Abstract
This perspective discusses the convergence of digital twin (DT) technology and on-the-chip systems as pivotal innovations in precision medicine, substantially advancing drug discovery. DT leverages extensive health data to create dynamic virtual patient models, enabling predictive insights and optimized treatment strategies. Concurrently, on-the-chip systems from the Carbon world replicate human biological processes on microfluidic platforms, providing detailed insights into disease mechanisms and pharmacological interactions. The convergence of these technologies promises to revolutionize drug development by enhancing therapeutic precision, accelerating discovery timelines, and reducing costs. Specifically, it assesses their role in drug development, from refining therapeutic precision to expediting discovery timelines and reducing the final price. Nevertheless, integrating these technologies faces challenges, including data collection and privacy concerns, technical intricacies, and clinical adoption barriers. This manuscript argues for interdisciplinary cooperation to navigate these challenges, positing DTs and on-the-chip technologies as foundational elements in personalized healthcare and drug discovery.
Subject terms: Drug discovery, Medical research, Computer modelling
Background
The realm of precision medicine − the new term for personalized medicine1 − is witnessing a monumental shift with the emergence of digital twin (DT) technology and on-the-chip systems. This confluence represents a significant leap towards a future where healthcare is not only customized to the individual’s genetic and environmental context but also predictive and precision-driven. This perspective delves into the implications of these technologies for drug discovery, a field poised to benefit immensely from such advancements.
Digital twin technology: revolutionizing patient-specific treatment
Generally, DT technology refers to creating virtual models that replicate real-world entities, systems, or processes using real-time data. These digital replicas enable simulations and predictions by continuously updating and synchronizing with their physical counterparts. Biomedical DTs are precise digital replicas of biological systems that aim to model complex biological processes across various scales, from molecules to entire organs2. These virtual models integrate diverse datasets, encompassing multi-omics data, environmental exposures, and real-time physiological data, to dynamically mirror and predict individual health outcomes and treatment responses. In the context of drug discovery, DTs serve as a powerful tool for simulating drug interactions at the molecular level, predicting potential side effects, and assessing efficacy before clinical trials. This reduces the time and cost associated with bringing new drugs to market. By creating patient-specific DT, healthcare providers can simulate how different treatment options would affect an individual, leading to more accurate and effective personalized treatment plans, marking a departure from the one-size-fits-all paradigm. Pharmaceutical companies can use DTs to generate data required for regulatory submissions, potentially replacing some in vivo studies with in silico trials, and speeding up the approval process for new therapies3,4.
As of now, various companies have reported establishing several DT models of single-cells5,6, some bioprocesses like cell culture7, organs8–10, and even the cardiovascular system11,12. For instance, the Living Heart Project10 represents a groundbreaking initiative in using DT technology to advance cardiovascular science. The project focuses on creating a highly detailed virtual model of a human heart, referred to as the Living Heart Human Model, that can simulate various physical and biological processes of the heart, including electrical activity, blood flow, and tissue mechanics. It aims to revolutionize how heart-related medical devices are developed and tested, using these simulations to predict how new devices interact with the human heart. However, this project has some limitations in mimicking the human heart function completely, including a lack of heterogeneity between cells’ response to electrical conduction, poor parameter identification for muscle contraction, and employing a simplified fluid resistance model. Another example is the skin DT which mimics the skin barrier function and is beneficial in pharmaceutical research, especially transdermal drug delivery13,14. Additionally, several DT projects have been launched for cancer research and personalized treatment approaches15,16. However, these are mainly available as commercial offerings with limited methodological details that are publicly accessible17. It should be noted that although it has potential in medicine and health care, the use of this technology is still in its infancy18. The development and implementation of digital twins in biomedicine still face significant challenges. Therefore, it is crucial to acknowledge that current DT models, despite their capabilities, cannot fully replicate the complexity of biological systems and are replicas of a small part of their whole functions. For instance, DeepLife and Turbine both report they established DTs of single cells, but their models incorporate multi-omics data to replicate and analyze cell pathways related to drug response5,6. Possible reasons for this include difficulties in collecting data, technical hurdles, interdisciplinary collaboration and funding challenges, and ethical issues3. However, ongoing advancements in the field continue to push the boundaries of what DTs can achieve.
