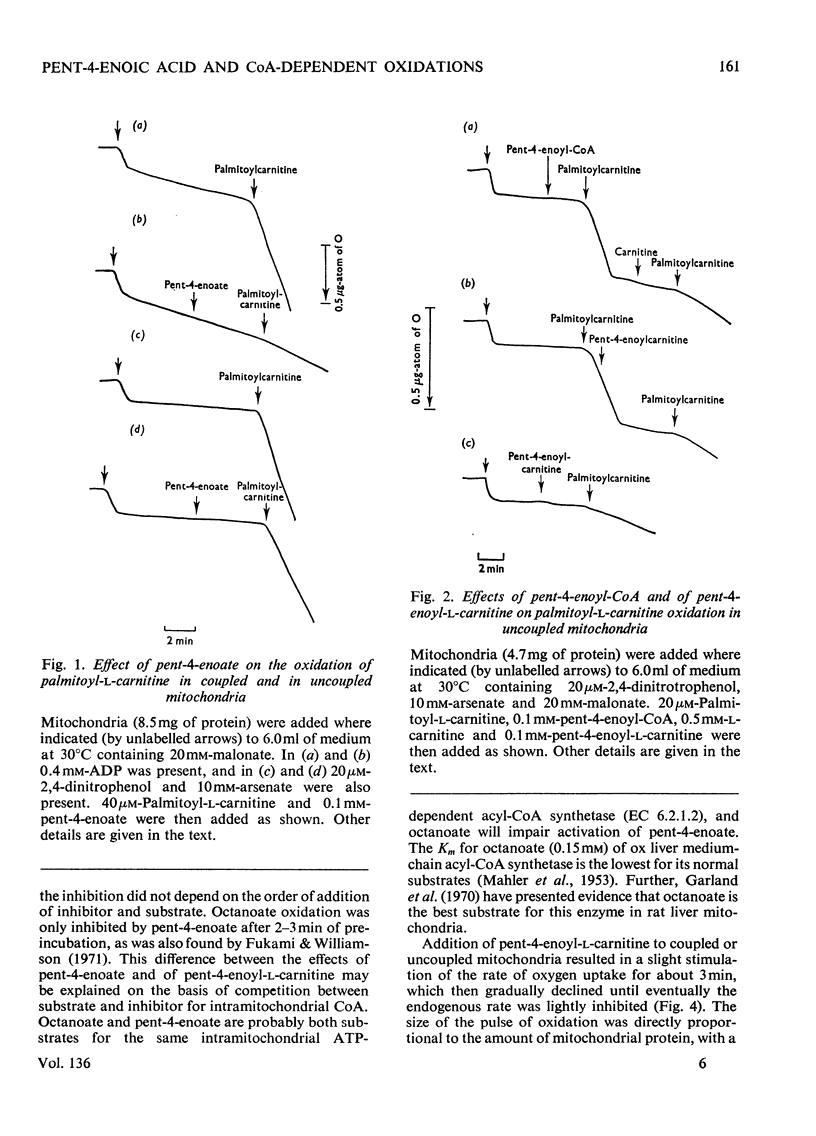
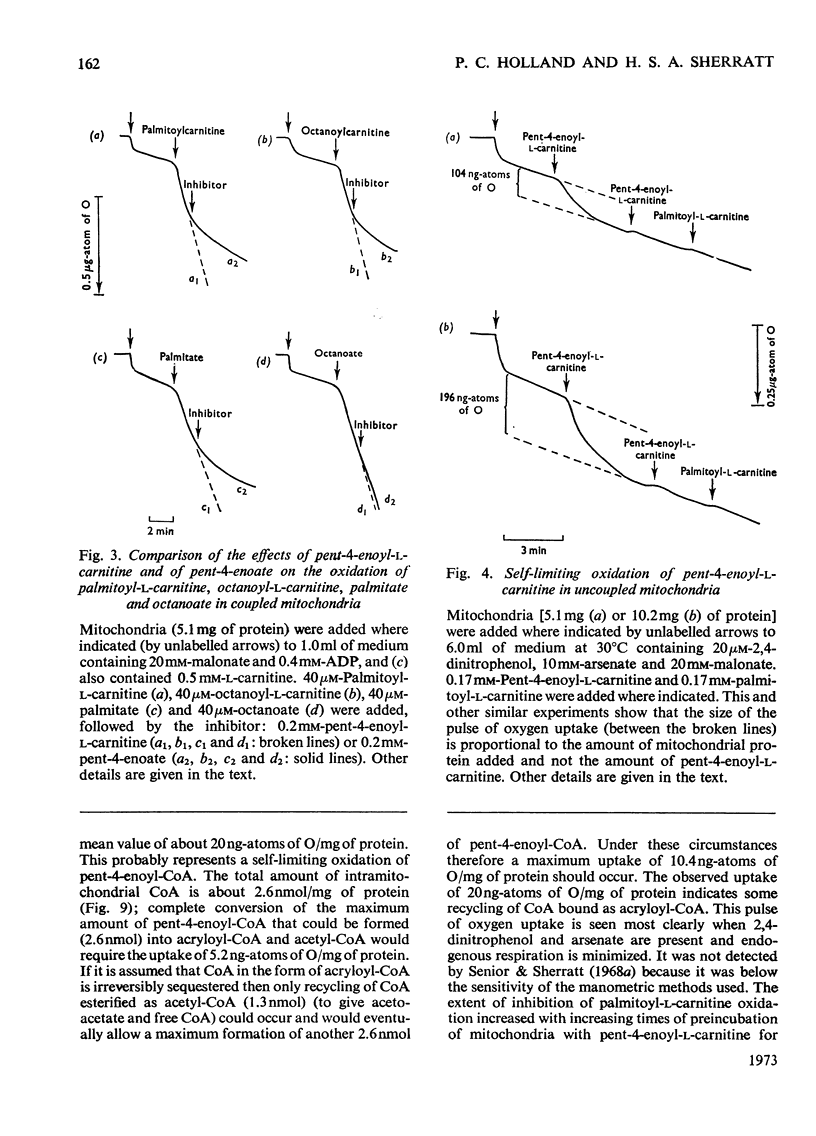
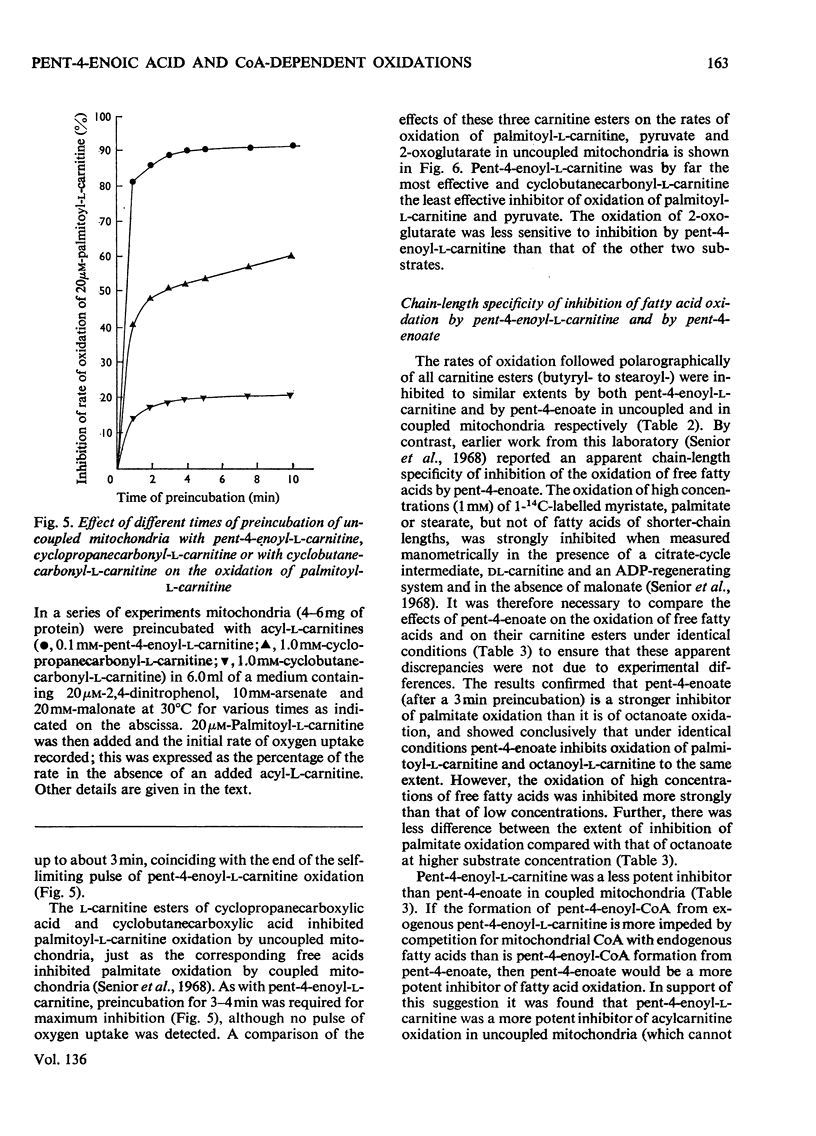
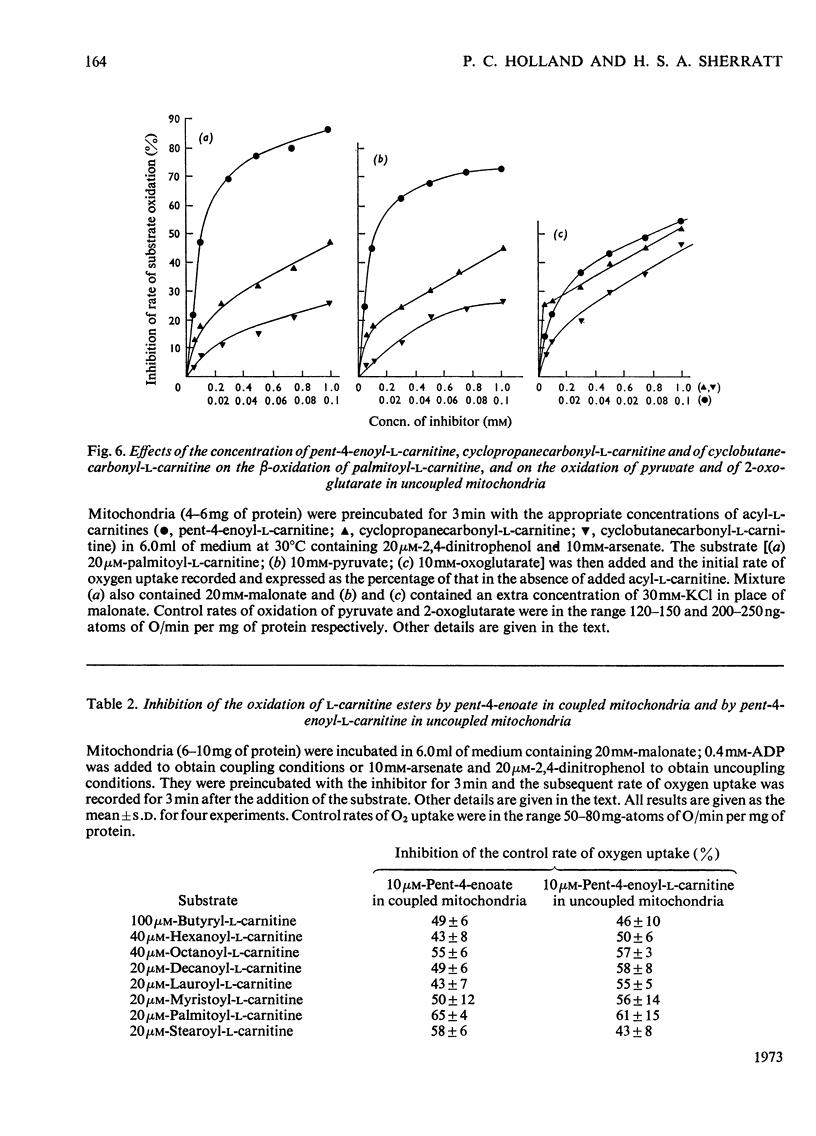
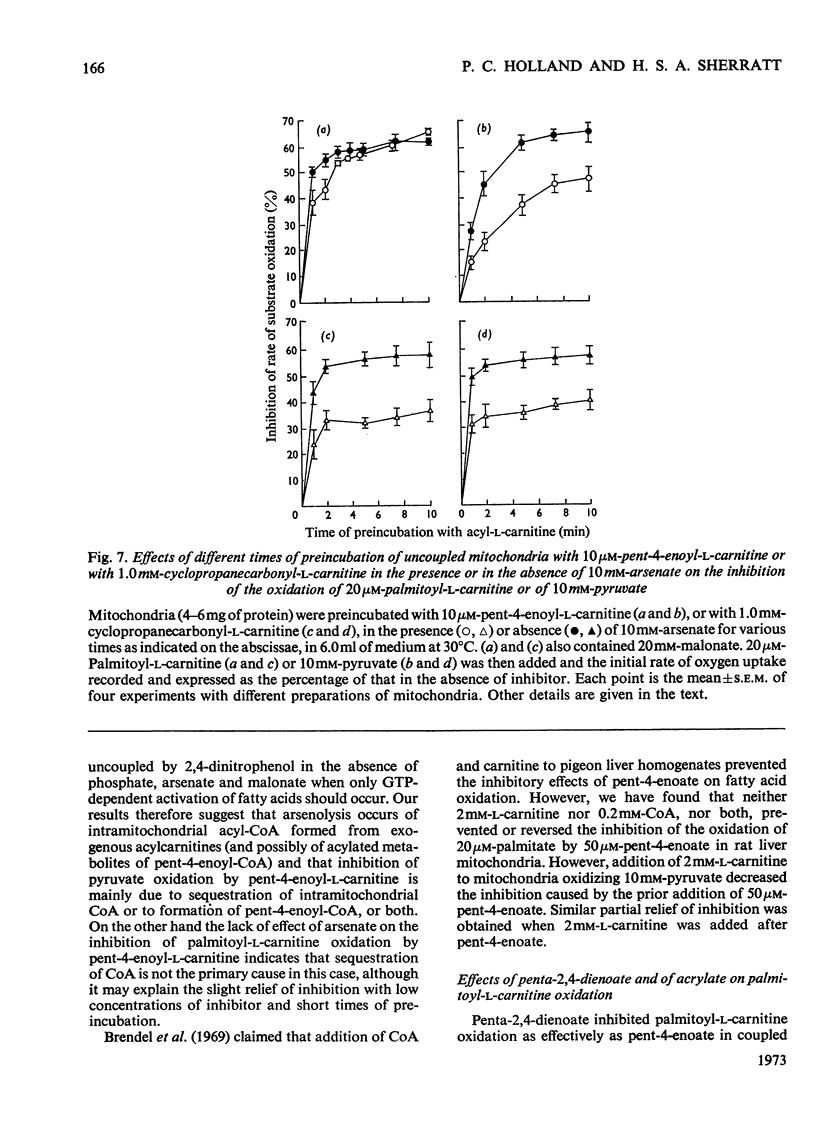
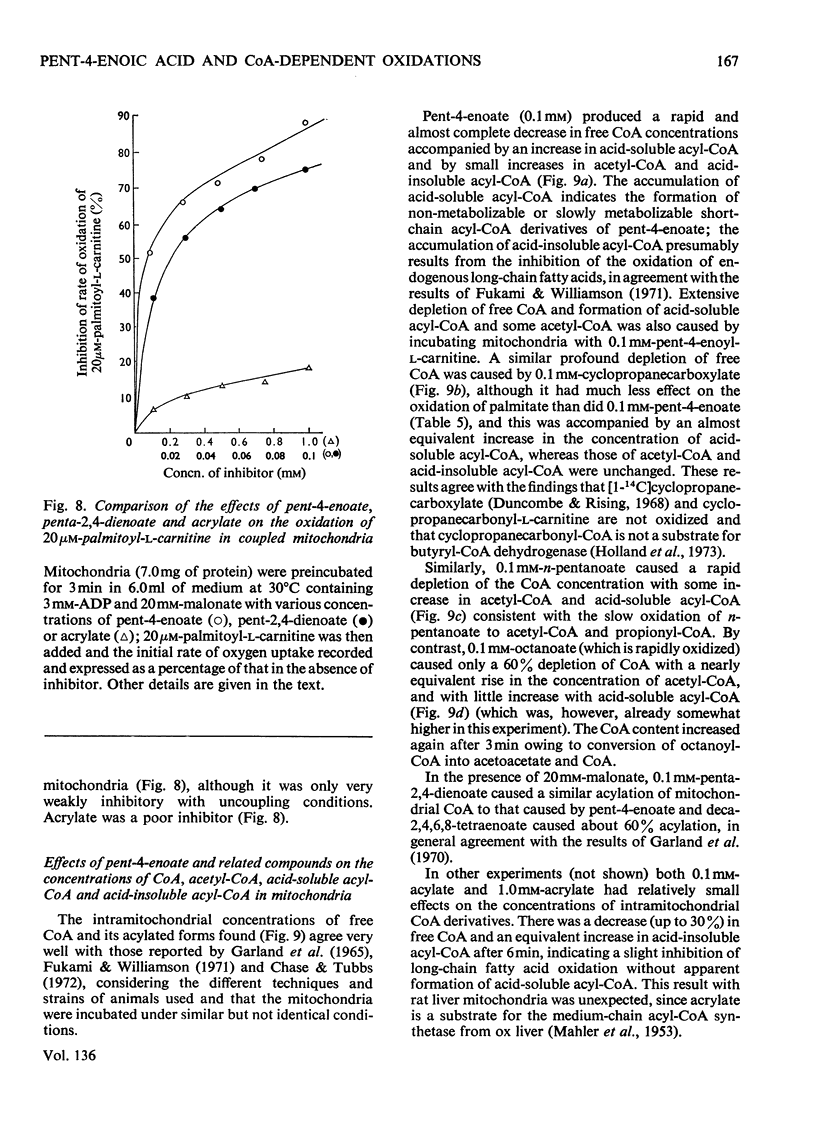
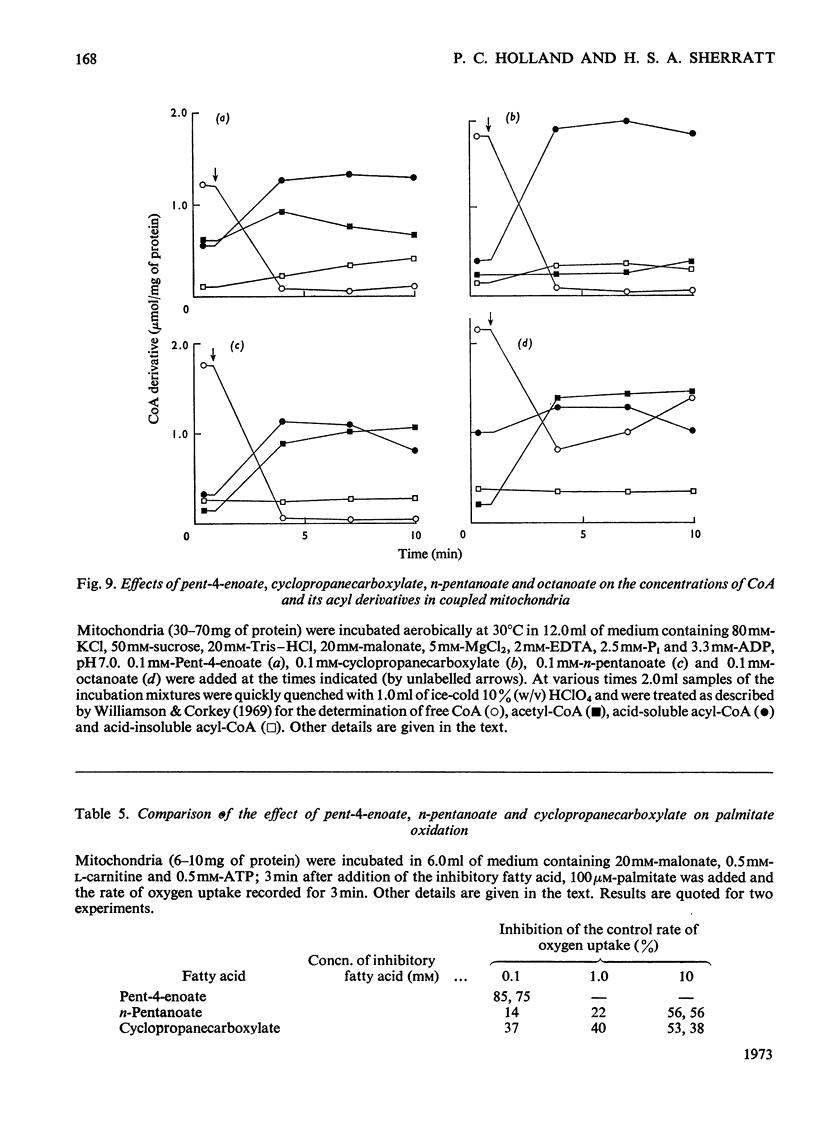
1. The synthesis of pent-4-enoyl-l-carnitine, cyclopropanecarbonyl-l-carnitine and cyclobutanecarbonyl-l-carnitine is described. 2. Pent-4-enoate strongly inhibits palmitoyl-l-carnitine oxidation in coupled but not in uncoupled mitochondria. Pent-4-enoyl-l-carnitine strongly inhibits palmitoyl-l-carnitine oxidation in uncoupled mitochondria. Prior intramitochondrial formation of pent-4-enoyl-CoA is therefore necessary for inhibition. 3. There was a small self-limiting pulse of oxidation of pent-4-enoyl-l-carnitine during which the ability to inhibit the oxidation of subsequently added palmitoyl-l-carnitine developed. 4. Pent-4-enoate and pent-4-enoyl-l-carnitine are equally effective inhibitors of the oxidation of all even-chain acylcarnitines of chain length C4–C16. Pent-4-enoyl-l-carnitine also inhibits the oxidation of pyruvate and of 2-oxoglutarate. 5. Pent-4-enoate strongly inhibits the oxidation of palmitate but not that of octanoate. This is presumably due to competition between octanoate and pent-4-enoate for medium-chain acyl-CoA ligase. 