Abstract
Purpose
Stage III colorectal cancer (CRC) is typically treated with surgery; however, it has a high recurrence rate and inconsistent benefits from postoperative chemotherapy. Inflammatory markers like the neutrophil-to-lymphocyte ratio (NLR) have shown prognostic value in various cancers. However, the prognostic significance of NLR measured before and after CRC surgery is not clear. This study aims to clarify the prognostic value of the combination of pre- and post-surgery NLR in stage III CRC patients.
Methods
Patients with stage III CRC treated between 2001 and 2022 were retrospectively analyzed using data from the Chang Gung Medical Research Database. Patients were categorized into 4 groups based on their pre- and post-operative NLR levels. Kaplan–Meier survival analysis and Cox proportional hazard models were used to assess the associations between NLR levels and overall survival (OS), disease-free survival (DFS), and cancer-specific survival (CSS).
Results
Data from 2,742 patients, median age of 62 years and 54% male, were analyzed. After adjustment, patients in Group IV, with high NLR values both before and after surgery, had greater risks of worse DFS (adjusted hazard ratio [aHR] = 1.30, 95% confidence interval [CI]: 1.13–1.50), OS (aHR = 1.36, 95% CI: 1.14–1.63), and CSS (aHR = 1.27, 95% CI: 1.04–1.55) compared to Group I.
Conclusions
High NLR levels before and after surgery is a strong predictor of poor outcomes in stage III CRC patients. The findings suggest that monitoring NLR at both time points can be a valuable prognostic tool, guiding postoperative care and treatment strategies to improve patient outcomes.
Keywords: Neutrophil-to-lymphocyte ratio (NLR), Prognosis, Overall survival (OS), Colorectal cancer (CRC), Disease-free survival (DFS)
Introduction
Colorectal cancer (CRC) remains one of the most common cancers globally. Over 1.9 million new cases of CRC and approximately 930,000 deaths were estimated globally in 2020 [1]. In the United States, CRC is the second leading cause of cancer-related deaths. In 2023, approximately 153,000 persons were diagnosed with CRC, and 52,500 died from the disease [2]. Among patients with stage III CRC, 33% experience cancer recurrence, with 82% of the recurrences occurring within the first 3 years after diagnosis, peaking between 1 and 2 years after initial treatment [3]. The standard treatment for stage III CRC includes fluoropyrimidine- and oxaliplatin-based chemotherapy following surgery; however, postoperative chemotherapy benefits less than 20% of patients with stage III CRC, and more than 50% develop distant metastases [4]. For stage II/III CRC, the optimal adjuvant therapeutic strategies after curative surgery remain a topic of debate [5]. Therefore, precise and individualized treatment approaches are essential.
Recent research has emphasized the prognostic value of inflammatory markers and blood indices, such as the neutrophil-to-lymphocyte ratio (NLR), in various cancers [6–8]. Elevated NLR values often indicate a heightened inflammatory response, which can promote tumor progression and metastasis [9–11]. Several analyses have also indicated that solid tumors, whether metastatic or not, have a poor prognosis when accompanied by an elevated NLR [12, 13]. However, there is a significant gap in the literature regarding the prognostic value of the NLR measured before and after surgical resection of CRC. This is crucial because surgery is a cornerstone in the management of stage III CRC.
Notably, no study has assessed the prognostic value of combined pre- and post-operative NLR measurements, particularly in CRC. Therefore, this study aims to evaluate the prognostic value of the NLR measured before and after surgery in patients with stage III CRC. By analyzing a large database, we seek to determine whether the NLR can serve as a reliable marker for predicting long-term outcomes and guiding clinical decision-making in the perioperative setting, ultimately optimizing post-operative care strategies.
Methods
Data source
This population-based retrospective study utilized data from the Cancer Registry of the Chang Gung Medical Research Database (CGRD), established by Taiwan's Chang Gung Medical Foundation. This database serves as a vital resource for clinical and epidemiological research. The CGRD is compiled from the extensive Chang Gung Memorial Hospital network, which includes 4 medical centers and is the largest in Taiwan. The database contains comprehensive patient demographic information, medical history, treatments, and outcomes [14, 15].
Study design and patient selection
We retrospectively reviewed data of patients with pathologically confirmed stage III CRC verified by catastrophic illness certifications from the Cancer Registry, treated between January 2001 and April 2022. Patients were excluded if: 1) Their surgery date did not match the payment date; 2) Absent preoperative and/or postoperative NLR measurements within the defined time window; 3) Without complete data of interest; 4) Primary tumor was located in overlapping lesions of the colon (ICD-10 code: C18.8) or classified as a malignant neoplasm of the colon, unspecified (ICD-10 code: C18.9); 5) Pathological staging data were missing.
Ethics statement
This study was approved by the Institutional Review Board (IRB) of the Chang-Gung Medical Center (approval date: September 14, 2017; IRB No.: 20171098). We analyzed a pre-collected dataset that had been de-identified to ensure anonymity and protect the privacy of the patients. All patient data were handled in strict compliance with ethical guidelines to maintain confidentiality, and ensure that no individual could be identified from the data used. The requirement for patient informed consent was waived by the IRB of Chang-Gung Medical Center due to the retrospective nature of the study.
Outcomes and variables
The primary outcomes were overall survival (OS), disease-free survival (DFS), and cancer-specific survival (CSS). DFS was defined as the length of time from the date of surgery until the date of recurrence of CRC or death from any cause, whichever occurred first. OS was the length of time from the date of surgery until the date of death from any cause. CSS was the length of time from the date of surgery until the date of death due to CRC.
The NLR was measured before and after surgery. The preoperative NLR was ideally measured on the day before the surgery. However, if this measurement was not available the measurement taken within 30 days before the surgery and closest to the day before surgery was used. The postoperative NLR value used was measured closest to 90 days after surgery, within 14 days before or after 90 days postoperatively.
Patients were divided into 4 groups based on the median values of NLR (preoperative: 2.5, postoperative: 1.5) of the study cohort: Group I (low preoperative NLR and low postoperative NLR), Group II (low preoperative NLR and high postoperative NLR), Group III (high preoperative NLR and low postoperative NLR), and Group IV (high preoperative NLR and high postoperative NLR). Since there was no universal consensus on the optimal NLR cutoff in the context of colorectal cancer prognosis, we adopted the use of the median value as the cutoff. This decision was made after considering the variability in previously reported cutoff values and their dependence on study populations, methodologies, and clinical settings. Using the median ensures a balanced distribution of patients across groups, which facilitates more robust statistical comparisons.