On-the-chip systems: bridging the gap between bench and bedside
On-the-chip technologies, encompassing cell-on-the-chip, organ-on-the-chip, and human-on-the-chip, refer to physical microfluidic devices designed to mimic the biological and physiological functions of cells, tissues, or organ systems in a controlled environment19. These technologies allow for precise control and observation of biological processes, offering insights into disease mechanisms and drug responses and providing scalable and ethically viable alternatives to animal testing20,21. Scientists struggle to overcome on-the-chip technology challenges and boost its applications. One of the most critical hurdles is vascularization, which involves developing a universal medium with all nutrients and growth factors for various cell types to mimic blood22. Now, with the help of multi-omics and DTs, it is possible to create a single medium containing all the necessary nutrients and growth factors for various cell types. By using the genome-scale metabolic model (GEM), we can visualize global networks of metabolic pathways and identify crucial bioreactions and components for all the involved cells23. Maintaining cellular interactions over extended periods is another challenge. DT models could potentially address this by simulating the precise nutrient flow necessary to sustain cell cultures within these systems. This allows researchers to predict and prevent issues before they arise24. Another critical obstacle to industrializing the technology is the lack of a standard manufacturing protocol. This not only hinders large-scale and cost-effective production but impedes the process of drug testing22,25. For on-chip systems, a standard manufacturing protocol heavily relies on designing and conducting thousands of experiments to identify the most suitable components (i.e., biomaterials, biosensors, or living cells) that bring the most promising results26. It is evident that one of the most advantageous aspects of DTs is their rapid processing ability. As a result, we can simulate hundreds of experiments in a virtual environment and analyze the results in a shorter time frame. Additionally, Some key factors that affect the reliability of on-chip results, including real-time monitoring, considering genomic diversity, and incorporating mechanical forces like shear stress, are largely absent from the on-chip technologies currently available24,27. However, these issues could be overcome by integrating DT technology as it can use the results from the on-chip experiment, evaluate the effect of other factors on them, and provide us more precise and realistic outcomes.
Cell-on-the-Chip: These tools are designed to mimic the behavior and interactions of cells within a controlled micro-environment. By providing a platform for studying cellular responses to various stimuli, cell-on-the-chip technology plays a crucial role in uncovering fundamental disease mechanisms and screening potential therapeutic agents at the cellular level. Besides routine topics, cell-on-the-chips are unique choices for extreme environment and aerospace medical research since scientists can mimic extreme temperature and microgravity conditions through them28.
Organ-on-the-Chip: This represents a more sophisticated approach where specific organ functions are replicated on microfluidic chips. Organ-on-the-chip systems offer a detailed view of how organs respond to drugs, toxins, and other treatments, bridging the gap between insights gained at the cellular level and their implications for organ health and function. This technology holds immense promise for advancing our understanding of organ-specific diseases and developing targeted therapies. Organ-on-chip technology offers greater clinical relevance when compared to traditional two-dimensional single-cell or cell-line biology models. This was highlighted in a 2017 study conducted by AstraZeneca, which assessed 110 compounds for drug-induced liver injury24,29. The EVATAR is a groundbreaking 3D organ-on-a-chip platform designed to simulate the female reproductive system and liver, replicating the human 28-day menstrual cycle through hormone regulation. The EVATAR is valuable for research on fertility, hormonal drug interactions, and diseases like cervical cancer and endometriosis. It also supports drug development, toxicological studies, and advances in contraception and infertility treatments by enabling controlled, in-depth analysis of single and multi-tissue interactions30.
Human-on-the-Chip: As the most comprehensive application, human-on-the-chip models could integrate data from multiple cellular and organ chips to simulate a holistic version of the physiological system of the human body. These systems can replicate the genetic and phenotypic variations seen in humans, enabling the prediction of holistic simulations of health outcomes, disease progression, and treatment efficacy personalized to individual patients. This innovation aims to enhance patient care, minimize adverse drug effects, and improve healthcare efficiency. Additionally, human-on-chip platforms provide a more accurate and cost-effective alternative to traditional animal models in drug testing and toxicity assessments, addressing key challenges in the pharmaceutical industry31,32.