6. There was less inhibition of the oxidation of pyruvate by pent-4-enoyl-l-carnitine, and of palmitoyl-l-carnitine by cyclopropanecarbonyl-l-carnitine, after pre-incubation with 10mm-arsenate. This suggests that these inhibitions were caused either by depletion of free CoA or by increase of acyl-CoA concentrations, since arsenate deacylates intramitochondrial acyl-CoA. There was little effect on the inhibition of palmitoyl-l-carnitine oxidation by pent-4-enoyl-l-carnitine. 7. Penta-2,4-dienoate strongly inhibited palmitoyl-l-carnitine oxidation in coupled mitochondria; acrylate only inhibited slightly. 8. Pent-4-enoate (0.1mm) caused a rapid and almost complete decrease in free CoA and a large increase in acid-soluble acyl-CoA when incubated with coupled mitochondria. Cyclopropanecarboxylate caused a similar decrease in CoA, with an equivalent rise in acid-soluble acyl-CoA concentrations. n-Pentanoate caused extensive lowering of CoA and a large increase in acid-soluble acyl-CoA and acetyl-CoA concentrations. Octanoate caused a 50% lowering of CoA and an increase in acid-soluble acyl-CoA and acetyl-CoA concentrations. 9. Cyclopropanecarboxylate and n-pentanoate were less potent inhibitors of palmitate oxidation than was pent-4-enoate. 10. It is concluded that pent-4-enoate causes a specific inhibition of β-oxidation after the formation intramitochondrially of its metabolites.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allred J. B., Guy D. G. Determination of coenzyme A and acetyl CoA in tissue extracts. Anal Biochem. 1969 May;29(2):293–299. doi: 10.1016/0003-2697(69)90312-1. [DOI] [PubMed] [Google Scholar]
