Abstract
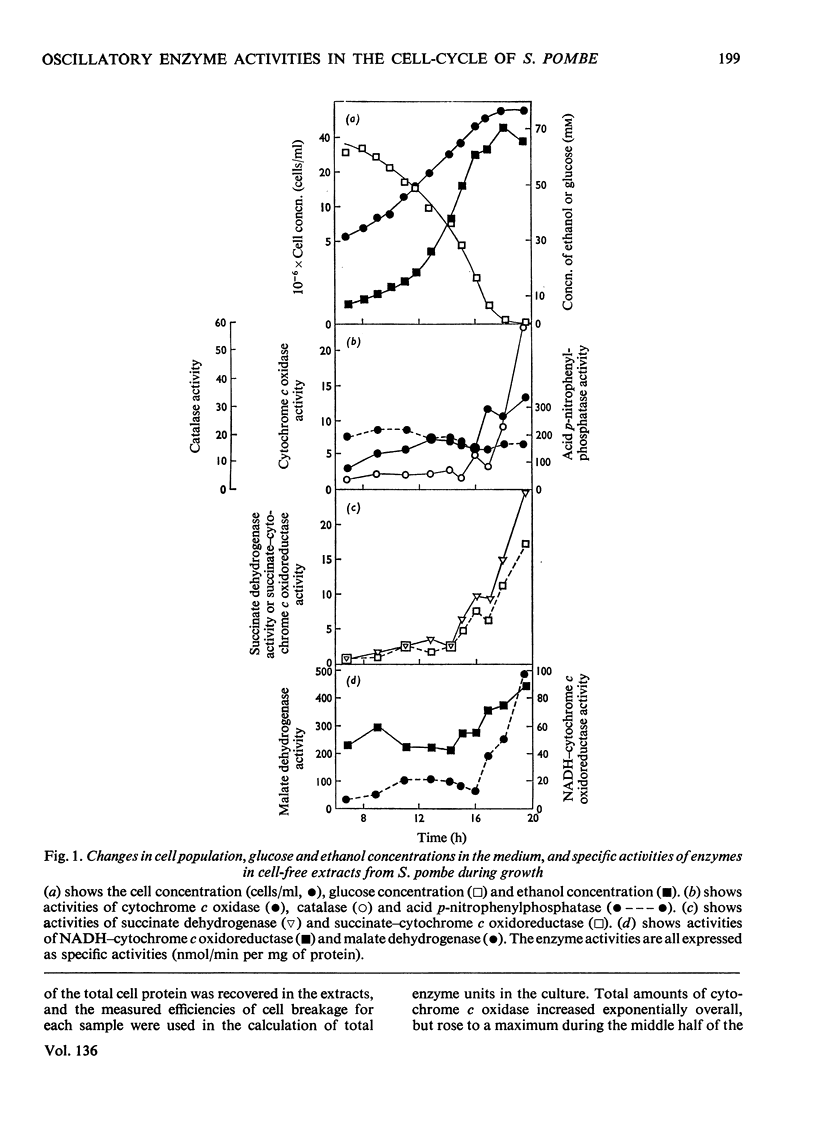
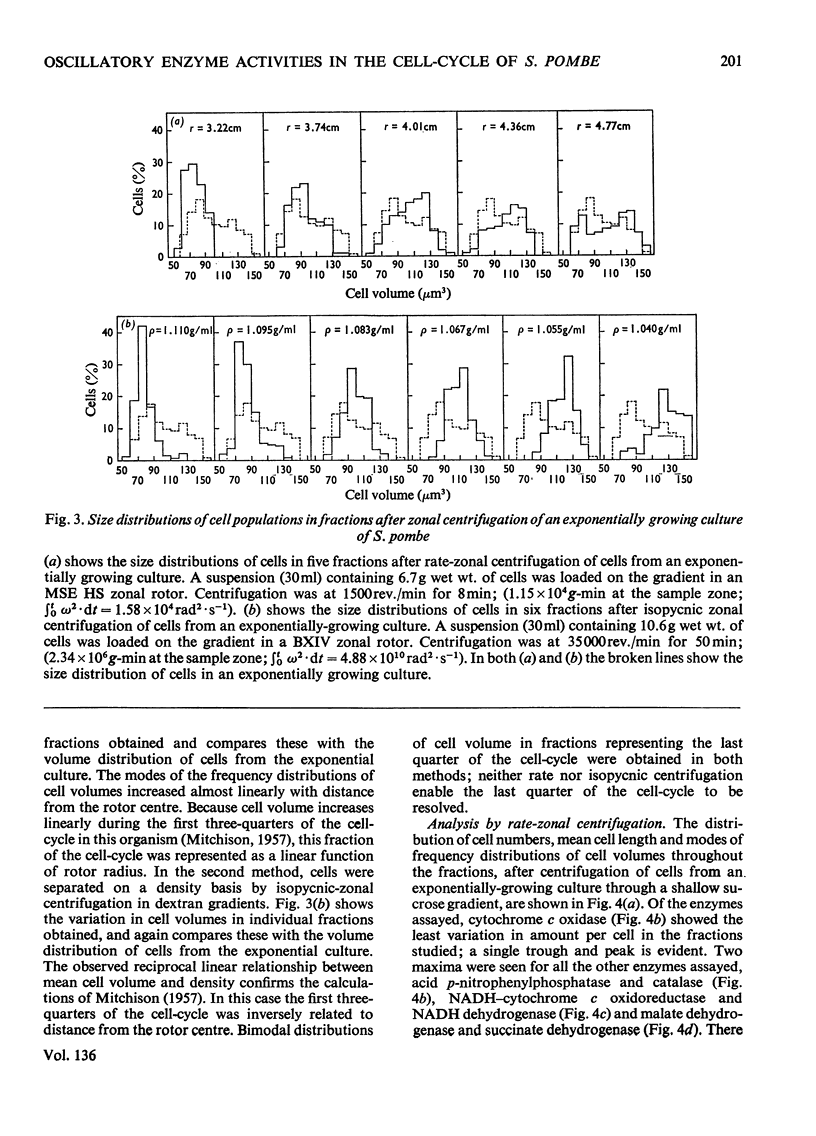
1. Increased specific activities of cytochrome c oxidase, catalase, succinate dehydrogenase, succinate–cytochrome c oxidoreductase, NADH–cytochrome c oxidoreductase and malate dehydrogenase were observed during glucose de-repression of Schizosaccharomyces pombe. 2. The cell-cycle of this organism was analysed by three different methods: (a) harvesting of cells at intervals from a synchronous culture, (b) separation of cells by rate-zonal centrifugation into different size classes and (c) separation of cells by isopycnic-zonal centrifugation into different density classes. 3. Measurement of enzyme activities during the cell-cycle showed that all the enzymes assayed [cytochrome c oxidase, catalase, acid p-nitrophenylphosphatase, NADH-dehydrogenase, NADH–cytochrome c oxidoreductase, NADPH–cytochrome c oxidoreductase, succinate dehydrogenase, malate dehydrogenase, isocitrate dehydrogenase (NADP) and fumarate hydratase] show periodic expression as `peaks'. 4. Cytochrome c oxidase shows a single maximum at 0.67 of a cycle, whereas succinate dehydrogenase exhibits two maxima separated by 0.5 of a cell-cycle. 5. All other enzymes assayed showed two distinct maxima per cell-cycle; for catalase, malate dehydrogenase and NADPH–cytochrome c oxidoreductase there is the possibility of multiple fluctuations. 6. The single maximum of cytochrome c oxidase appears at a similar time in the cycle to one maximum of each of the other enzymes studied, except for NADH dehydrogenase. 7. These results are discussed with reference to previous observations on the expression of enzyme activities during the cell-cycle of yeasts.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLUMENTHAL L. K., ZAHLER S. A. Index for measurement of synchronization of cell populations. Science. 1962 Mar 2;135(3505):724–724. doi: 10.1126/science.135.3505.724. [DOI] [PubMed] [Google Scholar]