Optimizing interaction for enhanced medical insights
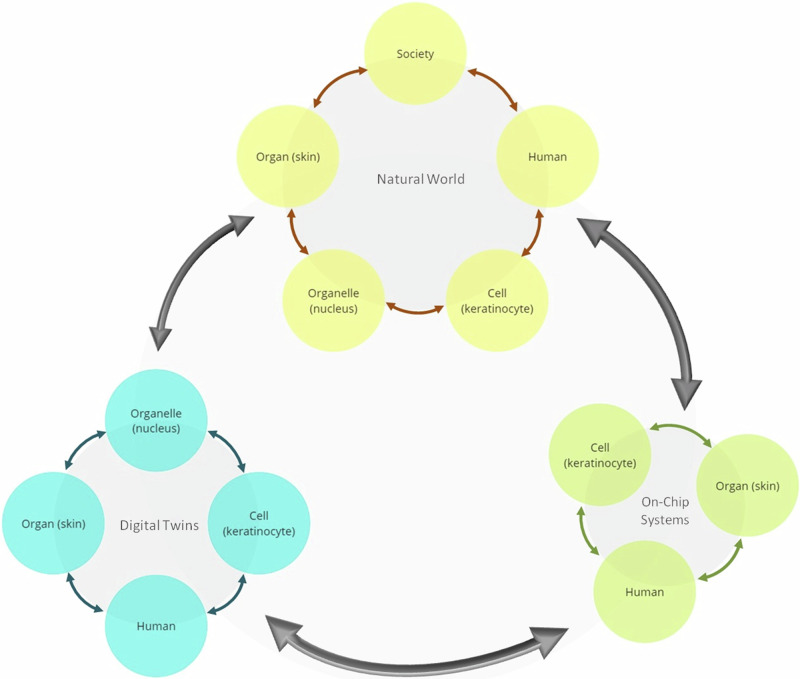
Integrating DTs and on-chip systems advances precision medicine. While ML algorithms enhance DT predictions, DTs can also use rule-based or hybrid approaches. The accuracy of these models depends on the quality and relevance of input data. Collecting such data is costly and time-consuming, so on-chip systems, capable of mimicking real-world processes efficiently, are critical for refining DT models. According to these, we suggest an integration of on-the-chip technologies and DTs to create a feedback loop that enriches both domains (Fig. 1), ensures efficient use of resources, and maximizes the potential for discovery and innovation:
From Chip to DT: Data generated from on-the-chip experiments provide real-world insights that validate and refine DT models’ assumptions and algorithms. This empirical foundation enhances the accuracy of digital simulations, improving predictive power and reliability.
From DT to Chip: Conversely, DTs can identify potential biological interactions and treatment outcomes that may not be readily apparent, guiding the design and focus of on-the-chip experiments.
Fig. 1. Intricate associations between the different levels of human, digital twins, and on-chip technologies.
The diagram illustrates the complex and interconnected relationships between different biological levels, from organelles to the human body as a whole, and their associated digital twins (DTs) and on-chip technologies. These connections are crucial for advancing drug discovery, as integrating DTs with on-chip systems allows for more precise and efficient simulations and experiments. The arrows in the diagram emphasize the dynamic interaction between these biological levels, their DT counterparts, and the on-chip systems. This integration creates a feedback loop, where insights gained from DTs inform on-chip experiments, and findings from these experiments, in turn, refine the DT models. This continuous process optimizes resource utilization, accelerates discoveries, and drives innovation in drug development.
Through these years, several companies and research groups have made some advancements in relatively integrating DTs and on-the-chip technologies. In the case of drug development, the DigiLoCS framework represents a significant advancement in predictive pharmacokinetics. DigiLoCS combines the detailed biological data from liver-on-chip systems with sophisticated mathematical models to visually represent the liver’s drug processing functions. DigiLoCS models complex biological processes, such as drug metabolism, permeability, and partitioning, within the liver-on-chip system, and it has been shown to outperform traditional methods in predicting human liver clearance. One of the main challenges in integrating DTs with organ-on-chip systems is accurately modeling the complex interactions within the liver. Traditional models often oversimplify these processes, leading to significant underprediction of drug clearance. This is important because inadequate understanding of drug clearance can lead to underdosing or toxicity33,34.