Demographic, clinical, and laboratory parameters collected from the database included patient age and sex, chemotherapy regimen, comorbidities, tumor location, T stage, N stage, histologic grade, and laboratory data. The laboratory data included white blood cell (WBC) count, red blood cell (RBC) count, platelet count, red cell distribution width (RDW), hemoglobin level, mean corpuscular volume (MCV), neutrophil segment percentage, lymphocyte percentage, albumin level, carcinoembryonic antigen (CEA) level, and platelet-to-lymphocyte ratio (PLR).
Statistical analysis
Continuous variables were presented as median and interquartile range (IQR), and categorical variables as count and percentage. To compare clinical characteristics among the 4 NLR groups, the Kruskal–Wallis test was used to compare continuous variables, and the chi-square test was used for categorical variables. The DFS, OS, and CSS of different NLR groups were analyzed using the Kaplan–Meier method and compared with the log-rank test. Cox proportional hazard models were employed to measure the hazard ratios (HRs) and 95% confidence intervals (CIs) for DFS, OS, and CSS in univariate and multivariable models. The adjusted covariates in the multivariable model were using stepwise model selection techniques with the candidate factors including age, sex, chemotherapy, all comorbidities, tumor location, T stage, N stage, histologic grade, CEA, hemoglobin, and albumin to estimate the effect of NLR for outcomes. All statistical analyses were conducted using SAS software version 9.4 (SAS Institute Inc., Cary, NC, USA). A 2-sided p-value of < 0.05 indicated statistical significance.
Results
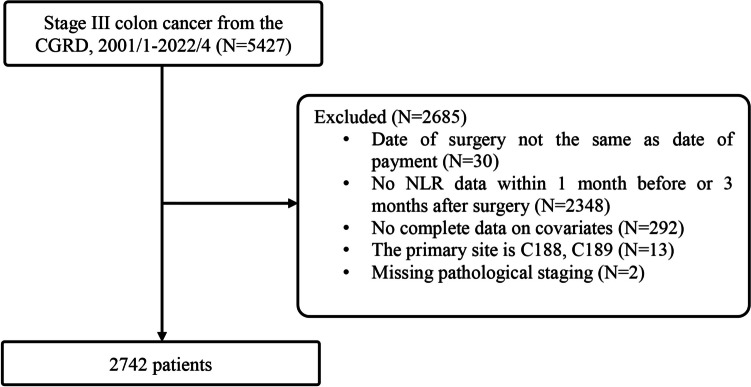
Data were extracted for a total of 5,427 patients with pathological stage III CRC treated between January 2001 and April 2022. A total of 2,685 patients were excluded: surgery date did not match the billing surgery date (n = 30); absent NLR data (n = 2,348); absent covariate data (n = 292); primary tumors overlapping sites of the colon (C18.8) or malignant neoplasm of the colon, unspecified (C18.9) (n = 13); pathological T stage recorded as X and N stage 0 (n = 2). Thus, 2,742 patients were included in the statistical analysis. The study flowchart is shown in Fig. 1.
Fig. 1.
Flow diagram of the patient selection process
Patient characteristics
A summary of patient characteristics is shown in Table 1. The median age of the patients was 62 years, and 54% were male. About 22% of the patients had diabetes and 40% hypertension, and about 57% of the patients had left-sided colon tumors. With respect to the pathological T stage, 8.5% of patients were stage 1 or 2, 58% were stage 3, and 34% were stage 4. About 62% of patients were N stage 1 and 38% were N stage 2. Histologic grading indicated that 64% of tumors were well or moderately differentiated. Significant differences were observed across most variables among the 4 NLR groups, except for sex, coronary artery disease (CAD), and N stage.
Table 1.
Patient characteristics categorized by preoperative and postoperative NLR
| Characteristics | Total | Group I Preoperative NLR ≤ 2.5 Postoperative NLR ≤ 1.5 |
Group II Preoperative NLR ≤ 2.5 Postoperative NLR > 1.5 |
Group III Preoperative NLR > 2.5 Postoperative NLR ≤ 1.5 |
Group IV Preoperative NLR > 2.5 Postoperative NLR > 1.5 |
p-value |
|---|---|---|---|---|---|---|
| (N = 2742) | (n = 858) | (n = 478) | (n = 511) | (n = 895) | ||
| Age, years | 62.0 (53.0–70.0) | 60.0 (53.0–67.0) | 63.0 (54.0–71.0) | 61.0 (50.0–68.0) | 63.0 (53.0–72.0) | < 0.001 |
| 18–64 | 1646 (60.0) | 566 (66.0) | 266 (55.6) | 320 (62.6) | 494 (55.2) | < 0.001 |
| ≥ 65 | 1096 (40.0) | 292 (34.0) | 212 (44.4) | 191 (37.4) | 401 (44.8) | |
| Sex | 0.319 | |||||
| Male | 1474 (53.8) | 454 (52.9) | 246 (51.5) | 271 (53.0) | 503 (56.2) | |
| Female | 1268 (46.2) | 404 (47.1) | 232 (48.5) | 240 (47.0) | 392 (43.8) | |
| Chemotherapy | < 0.001 | |||||
| IRI, OXA | 1790 (65.3) | 602 (70.2) | 299 (62.6) | 373 (73.0) | 516 (57.7) | |
| 5FU, others | 580 (21.2) | 173 (20.2) | 111 (23.2) | 95 (18.6) | 201 (22.5) | |
|
Chemotherapy < 4 times/ duration < 3 months/ no chemotherapy |
372 (13.6) | 83 (9.7) | 68 (14.2) | 43 (8.4) | 178 (19.9) | |
| Comorbidity | ||||||
| DM | 607 (22.1) | 156 (18.2) | 122 (25.5) | 93 (18.2) | 236 (26.4) | < 0.001 |
| HTN | 1094 (39.9) | 291 (33.9) | 217 (45.4) | 172 (33.7) | 414 (46.3) | < 0.001 |
| CAD | 144 (5.3) | 35 (4.1) | 28 (5.9) | 23 (4.5) | 58 (6.5) | 0.111 |