- Bohmer T., Bremer J. Propionylcarnitine. Physiological variations in vivo. Biochim Biophys Acta. 1968 May 1;152(3):559–567. doi: 10.1016/0005-2760(68)90096-9. [DOI] [PubMed] [Google Scholar]
- Bremer J. Comparison of acylcarnitines and pyruvate as substrates for rat-liver mitochondria. Biochim Biophys Acta. 1966 Feb 1;116(1):1–11. doi: 10.1016/0005-2760(66)90087-7. [DOI] [PubMed] [Google Scholar]
- Brendel K., Corredor C. F., Bressler R. The effect of 4-pentenoic acid on fatty acid oxidation. Biochem Biophys Res Commun. 1969 Feb 7;34(3):340–347. doi: 10.1016/0006-291x(69)90838-9. [DOI] [PubMed] [Google Scholar]
- Bressler R., Corredor C., Brendel K. Hypoglycin and hypoglycin-like compounds. Pharmacol Rev. 1969 Jun;21(2):105–130. [PubMed] [Google Scholar]
- CHEN K. K., ANDERSON R. C., McCOWEN M. C., HARRIS P. N. Pharmacologic action of hypoglycin A and B. J Pharmacol Exp Ther. 1957 Nov;121(3):272–285. [PubMed] [Google Scholar]
- Chase J. F., Tubbs P. K. Some kinetic studies on the mechanism of action of carnitine acetyltransferase. Biochem J. 1966 Apr;99(1):32–40. doi: 10.1042/bj0990032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase J. F., Tubbs P. K. Specific inhibition of mitochondrial fatty acid oxidation by 2-bromopalmitate and its coenzyme A and carnitine esters. Biochem J. 1972 Aug;129(1):55–65. doi: 10.1042/bj1290055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciman M., Rossi C. R., Siliprandi N. On the mechanism of the antiketogenic action of propionate and succinate in isolated rat liver mitochondria. FEBS Lett. 1972 Apr 15;22(1):8–10. doi: 10.1016/0014-5793(72)80205-9. [DOI] [PubMed] [Google Scholar]