- Bosmann H. B. Cellular membranes: membrane marker enzyme activities in synchronized mouse leukemic cells L5178Y. Biochim Biophys Acta. 1970 Apr 21;203(2):256–260. doi: 10.1016/0005-2736(70)90139-2. [DOI] [PubMed] [Google Scholar]
- Bosmann H. B. Mitochondrial biochemical events in a synchronized mammalian cell population. J Biol Chem. 1971 Jun 25;246(12):3817–3823. [PubMed] [Google Scholar]
- Cartledge T. G., Lloyd D. Subcellular fractionation by differential and zonal centrifugation of aerobically grown glucose-de-repressed Saccharomyces carlsbergensis. Biochem J. 1972 Jan;126(2):381–393. doi: 10.1042/bj1260381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottrell S. F., Avers C. J. Evidence of mitochondrial synchrony in synchronous cell cultures of yeast. Biochem Biophys Res Commun. 1970 Mar 12;38(5):973–980. doi: 10.1016/0006-291x(70)90817-x. [DOI] [PubMed] [Google Scholar]
- ELLS H. A. A colorimetric method for the assay of soluble succinic dehydrogenase and pyridinenucleotide-linked dehydrogenases. Arch Biochem Biophys. 1959 Dec;85:561–562. doi: 10.1016/0003-9861(59)90527-2. [DOI] [PubMed] [Google Scholar]
- Gifford G. D., Pritchard G. G. Toxicity of hyperbaric oxygen to yeasts displaying periodic enzyme synthesis. J Gen Microbiol. 1969 May;56(2):143–149. doi: 10.1099/00221287-56-2-143. [DOI] [PubMed] [Google Scholar]
- Halvorson H. O., Carter B. L., Tauro P. Synthesis of enzymes during the cell cycle. Adv Microb Physiol. 1971;6(0):47–106. [PubMed] [Google Scholar]