Individualized and precision medicine has also progressed at a fast pace; for instance, DIGIPREDICT has established a model to assess disease progression in COVID-19 patients and the need for early intervention in case of serious complications. This technology uses organ-on-the-chip to select the suitable biomarkers combination for predicting cytokine storm by its early signs in high-risk patients. This technology uses smart patches of nanosensors that analyze sweat to detect biomarkers and send data to a smartphone35. While the DIGIPREDICT project has made commendable progress in integrating digital twin technology with clinical practice, it is important to acknowledge that it has not yet produced groundbreaking outcomes. The project is still developing, and significant work remains to be done before its full potential can be realized.
Another area that benefits from integrating DT technology and on-the-chip systems is cancer therapy, which requires personalized treatment approaches due to the variability in patient responses. Furthuremore, by combining DTs with microfluidic platforms such as cancer on-a-chip and radiopharmaceutical on-a-chip researchers can simulate and optimize radiopharmaceutical therapy based on patient-specific data36. This integration allows for precise predictions of treatment outcomes, enabling tailored therapy plans that improve effectiveness and reduce side effects.
Although even the DT concept is newfound in the healthcare era, integrating it with other systems like on-the-chip technology and enhancing its application is progressing expeditiously. Therefore, it is imaginable that the early application of these technologies will probably be in the next few years. However, the widespread clinical adoption of these technologies will likely take longer, requiring significant advancements in technology and regulatory frameworks.
Potential areas of implementation in medicine
Catalyzed Pharmaceutical Development: DTs and on-the-chip technologies enable the rapid screening of new medicinal compounds without the ethical and logistical complexities of traditional testing methods, including probable fatal unexpected adverse effects of new drugs and drug toxicity. Additionally, the estimated time of bringing a new drug to market is about 10 years and the attrition rate for drug targets is as much as 96%. The convergence of these technologies is able to choose compounds with a higher chance of effectiveness to enter the trials and reduce time and costs2. However, employing these requires a tremendous intellectual and financial resource. One solution to solve this problem is to collaborate with the pharmaceutical industry. As we mentioned before, DigiLoCS can be a good example in this area33,34.
Personalized Treatment Regimens: Merging these two technologies could potentially boost the feasibility of personalized medicine based on individual models in carbon and silicon worlds. In this way, patients are prescribed more effective drugs with lower doses and fewer side effects which increases their treatment compliance and decreases the burden on the health system37,38. For this area, the integrated model developed by Abdollahi et al. is an example that helps cancer patients36.
Enhanced Disease Modeling: The combination of DTs with on-the-chip systems allows for sophisticated disease modeling, facilitating a deeper understanding of disease mechanisms and the identification of novel therapeutic targets. One potential model for the proposed integrated technology is DIGIPREDICT. It aims to help early diagnosis of COVID-19 complications and better understand the disease35.
Discontinued Animal Testing: Using animals for drug discovery has always been an excellent debate for ethical and practical reasons. Frequent failure of animal models to predict drug responses in humans also puts their use in basic research in doubt19. In this case, the integration of DT and on-the-chip systems provides more precise and realistic results and will impede animal testing and euthanization.
Model for application
As we mentioned before, any field in medical research or health care system has the potential to take its outcome quality to the next level of excellence using DTs and on-the-chip technology integration. Here, we choose dermatology merely as an example of broader implications and based on our field of interest and its unique challenges and direct impact on patient quality of life to concretize this framework. Considering the application to atopic dermatitis (AD), a complex skin condition characterized by inflammation and barrier disruption, the steps for our simplified approach would be:
Stage 1: Data Collection and Model Development: Developing a DT of the AD patient’s skin by integrating genomic data, behavioral factors, historical treatment responses, and current skin conditions. Simultaneously, creating a skin-on-the-chip model that replicates the AD-affected skin environment.
Stage 2: Simulation and Experimentation: Simulate treatment responses using the DTs, identifying potential therapeutic agents. Experimenting with these agents on the skin-on-the-chip, observing direct effects on skin barrier function and inflammatory markers.
Stage 3: Feedback and Refinement: This step compares simulation and experimentation results to identify discrepancies and refines the DT model accordingly. It enhances the model’s predictive accuracy for future treatments.
Stage 4: Clinical Application and Monitoring: Implementing and testing the most promising treatments identified through the integrated simulation-experimentation approach in clinical trials. Monitoring patient outcomes via wearable technology and relevant medical IoT (Internet of Things), using data to personalize the DT further and adjust treatments as necessary.