| CVA | 117 (4.3) | 22 (2.6) | 23 (4.8) | 11 (2.2) | 61 (6.8) | < 0.001 |
| CKD | 102 (3.7) | 9 (1.0) | 18 (3.8) | 13 (2.5) | 62 (6.9) | < 0.001 |
| COPD/Asthma | 128 (4.7) | 22 (2.6) | 19 (4.0) | 25 (4.9) | 62 (6.9) | < 0.001 |
| Tumor location | < 0.001 | |||||
| Right | 1182 (43.1) | 327 (38.1) | 180 (37.7) | 254 (49.7) | 421 (47.0) | |
| Left | 1560 (56.9) | 531 (61.9) | 298 (62.3) | 257 (50.3) | 474 (53.0) | |
| T stage | < 0.001 | |||||
| 1, 2 | 233 (8.5) | 101 (11.8) | 63 (13.2) | 16 (3.1) | 53 (5.9) | |
| 3 | 1585 (57.8) | 534 (62.2) | 281 (58.8) | 263 (51.5) | 507 (56.6) | |
| 4 | 924 (33.7) | 223 (26.0) | 134 (28.0) | 232 (45.4) | 335 (37.4) | |
| N stage | 0.955 | |||||
| 1 | 1705 (62.2) | 527 (61.4) | 298 (62.3) | 319 (62.4) | 561 (62.7) | |
| 2 | 1037 (37.8) | 331 (38.6) | 180 (37.7) | 192 (37.6) | 334 (37.3) | |
| Histologic Grade | < 0.001 | |||||
| Well, moderately differentiated | 1750 (63.8) | 595 (69.3) | 320 (66.9) | 301 (58.9) | 534 (59.7) | |
| Poorly differentiated, Undifferentiated | 301 (11.0) | 67 (7.8) | 43 (9.0) | 75 (14.7) | 116 (13.0) | |
| Unknown | 691 (25.2) | 196 (22.8) | 115 (24.1) | 135 (26.4) | 245 (27.4) | |
| CEA, ng/mL | < 0.001 | |||||
| Normal (< 5) | 1381 (50.4) | 454 (52.9) | 281 (58.8) | 243 (47.6) | 403 (45.0) | |
| Elevated (≥ 5) | 862 (31.4) | 230 (26.8) | 101 (21.1) | 197 (38.6) | 334 (37.3) | |
| Unknown | 499 (18.2) | 174 (20.3) | 96 (20.1) | 71 (13.9) | 158 (17.7) | |
| WBC, 103/µL | 7 (5.8–8.6) | 6.3 (5.3–7.4) | 6.2 (5.3–7.4) | 8.2 (6.6–10.3) | 7.8 (6.3–9.8) | < 0.001 |
| RBC, 106/µL | 4.41 (4.0–4.8) | 4.5 (4.1–4.8) | 4.39 (4.0–4.8) | 4.41 (4.0–4.8) | 4.33 (3.9–4.8) | < 0.001 |
| Platelet, 103/µL | 272.0 (220.0–341.0) | 256.5 (211.0–319.0) | 251.5 (209.0–312.0) | 303 (241.0–387.0) | 284 (225.0–354.0) | < 0.001 |
| RDW, % | 13.8 (13.0–16.1) | 13.5 (12.9–14.9) | 13.5 (12.8–15.0) | 14.5 (13.2–17.3) | 14.4 (13.1–17.0) | < 0.001 |
| Hemoglobin, g/dL | 12.1 (10.0–13.7) | 12.7 (11.0–14.0) | 12.4 (10.6–13.7) | 11.6 (9.6–13.3) | 11.5 (9.5–13.4) | < 0.001 |
| MCV, fL | 85.9 (77.2–90.2) | 87.5 (80–91.1) | 87.0 (79.5–90.4) | 83.9 (74.8–89.4) | 84.8 (76.1–89.5) | < 0.001 |
| Segment, % | 65.4 (58.7–72.3) | 57.5 (52.5–61.7) | 60.0 (56.0–63.0) | 71.9 (67.9–77.0) | 72.2 (68.4–77.7) | < 0.001 |
| Lymphocyte, % | 25.8 (18.9–31.8) | 32.9 (29.6–37.8) | 30.4 (28.0–34.0) | 19.7 (14.9–23.6) | 18.8 (14.0–22.5) | < 0.001 |
| Albumin, g/dL | 4.2 (3.8–4.4) (n = 2587) | 4.3 (4.0–4.5) (n = 834) | 4.3 (4.0–4.5) (n = 460) | 4.0 (3.5–4.3) (n = 467) | 4.0 (3.6–4.4) (n = 826) | < 0.001 |
| CEA, ng/mL | 3.4 (1.7–9.0) (n = 2243) | 3.0 (1.5–6.9) (n = 684) | 2.6 (1.4–5.3) (n = 382) | 4.1 (1.8–11.3) (n = 440) | 4.2 (2.1–13.2) (n = 737) | < 0.001 |
| NLR | 2.5 (1.9–3.8) | 1.8 (1.4–2.1) | 2.0 (1.7–2.3) | 3.65 (2.9–5.1) | 3.9 (3.0–5.5) | < 0.001 |
| PLR | 1.6 (1.2–2.3) | 1.2 (0.9–1.6) | 1.3 (1.0–1.7) | 2.1 (1.6–2.8) | 2.1 (1.5–2.9) | < 0.001 |
| Hemoglobin, g/dL a | < 0.001 | |||||
| Low | 1643 (59.9) | 429 (50.0) | 266 (55.6) | 341 (66.7) | 607 (67.8) | |
| Normal | 1099 (40.1) | 429 (50.0) | 212 (44.4) | 170 (33.3) | 288 (32.2) | |
| Albumin, g/dL | < 0.001 | |||||
| > 3.5 | 2194 (80.0) | 788 (91.8) | 426 (89.1) | 353 (69.1) | 627 (70.1) | |
| ≤ 3.5 | 393 (14.3) | 46 (5.4) | 34 (7.1) | 114 (22.3) | 199 (22.2) | |
| Unknown | 155 (5.7) | 24 (2.8) | 18 (3.8) | 44 (8.6) | 69 (7.7) |
Continuous variables are presented as median (IQR); categorical variables are presented as count (percentage)
p-values < 0.05 are shown in bold
DM diabetes mellitus, HTN hypertension, CAD coronary artery disease, CVA cardiovascular accident, CKD chronic kidney disease, COPD chronic obstructive pulmonary disease, CEA carcinoembryonic antigen, IRI irinotecan, OXA oxaliplatin, HR hazard ratio, aHR adjusted hazard ratio, CI confidence interval, RDW red cell distribution width, NLR neutrophil-to-lymphocyte ratio, PLR platelet-to-lymphocyte ratio, WBC white blood cell, RBC red blood cell, MCV mean corpuscular volume
aMale ≤ 13.5 g/dL, female ≤ 12 g/Dl
Approximately half of the patients (50.4%) had a normal CEA level. Of the group with a low preoperative NLR and a high postoperative NLR, 59% had a normal CEA level. Conversely, in the high preoperative NLR group, approximately 67% of patients had a low hemoglobin level (male ≤ 13.5 g/dL, female ≤ 12 g/dL), while a smaller proportion (around 22%) had a low albumin level. Significant differences were observed across all laboratory test data among the 4 NLR groups.