- Corredor C., Brendel K., Bressler R. Effects of 4-pentenoic acid on carbohydrate metabolism in pigeon liver homogenate. J Biol Chem. 1969 Mar 10;244(5):1212–1219. [PubMed] [Google Scholar]
- Corredor C., Brendel K., Bressler R. Studies of the mechanism of the hypoglycemic action of 4-pentenoic acid. Proc Natl Acad Sci U S A. 1967 Dec;58(6):2299–2306. doi: 10.1073/pnas.58.6.2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncombe W. G., Rising T. J. Biosynthesis of cyclopropyl long-chain fatty acids from cyclopropanecarboxylic acid by mammalian tissues in vitro. Biochem J. 1968 Sep;109(3):449–455. doi: 10.1042/bj1090449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Entman M., Bressler R. The mechanism of action of hypoglycin on long-chain fatty acid oxidation. Mol Pharmacol. 1967 Jul;3(4):333–340. [PubMed] [Google Scholar]
- FRIEDMAN S., MCFARLANE J. E., BHATTACHARYYA P. K., FRAENKEL G. Quantitative separation and identification of quaternary ammonium bases. Arch Biochem Biophys. 1955 Dec;59(2):484–490. doi: 10.1016/0003-9861(55)90514-2. [DOI] [PubMed] [Google Scholar]
- FRITZ I. B., YUE K. T. LONG-CHAIN CARNITINE ACYLTRANSFERASE AND THE ROLE OF ACYLCARNITINE DERIVATIVES IN THE CATALYTIC INCREASE OF FATTY ACID OXIDATION INDUCED BY CARNITINE. J Lipid Res. 1963 Jul;4:279–288. [PubMed] [Google Scholar]
- Fukami M. H., Williamson J. R. On the mechanism of inhibition of fatty acid oxidation by 4-pentenoic acid in rat liver mitochondria. J Biol Chem. 1971 Mar 10;246(5):1206–1212. [PubMed] [Google Scholar]