Challenges and pathways to integration
Despite their promise, the path to integrating these technologies into clinical practice and drug discovery is laden with hurdles:
Data Quality, Collection, and Management: As we mentioned before, the strength of DTs is based on their structured data entry and their underlying algorithm. Therefore, gathered data should be standardized and reported digitally to facilitate comparability. Poor or missing data can lead to improper predictions and recommendations. Also, studies show DTs would exaggerate racial and other biases and reinforce healthcare inequalities if they trained with poor-quality data18. Conversely, some human traits, such as thinking, reactions, and behavior, are unpredictable and challenging to measure quantitatively. Moreover, a suitable interface is needed to integrate multi-dimensional information from various sources.
Data Privacy, Data Security, and Ethical Considerations: Managing sensitive patient data and ensuring privacy and consent are paramount concerns that require stringent regulatory frameworks. Patients need to be confident about data security and transparency of use; otherwise, these advanced technologies could cause mistrust in patients and destroy physician-patient rapport.
Technical Complexity and Resource Intensity: The development and maintenance of DT and on-the-chip models demand substantial computational resources and expertize, calling for collaborative efforts across disciplines and sectors. Current DTs mostly use ‘black box’ AI, which makes it difficult for healthcare providers to trust their outcomes. Thus, there is a need for developing more models based on interpretable algorithms.
Clinical Adoption, Regulatory Approval, and Financial Justification: Bridging the gap between technological innovation and clinical application involves navigating regulatory landscapes, adapting healthcare infrastructures, and managing financial burdens to accommodate these new technologies. For instance, in adapting to these new technologies, the FDA recently started the Medical Device Development Tools (MDDT) program to prequalify AI models and digital health tools and accelerate the approval process. In fact, due to the complexity and multidisciplinary nature of these technologies, regulatory frameworks have to use advisory committees consisting of data scientists, healthcare professionals, and engineers39. In terms of financial justification, although developing this cutting-edge technology requires massive funding and investment, ultimately, it could provide optimized care for patients and low final costs for industries. One main incentive for the industry section could be that the convergence of DTs and on-the-chip systems results in accelerated drug development and fewer discovered drugs failing in further clinical trials. Additionally, personalized treatment approaches employ the most valuable agents with the minimum optimal doses; consequently, fewer drugs will be prescribed, and patient outcomes will improve.
Feasibility: Regardless of logistical challenges, dosage adjustment would be required as there are differences between in vivo and in vitro settings, even with the most precise modeling techniques.
Looking ahead: a future defined by personalization
The integration of DT and on-the-chip technologies represents an evolution and a revolution in precision medicine. These technologies can significantly improve outcomes for patients with intricate conditions by enabling more precise, predictive, and patient-centered care. Although DT and on-the-chip technologies still face several challenges for their wide application, their integration can overcome shortcomings by providing a synergistic effect. However, the integration process is challenging and fraught with various obstacles, generally categorized as technology development and adoption regulation. Data collection and management, technical hurdles in product manufacturing, and the feasibility of clinical application are related to technology development challenges. Developing a system for registering and sorting standardized data is a principal and complex challenge, especially when dealing with multi-dimensional and divergent data sources. The collected data must represent the entire human race to avoid racial biases. Despite the assistance on-the-chip systems provide in collecting individual data, training the DTs with general human information is still necessary to generate more accurate predictions. Furthermore, developing a reliable DT and manufacturing a detailed on-the-chip system is crucial for making the proposed integrated technology feasible, and they require strong teamwork among experts from different disciplines. On the other side, data privacy and security, ethical considerations, clinical adoption, and regulatory approvals are related to technology adoption regulation challenges. Data privacy and security are fundamentals of any technology related to digital health. In this case, the autonomy of the patients is crucial and technology developers must carefully consider their consent while using their data. Patients may not be willing to accept these technologies unless they are confident about the transparency of usage and the security of their sensitive health data. This is especially important for data used in drug discovery research, as it has the potential to be misused for criminal purposes such as bioterrorism. This is why regulatory agencies play a significant role in the safe clinical adoption of new technologies. They must scrutinize all aspects of any new technology, identify potential pitfalls, and create proper laws. The road ahead will undoubtedly require a concerted effort to address the ethical, technical, and clinical challenges. However, the promise of delivering treatments that are as unique as the individuals they aim to help is an inspiring vision that drives the field forward.