Associations between study variables and DFS
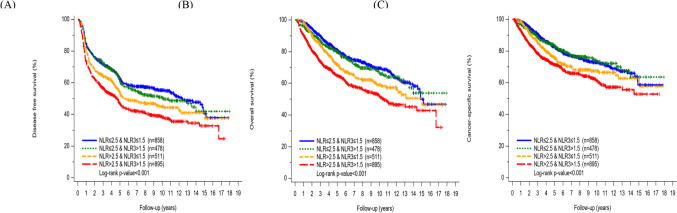
Kaplan–Meier curves for estimated survival demonstrated significant differences in DFS among the 4 NLR groups (log-rank p < 0.001) (Fig. 2A).
Fig. 2.
Kaplan–Meier curves for estimated survival. A) DFS. B) OS. C) CSS
In the univariate analysis, patients in NLR Group III had a significantly higher HR for DFS compared to those in Group I (HR = 1.30, 95% confidence interval [CI]: 1.10 to 1.52). Similarly, patients in Group IV had a significantly greater HR than those in Group I (HR = 1.63, 95% CI: 1.43 to 1.87). After adjusting for relevant covariates, including age, sex, chemotherapy agent, chronic kidney disease (CKD), T stage, N stage, histologic grade, CEA, and albumin, patients in NLR group IV had a significantly greater HR for DFS (Group IV vs. Group I: aHR = 1.30, 95% CI: 1.13 to 1.50) (Table 2).
Table 2.
Associations between study variables and DFS
| Study variables | HR (95% CI) | p-value | aHR (95% CI) | p-value |
|---|---|---|---|---|
| NLR groupa | ||||
| Group I | Reference | Reference | ||
| Group II | 1.07 (0.90, 1.27) | 0.448 | 1.00 (0.84, 1.19) | 0.997 |
| Group III | 1.30 (1.10, 1.52) | 0.001 | 1.09 (0.93, 1.29) | 0.293 |
| Group IV | 1.63 (1.43, 1.87) | < 0.001 | 1.30 (1.13, 1.50) | < 0.001 |
| Age, years (≥ 65 vs. 18–64) | 1.60 (1.44, 1.78) | < 0.001 | 1.36 (1.21, 1.52) | < 0.001 |
| Male sex (vs. female) | 1.10 (0.99, 1.23) | 0.074 | 1.12 (1.00, 1.25) | 0.042 |
| Chemotherapy | ||||
| IRI, OXA | 0.44 (0.38, 0.50) | < 0.001 | 0.48 (0.42, 0.56) | < 0.001 |
| 5FU, others | 0.51 (0.43, 0.60) | < 0.001 | 0.57 (0.48, 0.67) | < 0.001 |
|
Chemotherapy < 4 times/ duration < 3 months/no chemotherapy |
Reference | Reference | ||
| Comorbidity | ||||
| DM | 1.24 (1.09, 1.40) | 0.001 | ||
| HTN | 1.14 (1.02, 1.27) | 0.019 | ||
| CAD | 1.53 (1.24, 1.89) | < 0.001 | ||
| CVA | 1.51 (1.19, 1.92) | 0.001 | ||
| CKD | 2.44 (1.93, 3.08) | < 0.001 | 1.42 (1.12, 1.81) | 0.004 |
| COPD/asthma | 1.38 (1.10, 1.74) | 0.005 | ||
| Tumor location (left vs. right) | 0.84 (0.76, 0.94) | 0.002 | ||
| T stage | ||||
| 1, 2 | Reference | Reference | ||
| 3 | 1.50 (1.19, 1.89) | 0.001 | 1.28 (1.01, 1.62) | 0.039 |
| 4 | 2.08 (1.65, 2.64) | < 0.001 | 1.67 (1.31, 2.13) | < 0.001 |
| N stage | ||||
| 1 | Reference | Reference | ||
| 2 | 1.40 (1.26, 1.56) | < 0.001 | 1.45 (1.30, 1.62) | < 0.001 |
| Histologic grade | ||||
| Well, moderately | Reference | Reference | ||
| Poorly, undifferentiated | 1.38 (1.17, 1.63) | < 0.001 | 1.25 (1.05, 1.48) | 0.010 |
| Unknown | 2.29 (2.00, 2.61) | < 0.001 | 2.37 (2.06, 2.72) | < 0.001 |
| CEA, ng/mL | ||||
| Normal (< 5) | Reference | Reference | ||
| Elevated (≥ 5) | 1.52 (1.35, 1.72) | < 0.001 | 1.30 (1.15, 1.47) | < 0.001 |
| Unknown | 1.05 (0.91, 1.22) | 0.520 | 1.11 (0.95, 1.29) | 0.202 |
| Hemoglobin, g/dLb | ||||
| Normal | Reference | |||
| Low | 1.20 (1.08, 1.35) | 0.001 | ||
| Albumin, g/dL | ||||
| > 3.5 | Reference | Reference | ||
| ≤ 3.5 | 1.78 (1.55, 2.03) | < 0.001 | 1.31 (1.13, 1.51) | < 0.001 |
| Unknown | 1.30 (1.04, 1.63) | 0.021 | 1.13 (0.90, 1.41) | 0.308 |
p-values < 0.05 are shown in bold
DFS disease-free survival, DM diabetes mellitus, HTN hypertension, CAD coronary artery disease, CVA cardiovascular accident, CKD chronic kidney disease, COPD chronic obstructive pulmonary disease, CEA carcinoembryonic antigen, IRI irinotecan, OXA oxaliplatin, HR hazard ratio, aHR adjusted hazard ratio, CI confidence interval
aGroup I: preoperative NLR ≤ 2.5, postoperative NLR ≤ 1.5
Group II: preoperative NLR ≤ 2.5 postoperative NLR > 1.5
Group III: preoperative NLR > 2.5 postoperative NLR ≤ 1.5
Group IV: preoperative NLR > 2.5 postoperative NLR > 1.5
bMale ≤ 13.5 g/dL, female ≤ 12 g/dL
Associations between study variables, OS and CSS
Kaplan–Meier curves for estimated survival demonstrated significant differences in OS and CSS among the 4 NLR groups (log-rank p < 0.001) (Fig. 2B and 2C).
For OS, the estimated crude HR for Group III versus Group I was 1.40 (95% CI: 1.14 to 1.72), and for Group IV versus Group I was 1.95 (95% CI: 1.65 to 2.31). Multiple Cox models constructed via stepwise selection showed that only Group IV had a significantly worse OS compared to Group I (aHR = 1.36, 95% CI: 1.14 to 1.63) (Table 3).
Table 3.