- Galzigna L., Rossi C. R., Sartorelli L., Gibson D. M. A guanosine triphosphate-dependent acyl coenzyme A synthetase from rat liver mitochondria. J Biol Chem. 1967 May 10;242(9):2111–2115. [PubMed] [Google Scholar]
- Garland P. B. Control of citrate synthesis in mitochondria. Biochem Soc Symp. 1968;27:41–60. [PubMed] [Google Scholar]
- Garland P. B., Shepherd D., Yates D. W. Steady-state concentrations of coenzyme A, acetyl-coenzyme A and long-chain fatty acyl-coenzyme A in rat-liver mitochondria oxidizing palmitate. Biochem J. 1965 Nov;97(2):587–594. doi: 10.1042/bj0970587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland P. B., Yates D. W., Haddock B. A. Spectrophotometric studies of acyl-coenzyme A synthetases of rat liver mitochondria. Biochem J. 1970 Sep;119(3):553–564. doi: 10.1042/bj1190553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland P. C., Senior A. E., Sherratt H. S. Biochemical effects of the hypoglycaemic compound pent-4-enoic acid and related non-hypoglycaemic fatty acids. Effects of their coenzyme A esters on enzymes of fatty acid oxidation. Biochem J. 1973 Sep;136(1):173–184. doi: 10.1042/bj1360173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitsky D. O., Skulachev V. P. Carnitine: the carrier transporting fatty acyls into mitochondria by means of an electrochemical gradient of H + . Biochim Biophys Acta. 1972 Jul 12;275(1):33–50. doi: 10.1016/0005-2728(72)90022-9. [DOI] [PubMed] [Google Scholar]