- Heslot H., Goffeau A., Louis C. Respiratory metabolism of a "petite negative"yeast Schizosaccharomyces pombe 972h-. J Bacteriol. 1970 Oct;104(1):473–481. doi: 10.1128/jb.104.1.473-481.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MILNER H. W., LAWRENCE N. S., FRENCH C. S. Colloidal dispersion of chloroplast material. Science. 1950 Jun 9;111(2893):633–634. doi: 10.1126/science.111.2893.633. [DOI] [PubMed] [Google Scholar]
- MITCHISON J. M. The growth of single cells. I. Schizosaccharomyces pombe. Exp Cell Res. 1957 Oct;13(2):244–262. doi: 10.1016/0014-4827(57)90005-8. [DOI] [PubMed] [Google Scholar]
- Mitchison J. M., Creanor J. Linear synthesis of sucrase and phosphatases during the cell cycle of Schizosaccharomyces pombe. J Cell Sci. 1969 Sep;5(2):373–391. doi: 10.1242/jcs.5.2.373. [DOI] [PubMed] [Google Scholar]
- Mitchison J. M. Enzyme synthesis in synchronous cultures. Science. 1969 Aug 15;165(3894):657–663. doi: 10.1126/science.165.3894.657. [DOI] [PubMed] [Google Scholar]
- Sebastian J., Carter B. L., Halvorson H. O. Use of yeast populations fractionated by zonal centrifugation to study the cell cycle. J Bacteriol. 1971 Dec;108(3):1045–1050. doi: 10.1128/jb.108.3.1045-1050.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TORRIANI A. Influence of inorganic phosphate in the formation of phosphatases by Escherichia coli. Biochim Biophys Acta. 1960 Mar 11;38:460–469. doi: 10.1016/0006-3002(60)91281-6. [DOI] [PubMed] [Google Scholar]
- Warmsley A. M., Phillips B., Pasternak C. A. The use of zonal centrifugation to study membrane formation during the life cycle of mammalian cells. Synthesis of 'marker' enzymes and other components of cellular organelles. Biochem J. 1970 Dec;120(4):683–688. doi: 10.1042/bj1200683. [DOI] [PMC free article] [PubMed] [Google Scholar]