Associations between study variables and OS
| Study variables | HR (95% CI) | p-value | aHR (95% CI) | p-value |
|---|---|---|---|---|
| NLR groupa | ||||
| Group I | Reference | Reference | ||
| Group II | 1.10 (0.88, 1.37) | 0.410 | 0.96 (0.77, 1.20) | 0.733 |
| Group III | 1.40 (1.14, 1.72) | 0.001 | 1.04 (0.84, 1.29) | 0.727 |
| Group IV | 1.95 (1.65, 2.31) | < 0.001 | 1.36 (1.14, 1.63) | 0.001 |
| Age, years (≥ 65 vs. 18–64) | 2.04 (1.78, 2.33) | < 0.001 | 1.64 (1.42, 1.89) | < 0.001 |
| Male sex (vs. female) | 1.20 (1.05, 1.37) | 0.009 | 1.22 (1.06, 1.40) | 0.005 |
| Chemotherapy | ||||
| IRI, OXA | 0.29 (0.25, 0.35) | < 0.001 | 0.35 (0.30, 0.43) | < 0.001 |
| 5FU, others | 0.46 (0.38, 0.55) | < 0.001 | 0.47 (0.39, 0.57) | < 0.001 |
|
Chemotherapy < 4 times/ duration < 3 months/no chemotherapy |
Reference | Reference | ||
| Comorbidity | ||||
| DM | 1.39 (1.19, 1.62) | < 0.001 | ||
| HTN | 1.35 (1.18, 1.55) | < 0.001 | ||
| CAD | 1.66 (1.28, 2.15) | < 0.001 | ||
| CVA | 1.83 (1.37, 2.45) | < 0.001 | ||
| CKD | 3.30 (2.51, 4.34) | < 0.001 | 1.94 (1.46, 2.58) | < 0.001 |
| COPD/asthma | 1.56 (1.18, 2.06) | 0.002 | ||
| Tumor location (left vs. right) | 0.76 (0.66, 0.87) | < 0.001 | ||
| T stage | ||||
| 1, 2 | Reference | Reference | ||
| 3 | 1.84 (1.32, 2.58) | < 0.001 | 1.43 (1.02, 2.01) | 0.040 |
| 4 | 3.10 (2.21, 4.34) | < 0.001 | 2.15 (1.52, 3.04) | < 0.001 |
| N stage | ||||
| 1 | Reference | Reference | ||
| 2 | 1.80 (1.58, 2.06) | < 0.001 | 1.81 (1.58, 2.08) | < 0.001 |
| Histologic grade | ||||
| Well, Moderate | Reference | Reference | ||
| Poorly, Undifferentiated | 1.50 (1.25, 1.80) | < 0.001 | 1.29 (1.07, 1.55) | 0.009 |
| Unknown | 0.80 (0.63, 1.02) | 0.078 | 0.85 (0.67, 1.09) | 0.210 |
| CEA, ng/mL | ||||
| Normal (< 5) | Reference | Reference | ||
| Elevated (≥ 5) | 1.65 (1.41, 1.92) | < 0.001 | 1.28 (1.10, 1.50) | 0.002 |
| Unknown | 1.41 (1.18, 1.68) | < 0.001 | 1.19 (0.99, 1.43) | 0.058 |
| Hemoglobin, g/dLb | ||||
| Normal | Reference | |||
| Low | 1.41 (1.22, 1.63) | < 0.001 | ||
| Albumin, g/dL | ||||
| > 3.5 | Reference | Reference | ||
| ≤ 3.5 | 2.64 (2.26, 3.08) | < 0.001 | 1.65 (1.39, 1.95) | < 0.001 |
| Unknown | 1.54 (1.17, 2.03) | 0.002 | 1.29 (0.97, 1.71) | 0.081 |
P-values < 0.05 are shown in bold
aGroup I: pre-op NLR ≤ 2.5 and post-op NLR ≤ 1.5; Group II: pre-op NLR ≤ 2.5 and post-op NLR > 1.5; Group III: pre-op NLR > 2.5 and post-op NLR ≤ 1.5; Group IV: pre-op NLR > 2.5 and post-op NLR > 1.5
bMale ≤ 13.5 g/dL, female ≤ 12 g/dL
Similar results were found for CSS. In the univariate analysis, the crude HR of Group III was greater than that of Group I (HR = 1.31, 95% CI: 1.04 to 1.64), and for Group IV versus Group I was 1.72 (95% CI: 1.42 to 2.07). After adjustment, Group IV still had significantly worse CSS as compared with Group I (aHR = 1.27, 95% CI: 1.04 to 1.55) (Table 4).
Table 4.
Associations between study variables and CSS
| Study variables | HR (95% CI) | p-value | aHR (95% CI) | p-value |
|---|---|---|---|---|
| NLR group | ||||
| Group I | Reference | Reference | ||
| Group II | 0.98 (0.77, 1.26) | 0.884 | 0.90 (0.70, 1.15) | 0.398 |
| Group III | 1.31 (1.04, 1.64) | 0.021 | 0.97 (0.77, 1.23) | 0.823 |
| Group IV | 1.72 (1.42, 2.07) | < 0.001 | 1.27 (1.04, 1.55) | 0.017 |
| Age, years (≥ 65 vs. 18–64) | 1.63 (1.40, 1.89) | < 0.001 | 1.37 (1.17, 1.61) | < 0.001 |
| Sex (male vs. female) | 1.13 (0.97, 1.32) | 0.107 | ||
| Chemotherapy | ||||
| IRI, OXA | 0.33 (0.27, 0.40) | < 0.001 | 0.38 (0.31, 0.46) | < 0.001 |
| 5FU, others | 0.47 (0.38, 0.58) | < 0.001 | 0.47 (0.38, 0.59) | < 0.001 |
|
Chemotherapy < 4 times/ duration < 3 months/no chemotherapy |
Reference | Reference | ||
| Comorbidity | ||||
| DM | 1.18 (0.99, 1.41) | 0.071 | ||
| HTN | 1.06 (0.91, 1.24) | 0.451 | ||
| CAD | 1.44 (1.06, 1.96) | 0.021 | ||
| CVA | 1.46 (1.03, 2.09) | 0.036 | ||
| CKD | 2.46 (1.74, 3.48) | < 0.001 | 1.63 (1.14, 2.33) | 0.007 |
| COPD/asthma | 1.19 (0.83, 1.69) | 0.341 | ||
| Tumor location (left vs. right) | 0.75 (0.64, 0.87) | < 0.001 | ||
| T stage | ||||
| 1, 2 | Reference | Reference | ||
| 3 | 2.00 (1.34, 2.98) | 0.001 | 1.52 (1.02, 2.28) | 0.042 |
| 4 | 3.73 (2.50, 5.56) | < 0.001 | 2.58 (1.71, 3.89) | < 0.001 |
| N stage | ||||
| 1 | Reference | Reference | ||
| 2 | 2.03 (1.74, 2.36) | < 0.001 | 2.01 (1.73, 2.35) | < 0.001 |
| Histologic grade | ||||
| Well, moderately | Reference | Reference | ||
| Poorly, undifferentiated | 1.51 (1.23, 1.86) | < 0.001 | 1.33 (1.08, 1.63) | 0.008 |
| Unknown | 0.69 (0.52, 0.91) | 0.009 | 0.73 (0.55, 0.97) | 0.031 |
| CEA, ng/mL | ||||
| Normal (< 5) | Reference | Reference | ||
| Elevated (≥ 5) | 1.72 (1.45, 2.05) | < 0.001 | 1.36 (1.14, 1.63) | 0.001 |
| Unknown | 1.46 (1.20, 1.78) | < 0.001 | 1.22 (0.99, 1.50) | 0.059 |
| Hemoglobin, g/dL | ||||
| Normal | Reference | |||
| Low | 1.28 (1.09, 1.49) | 0.003 | ||
| Albumin, g/dL | ||||
| > 3.5 | Reference | Reference | ||
| ≤ 3.5 | 2.28 (1.90, 2.72) | < 0.001 | 1.44 (1.18, 1.75) | < 0.001 |
| Unknown | 1.53 (1.12, 2.07) | 0.007 | 1.23 (0.90, 1.68) | 0.197 |
P-values < 0.05 are shown in bold
CSS cancer-specific survival, DM diabetes mellitus, HTN hypertension, CAD coronary artery disease, CVA cardiovascular accident, CKD, chronic kidney disease, COPD chronic obstructive pulmonary disease, CEA carcinoembryonic antigen, IRI irinotecan, OXA oxaliplatin, HR hazard ratio, aHR adjusted hazard ratio, CI confidence interval