Conclusion
As we embrace these transformative technologies, it is clear that the future of precision medicine and pharmaceutical development lies in our ability to harness the full potential of digital twins and on-the-chip systems. This journey, though complex, paves the way for a new era of healthcare, where drug discovery is accelerated, treatments are demystified, and patient care is profoundly personalized. The collaboration of scientists, clinicians, ethicists, and policymakers will be crucial in realizing this future, ensuring that as we advance, we do so with a commitment to improving patient outcomes, ethical standards, and equitable access for all.
Acknowledgements
This study did not receive any external funding. All resources and facilities used were provided by the authors themselves or their respective institutions.
Author contributions
H.A. conceived of the presented idea and made the initial draft. J.K., S.P., and A.H. expanded and developed the concept. H.A. and M.S. wrote the manuscript. H.A., M.S., S.P., and A.H. edited the final version. J.K. supervised the whole work. Every contributor has read and approved the final version of the manuscript.
Competing interests
The authors declare no competing interests.
Ethics Approval
As the nature of this study did not involve any human or animal subjects, interventions, or data collection, ethics committee approval was not required.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Delpierre, C. & Lefèvre, T. Precision and personalized medicine: What their current definition says and silences about the model of health they promote. Implication for the development of personalized health. Front. Sociol.8, 1112159 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akbarialiabad, H., Pasdar, A. & Murrell, D.F. Digital twins in dermatology, current status, and the road ahead. npj Digit. Med.7, 228 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barros, M. T. et al. From multiscale biophysics to digital twins of tissues and organs: future opportunities for in-silico pharmacology. IEEE Trans. Mol. Biolog. Multi-Scale Commun.10.48550/arXiv.2306.02369 (2024).
- 4.Laubenbacher, R., Mehrad, B., Shmulevich, I. & Trayanova, N. Digital twins in medicine. Nat. Comput. Sci.4, 184–191 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baptista, J. How digital twins of human cells are accelerating drug discovery. Biopharma Dealmakers (2022). https://www.nature.com/articles/d43747-022-00108-3 (accessed 2 Dec 2024).
- 6.Nagy, S. AI-powered cancer cell simulations will be used to identify novel disease positioning strategies in Cancer Research UK’s latest biotech partnership. Turbine (2023). https://turbine.ai/news/ai-powered-cancer-cell-simulations-will-be-used-to-identify-novel-disease-positioning-strategies-in-cancer-research-uks-latest-biotech-partnership/ (accessed 2 Dec 2024).
- 7.Mauch, K. Insilico launches comprehensive portfolio of predictive Digital Twins for cell culture process development. Insilico Biotechnology (2019). https://www.insilico-biotechnology.com/news/insilico-launches-comprehensive-portfolio-of-predictive-digital-twins (accessed 2 Dec 2024).
- 8.Mansi, T. A Digital Twin of the Heart. Siemens (2019). https://www.siemens.com/global/en/company/about/history/specials/175-years/digital-twin-of-the-heart.html (accessed 2 Dec 2024).
- 9.Lu, W. et al. Digital Twin Brain: a simulation and assimilation platform for whole human brain. arXiv preprint arXiv:2308.01241, 10.48550/arXiv.2308.01241 (2023).