- MAHLER H. R., WAKIL S. J., BOCK R. M. Studies on fatty acid oxidation. I. Enzymatic activation of fatty acids. J Biol Chem. 1953 Sep;204(1):453–468. [PubMed] [Google Scholar]
- Marley J., Sherratt H. S. The apparent failure of L-carnitine to prevent the hypoglycaemia and hypothermia caused by hypoglycin or by pent-4-enoic acid in mice. Biochem Pharmacol. 1973 Jan 15;22(2):281–284. doi: 10.1016/0006-2952(73)90284-0. [DOI] [PubMed] [Google Scholar]
- RENDINA G., COON M. J. Enzymatic hydrolysis of the coenzyme a thiol esters of beta-hydroxypropionic and beta-hydroxyisobutyric acids. J Biol Chem. 1957 Mar;225(1):523–534. [PubMed] [Google Scholar]
- Ruderman N., Shafrir E., Bressler R. Relation of fatty acid oxidation tgluconeogenesis: effect of pentenoic acid. Life Sci. 1968 Oct 15;7(20):1083–1089. doi: 10.1016/0024-3205(68)90145-8. [DOI] [PubMed] [Google Scholar]
- SNYDER F., STEPHENS N. A simplified spectrophotometric determination of ester groups in lipids. Biochim Biophys Acta. 1959 Jul;34:244–245. doi: 10.1016/0006-3002(59)90255-0. [DOI] [PubMed] [Google Scholar]
- STRACK E., LORENZ I. Zur Bestimmung des Carnitins. Hoppe Seylers Z Physiol Chem. 1954;298(1-2):27–33. [PubMed] [Google Scholar]
- Senior A. E., Robson B., Sherratt H. S. Biochemical effects of the hypoglycaemic compound pent--4-enoic acid and related non-hypoglycaemic fatty acids. Biochem J. 1968 Dec;110(3):511–519. doi: 10.1042/bj1100511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senior