aGroup I: pre-op NLR ≤ 2.5 and post-op NLR ≤ 1.5; Group II: pre-op NLR ≤ 2.5 and post-op NLR > 1.5; Group III: pre-op NLR > 2.5 and post-op NLR ≤ 1.5; Group IV: pre-op NLR > 2.5 and post-op NLR > 1.5
bMale ≤ 13.5 g/dL, female ≤ 12 g/dL
Discussion
The study demonstrated that a high NLR before and after surgery is a strong predictor of poor outcomes in stage III CRC patients. Patients with high preoperative and postoperative NLR values (Group IV) had notably poorer survival outcomes in terms of DFS, OS, and CSS compared to those with low preoperative and postoperative NLR values (Group I). These findings suggest that measuring the NLR preoperatively and postoperatively can effectively predict long-term outcomes, thereby aiding in the optimization of postoperative care and treatment plans for CRC patients.
In the recent decade, many researchers have sought to use various blood indices as prognostic factors for surgical outcomes, and the outcomes of medical treatments for various conditions. Other indices like the NLR include the platelet-to-lymphocyte ratio (PLR), and the lymphocyte-to-monocyte ratio (LMR) [16]. Various indices have been found to have prognostic value in a number of malignancies including breast cancer, lung cancer, melanoma, and other solid tumor malignancies [7, 8, 11, 12]. The NLR is well studied with respect to malignancies and prognosis [17] and has also shown value in predicting postoperative complications in patients with CRC [18] and the severity of colorectal adenocarcinoma (NLR is directly proportional to tumor histological grade) [19].
A list of studies has examined the prognostic value of the NLR in patients with CRC. For instance, Iaciu et al. [20] studied 195 patients with CRC and reported a mean NLR of 3.42 ± 2.27. NLR value above 3 was classified as high based on the receiver operating characteristic (ROC) analysis. As a result, a high NLR was independently associated with significantly lower OS and PFS. Another study compared the prognostic value of the preoperative NLR with that of the PLR and LMR and reported that the NLR was the only independent prognostic factor for OS and PFS in patients with resectable CRC [21].
Our study examined the prognostic value of combining preoperative and postoperative NLRs; however, most studies have only examined the preoperative or the postoperative value separately except few. Xiao et al. [22] examined the value of the preoperative NLR, PLR, and LMR in patients undergoing surgery for CRC. The results showed that a high NLR or PLR was associated with significantly worse OS, while a high LMR was associated with improved OS. On the other hand, Yasui et al. [23] reported that the postoperative, but not the preoperative NLR was associated with the prognosis of CRC patients with stage III disease. Patients with high preoperative and postoperative NLR had significantly worse OS and RFS than those with low preoperative and postoperative values, and those with a high preoperative value but a low postoperative value (considered the “normalized” group). A recent systematic review and meta-analysis that included about 33,000 patients with CRC reported that a high pre-treatment NLR was significantly associated with poor clinical outcomes [24]. The pooled analysis of multivariate studies found an HR = 1.57 (p < 0.0001) for OS and HR = 1.38 (p = 0.0003) for the combined surrogate endpoints of compromising disease, recurrence, and PFS. In a study somewhat similar to ours, Guo et al. [25] examined the preoperative to postoperative change of NLR as a predictor of survival in patients with CRC. Multivariate analysis showed that preoperative NLR (p = 0.002) and ΔNLR (p = 0.037) were independent predictors of OS; however, they were not predictors of disease-free survival. Despite differences in study design and population characteristics, our findings are broadly consistent with previous studies, highlighting the importance of considering both pre- and postoperative NLR.
It is important to note that while our study focused exclusively on follow-up mortality outcomes in stage III CRC, it is well established that liver metastases are not uncommon in patients with CRC as the disease progresses. Notably, two recent systematic reviews and meta-analyses have reported that a high NLR is associated with a poor prognosis in patients with CRC and liver metastasis [26, 27].
It should be noted that, while there is no established consensus on the optimal NLR cutoff for prognostic purposes, studies have used various methods. For instance, Iaciu et al. classified an NLR value above 3 as high based on receiver operating characteristic (ROC) analysis [20]. Song et al. employed Harrell's concordance index (C-index) and Bayesian information criterion (BIC) to determine a cutoff of 2.0 [21], while Xiao et al. identified a cutoff of 2.81 through ROC analysis [22]. A meta-analysis by Naszai revealed that about half of the studies used data-driven methods, such as ROC curves, to define the NLR cutoff, while the other half did not [24]. In our analysis, the cutoff was determined by the median for its simplicity and objectivity. This method avoids bias from arbitrarily selected thresholds and classifies patients into two balanced groups. It also offers a reproducible approach that is adaptable to different populations.
Although it is not clear why indices such as the NLR and the PLR have prognostic value, it is generally believed that high values indicate an inflammatory state, and it is the inflammatory state that leads to poorer outcomes [28]. Notably, the immune response and the release of pro-inflammatory cytokines have been shown to have important roles in promoting tumor progression [28]. Moreover, surgical stress—especially the invasiveness of the procedure—can influence NLR levels, as they reflect the body's response to surgical stress. More invasive surgeries may exacerbate the inflammatory response, thereby further elevating NLR levels [29]. Hosseini et al. [30] compared the NLR and PLR in patients who received laparoscopy or laparotomy for CRC. The PLR and NLR were calculated preoperatively and on postoperative (POD) days 1 and 3. The results showed that both ratios were significantly increased in laparotomy patients on POD 1 compared with laparoscopy patients (p < 0.05); the preoperative and postoperative PLR were significantly different in the laparotomy group (p < 0.05) but not in the laparoscopy group (p > 0.05); the preoperative and postoperative NLR were significantly different in both laparoscopy and laparotomy groups (p < 0.05). These findings suggest that surgical stress associated with more invasive procedures like laparotomy may interact with and elevate NLR levels, further influencing patient outcomes. In a unique study, Xun et al. [31] studied the NLR in colorectal tissue of patients with CRC. The results showed that an elevated intratumoral and extratumoral NLR is associated with a poor prognosis. This further supports the notion that the inflammatory environment, both within and surrounding the tumor, plays a critical role in influencing disease outcomes.
Of note, one of the potential clinical implications of our findings is the ability to use pre- and postoperative NLR measurements to inform decisions such as adjuvant chemotherapy. For instance, after adjustment for relevant confounders, patients with a high preoperative NLR but a significantly reduced postoperative NLR demonstrated survival outcomes comparable to those with consistently low preoperative and postoperative NLR. This potentially indicates that adjuvant chemotherapy may be unnecessary for certain patients, and may have values in guiding more personalized treatment strategies in future practice.
Taken together, the results of our study and those of other studies suggest that the use of the NLR, particularly combining the pre- and postoperative measurements, may improve risk stratification for patients with CRC and thus tailor postoperative treatment strategies accordingly. In addition, while our study focused on patients with CRC the findings may have broader implications for other cancers where systemic inflammation plays a crucial role, and the use of dual time-point NLR assessment could be explored in future research across different cancer types to determine its generalizability as a prognostic marker.
Strengths and limitations
This study has several strengths. The large sample size of more than 2,000 patients with stage III CRC provides robust statistical power and enhances the reliability of the findings. The comprehensive data collection from the detailed Cancer Registry of the CGRD allowed for capturing extensive demographic, clinical, and laboratory information, facilitating a thorough analysis. Additionally, the inclusion of both preoperative and postoperative NLR measurements offers a novel approach, potentially providing a more comprehensive prognostic tool compared to previous studies that only considered one of these time points. However, this study has several limitations that should be considered. The retrospective design may introduce selection bias and limit the ability to establish causal relations. Additionally, because the data were from a single healthcare system the results may not be generalizable to other populations or healthcare settings. Further, the exclusion of patients without complete NLR data before and after surgery could introduce bias, as these patients may have different characteristics or outcomes as the included patients. Lastly, the findings have not been validated in an external cohort, which is necessary to confirm the utility of NLR as a prognostic marker in diverse clinical settings.
Conclusion
Our findings demonstrate that elevated NLR values preoperatively and postoperatively is a strong predictor of poor DFS, OS, and CSS. Accordingly, the incorporation of the combination of preoperative and postoperative NLR values into clinical practice may provide a more comprehensive prognostic tool, aiding in the optimization of postoperative care and treatment plans for CRC patients. Future studies should focus on validating these findings in diverse clinical settings and exploring the underlying mechanisms linking NLR to cancer progression and prognosis.
Acknowledgements
The authors appreciate the Biostatistics Center, Kaohsiung Chang Gung Memorial Hospital for statistical analysis.
Authors contribution
Conceptualization; Data curation; Formal Analysis; Investigation; Methodology; Project administration; Resources; Software; Supervision; Visualization; Writing – original draft; Writing – original draft: Yun Lee, Kung-Chuan Cheng Funding acquisition: Yun Lee, Kung-Chuan Cheng, Yueh-Ming Lin, Chien-Chang Lu, Ko-Chao Lee Validation: Kung-Chuan Cheng.
Funding
This study is funded by Chang Gung Medical Foundation (grant number: CFRPG8M0031).
Data availability
All of the data supporting underlying findings are included in the manuscript.
Declarations
Conflicts of interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Morgan E, Arnold M, Gini A et al (2023) Global burden of colorectal cancer in 2020 and 2040: incidence and mortality estimates from GLOBOCAN. Gut 72(2):338–344. 10.1136/gutjnl-2022-327736 [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Wagle NS, Cercek A, Smith RA (2023) Jemal A (2023) Colorectal cancer statistics. CA Cancer J Clin 73(3):233–254. 10.3322/caac.21772 [DOI] [PubMed] [Google Scholar]
- 3.Chang YT, Tsai HL, Chen YC et al (2023) Clinicopathological Features and Oncological Outcomes of Early and Late Recurrence in Stage III Colorectal Cancer Patients after Adjuvant Oxaliplatin-Based Therapy. J Oncol 2023:2439128. 10.1155/2023/2439128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stachtea X, Loughrey MB, Salvucci M et al (2022) Stratification of chemotherapy-treated stage III colorectal cancer patients using multiplexed imaging and single-cell analysis of T-cell populations. Mod Pathol 35(4):564–576. 10.1038/s41379-021-00953-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luo XJ, Zhao Q, Liu J et al (2021) Novel Genetic and Epigenetic Biomarkers of Prognostic and Predictive Significance in Stage II/III Colorectal Cancer. Mol Ther 29(2):587–596. 10.1016/j.ymthe.2020.12.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mady M, Prasai K, Tella SH et al (2020) Neutrophil to lymphocyte ratio as a prognostic marker in metastatic gallbladder cancer. HPB (Oxford) 22(10):1490–1495. 10.1016/j.hpb.2020.02.002 [DOI] [PubMed] [Google Scholar]