A. E., Sherratt H. S. Biochemical effects of the hypoglycaemic compound pent-4-enoic acid and related non-hypoglycaemic fatty acids. Carbohydrate metabolism. Biochem J. 1968 Dec;110(3):521–527. doi: 10.1042/bj1100521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senior A. E., Sherratt H. S. Biochemical effects of the hypoglycaemic compound pent-4-enoic acid and related non-hypoglycaemic fatty acids. Oxidative phosphorylation and mitochondrial oxidation of pyruvate, 3-hydroxybutyrate and tricarboxylic acid-cycle intermediates. Biochem J. 1968 Dec;110(3):499–509. doi: 10.1042/bj1100499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stucki J. W., Brawand F., Walter P. Regulation of pyruvate metabolim in rat-liver mitochondria by adenine nucleotides and fatty acids. Eur J Biochem. 1972 May;27(1):181–191. doi: 10.1111/j.1432-1033.1972.tb01824.x. [DOI] [PubMed] [Google Scholar]
- Tanaka K., Isselbacher K. J., Shih V. Isovaleric and -methylbutyric acidemias induced by hypoglycin A: mechanism of Jamaican vomiting sickness. Science. 1972 Jan 7;175(4017):69–71. doi: 10.1126/science.175.4017.69. [DOI] [PubMed] [Google Scholar]
- Toews C. J., Lowy C., Ruderman N. B. The regulation of gluconeogenesis. The effect of pent-4-enoic acid on gluconeogenesis and on the gluconeogenic metabolite concentrations of isolated perfused rat liver. J Biol Chem. 1970 Feb 25;245(4):818–824. [PubMed] [Google Scholar]
- VAGELOS P. R., EARL J. M., STADTMAN E. R. Propionic acid metabolism. II. Enzymatic synthesis of lactyl pantethine. J Biol Chem. 1959 Apr;234(4):765–769. [PubMed] [Google Scholar]
- Von Holt C. Methylenecyclopropaneacetic acid, a metabolite of hypoglycin. Biochim Biophys Acta. 1966 Aug 3;125(1):1–10. doi: 10.1016/0005-2760(66)90138-x. [DOI] [PubMed] [Google Scholar]
- West D. W., Chase J. F., Tubbs P. K. The separation and properties of two forms of carnitine palmitoyltransferase from ox liver mitochondria. Biochem Biophys Res Commun. 1971 Mar 5;42(5):912–918. doi: 10.1016/0006-291x(71)90517-1. [DOI] [PubMed] [Google Scholar]
- Williamson J. R., Fukami M. H., Peterson M. J., Rostand S. G., Scholz R. Effect of 4-pentenoic acid on coenzyme A metabolites in rat liver. Biochem Biophys Res Commun. 1969 Aug 7;36(3):407–413. doi: 10.1016/0006-291x(69)90579-8. [DOI] [PubMed] [Google Scholar]
- Williamson J. R., Rostand S. G., Peterson M. J. Control factors affecting gluconeogenesis in perfused rat liver. Effects of 4-pentenoic acid. J Biol Chem. 1970 Jun;245(12):3242–3251. [PubMed] [Google Scholar]
- Yardley H. J., Godfrey G. Some in vitro effects of hypoglycin on skin. Arch Dermatol. 1967 Jul;96(1):89–93. [PubMed] [Google Scholar]
- Ziegler H. J., Bruckner P., Binon F. O-acylation of dl-carnitine chloride. J Org Chem. 1967 Dec;32(12):3989–3991. doi: 10.1021/jo01287a057. [DOI] [PubMed] [Google Scholar]
- van Tol A., de Jong J. W., Hulsmann W. C. On fatty acid activation in rat liver mitochondria. Biochim Biophys Acta. 1969 Mar 4;176(2):414–416. doi: 10.1016/0005-2760(69)90200-8. [DOI] [PubMed] [Google Scholar]