- 7.Corbeau I, Jacot W, Guiu S (2020) Neutrophil to Lymphocyte Ratio as Prognostic and Predictive Factor in Breast Cancer Patients: A Systematic Review. Cancers (Basel) 12(4):958. 10.3390/cancers12040958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith D, Raices M, Cayol F, Corvatta F, Caram L, Dietrich A (2022) Is the neutrophil-to-lymphocyte ratio a prognostic factor in non-small cell lung cancer patients who receive adjuvant chemotherapy? Semin Oncol 49(6):482–489. 10.1053/j.seminoncol.2023.01.006 [DOI] [PubMed] [Google Scholar]
- 9.Zhang X, Wang X, Li W, Sun T, Diao D, Dang C (2022) Predictive value of neutrophil-to-lymphocyte ratio for distant metastasis in gastric cancer patients. Sci Rep 12(1):10269. 10.1038/s41598-022-14379-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harimoto N, Hoshino K, Muranushi R et al (2019) Prognostic significance of neutrophil-lymphocyte ratio in resectable pancreatic neuroendocrine tumors with special reference to tumor-associated macrophages. Pancreatology 19(6):897–902. 10.1016/j.pan.2019.08.003 [DOI] [PubMed] [Google Scholar]
- 11.Li Y, Meng Y, Sun H et al (2022) The Prognostic Significance of Baseline Neutrophil-to-Lymphocyte Ratio in Melanoma Patients Receiving Immunotherapy. J Immunother 45(1):43–50. 10.1097/CJI.0000000000000392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Templeton AJ, McNamara MG, Šeruga B et al (2014) Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst 106(6):dju124. 10.1093/jnci/dju124 [DOI] [PubMed] [Google Scholar]
- 13.Vano YA, Oudard S, By MA et al (2018) Optimal cut-off for neutrophil-to-lymphocyte ratio: Fact or Fantasy? A prospective cohort study in metastatic cancer patients. PLoS ONE 13(4):e0195042. 10.1371/journal.pone.0195042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shao SC, Chan YY, Kao Yang YH et al (2019) The Chang Gung Research Database-A multi-institutional electronic medical records database for real-world epidemiological studies in Taiwan. Pharmacoepidemiol Drug Saf 28(5):593–600. 10.1002/pds.4713 [DOI] [PubMed] [Google Scholar]
- 15.Huang YT, Chen YJ, Chang SH, Kuo CF, Chen MH (2022) Discharge status validation of the Chang Gung Research database in Taiwan. Biomed J 45(6):907–913. 10.1016/j.bj.2021.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Portale G, Bartolotta P, Azzolina D, Gregori D, Fiscon V (2023) Prognostic role of platelet-to-lymphocyte ratio, neutrophil-to-lymphocyte, and lymphocyte-to-monocyte ratio in operated rectal cancer patients: systematic review and meta-analysis. Langenbecks Arch Surg 408(1):85. 10.1007/s00423-023-02786-8 [DOI] [PubMed] [Google Scholar]
- 17.Heshmat-Ghahdarijani K, Sarmadi V, Heidari A, Falahati Marvasti A, Neshat S, Raeisi S (2023) The neutrophil-to-lymphocyte ratio as a new prognostic factor in cancers: a narrative review. Front Oncol 13:1228076. 10.3389/fonc.2023.1228076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fuss J, Voloboyeva A, Polovyj V, Yaremkevych R (2022) Neutrophil to lymphocyte ratio in predicting postoperative complications and prognosis in patients with colorectal cancer. Pol Przegl Chir 94(6):33–37. 10.5604/01.3001.0015.8385 [DOI] [PubMed] [Google Scholar]
- 19.Ali S, Shahab S, Rauf M et al (2022) Neutrophil To Lymphocyte Ratio As A Predictor Of Severity In Colorectal Adenocarcinoma. J Ayub Med Coll Abbottabad 34(3):431–437. 10.55519/JAMC-03-9590 [DOI] [PubMed] [Google Scholar]
- 20.Iaciu CI, Emilescu RA, Cotan HT, Nitipir C (2023) Systemic Neutrophil-to-Lymphocyte Ratio as a Prognostic Biomarker for Colon Cancer. Chirurgia (Bucur) 118(3):260–271. 10.21614/chirurgia.2023.v.118.i.3.p.260 [DOI] [PubMed] [Google Scholar]
- 21.Song Y, Yang Y, Gao P et al (2017) The preoperative neutrophil to lymphocyte ratio is a superior indicator of prognosis compared with other inflammatory biomarkers in resectable colorectal cancer. BMC Cancer 17(1):744. 10.1186/s12885-017-3752-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xiao Z, Wang X, Chen X et al (2023) Prognostic role of preoperative inflammatory markers in postoperative patients with colorectal cancer. Front Oncol 13:1064343. 10.3389/fonc.2023.1064343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yasui K, Shida D, Nakamura Y, Ahiko Y, Tsukamoto S, Kanemitsu Y (2021) Postoperative, but not preoperative, inflammation-based prognostic markers are prognostic factors in stage III colorectal cancer patients. Br J Cancer 124(5):933–941. 10.1038/s41416-020-01189-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Naszai M, Kurjan A, Maughan TS (2021) The prognostic utility of pre-treatment neutrophil-to-lymphocyte-ratio (NLR) in colorectal cancer: A systematic review and meta-analysis. Cancer Med 10(17):5983–5997. 10.1002/cam4.4143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guo D, Han A, Jing W et al (2018) Preoperative to postoperative change in neutrophil-to-lymphocyte ratio predict survival in colorectal cancer patients. Future Oncol 14(12):1187–1196. 10.2217/fon-2017-0659 [DOI] [PubMed] [Google Scholar]
- 26.Li Y, Xu T, Wang X, Jia X, Ren M, Wang X (2023) The prognostic utility of preoperative neutrophil-to-lymphocyte ratio (NLR) in patients with colorectal liver metastasis: a systematic review and meta-analysis. Cancer Cell Int 23(1):39. 10.1186/s12935-023-02876-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin N, Li J, Yao X et al (2022) Prognostic value of neutrophil-to-lymphocyte ratio in colorectal cancer liver metastasis: A meta-analysis of results from multivariate analysis. Int J Surg 107:106959. 10.1016/j.ijsu.2022.106959 [DOI] [PubMed] [Google Scholar]
- 28.Li J, Yang J, Xing R, Wang Y (2023) A novel inflammation-related signature for predicting prognosis and characterizing the tumor microenvironment in colorectal cancer. Aging (Albany NY) 15(7):2554–2581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu X, Wang Y, Fu Z (2020) Impact of enhanced recovery after surgery on postoperative neutrophil-lymphocyte ratio in patients with colorectal cancer. J Int Med Res 48(6):300060520925941. 10.1177/0300060520925941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hosseini SV, Maleknejad A, Salem SA, Pourahmad S, Zabangirfard Z, Zamani M (2022) The pre- and postoperative neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios: The comparison of laparoscopy and laparotomy in colorectal cancer patients. Asian J Endosc Surg 15(1):44–50. 10.1111/ases.12962 [DOI] [PubMed] [Google Scholar]
- 31.Xun F, Jiang W, Sha M et al (2024) Neutrophil-to-lymphocyte ratio in colorectal tissue affects prognosis in patients with colorectal cancer. Pathology 56(5):643–652. 10.1016/j.pathol.2024.03.003 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All of the data supporting underlying findings are included in the manuscript.