- 10.Baillargeon, B., Rebelo, N., Fox, D. D., Taylor, R. L. & Kuhl, E. The living heart project: a robust and integrative simulator for human heart function. Eur. J. Mech.-A/Solids48, 38–47 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mantilla, D. et al. Clinical impact of Sim & Size® simulation software in the treatment of patients with cerebral aneurysms with flow-diverter Pipeline stents. Interven. Neuroradiol.29, 47–55 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Contarino, C., Chifari, F., D'Souza, G. A. & Herbertson, L. H. Validation of a Multiscale Computational Model Using a Mock Circulatory Loop to Simulate Cardiogenic Shock. ASAIO J.69, e502–e512 (2023). [DOI] [PubMed] [Google Scholar]
- 13.Gupta, R., Dwadasi, B. S., Rai, B. & Mitragotri, S. Effect of chemical permeation enhancers on skin permeability: In silico screening using molecular dynamics simulations. Sci. Rep.9, 1–12 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Akbarialiabad, H. & Murrell, D. F. A new dawn for orphan diseases in dermatology: The transformative potential of digital twins. J. Eur. Acad. Dermatol. Venereol.38, 2309–2310 (2024). [DOI] [PubMed] [Google Scholar]
- 15.Stahlberg, E. A. et al. Exploring approaches for predictive cancer patient digital twins: Opportunities for collaboration and innovation. Front. Digit. Health.4, 1007784, (2022). [DOI] [PMC free article] [PubMed]
- 16.Min, R. Researchers in Spain are creating ‘digital twins’ to treat breast cancer.https://www.euronews.com/health/2023/12/05/researchers-in-spain-are-creating-digital-twins-to-treat-breast-cancer (2023).
- 17.Bordukova, M., Makarov, N., Rodriguez-Esteban, R., Schmich, F. & Menden, M. P. Generative artificial intelligence empowers digital twins in drug discovery and clinical trials. Expert Opin. Drug Discov.19, 33–42 (2024). [DOI] [PubMed] [Google Scholar]
- 18.Voigt, I. et al. Digital twins for multiple sclerosis. Front. Immunol.12, 669811 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ingber, D. E. Human organs-on-chips for disease modelling, drug development and personalized medicine. Nat. Rev. Genet.23, 467–491 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Teixeira Carvalho, D. J., Moroni, L. & Giselbrecht, S. Clamping strategies for organ-on-a-chip devices. Nat. Rev. Mater.8, 147–164 (2023). [Google Scholar]
- 21.Leung, C. M. et al. A guide to the organ-on-a-chip. Nat. Rev. Methods Prim.2, 33 (2022). [Google Scholar]
- 22.Srivastava, S. K., Foo, G. W., Aggarwal, N. & Chang, M. W. Organ-on-chip technology: Opportunities and challenges. Biotechnol. Notes5, 8–12 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Silberberg, Y. Development of Advanced Feed Media and Supplements (Webcast Recap).https://www.bioprocessintl.com/cell-culture-media/development-of-advanced-feed-media-and-supplements (2024).
- 24.Low, L. A., Mummery, C., Berridge, B. R., Austin, C. P. & Tagle, D. A. Organs-on-chips: into the next decade. Nat. Rev. Drug Discov.20, 345–361 (2021). [DOI] [PubMed] [Google Scholar]
- 25.Norden, A. The Future of Organ on a Chip: Technical and Intellectual Property Challenges.https://www.gje.com/resources/the-future-of-organ-on-a-chip-technical-and-intellectual-property-challenges/#_edn2 (2023).
- 26.Piergiovanni, M. et al. Organ on chip: building a roadmap towards standardisation. Report No. JRC126163, (JRC126163 Joint Research Centre (JRC) 2021).
- 27.Möller, J. & Pörtner, R. Digital twins for tissue culture techniques—concepts, expectations, and state of the art. Processes9, 447 (2021). [Google Scholar]
- 28.Vashi, A., Sreejith, K. R. & Nguyen, N.-T. Lab-on-a-chip technologies for microgravity simulation and space applications. Micromachines14, 116 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bentwich, I. Pharma’s Bio-AI revolution. Drug Discov. Today28, 103515 (2023). [DOI] [PubMed] [Google Scholar]
- 30.Xiao, S. et al. A microfluidic culture model of the human reproductive tract and 28-day menstrual cycle. Nat. Commun.8, 14584 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaushik, P., Kaushik, M., Jacob, S. & Parvez, S. in Microfluidics and Multi Organs on Chip 289-324 (Springer, 2022).
- 32.Wang, Y. et al. Emerging trends in organ-on-a-chip systems for drug screening. Acta Pharmaceutica Sin. B13, 2483–2509 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maass, C. Organ-On-Chips And Digital Twins. https://esqlabs.com/news-20230307-2-2-2/#:~:text=The%20Rise%20of%20Digital%20Twin%20Platforms&text=It%20involves%20creating%20a%20virtual,allowing%20for%20analysis%20and%20optimization. Access date: 02 Dec 2024.
- 34.Aravindakshan, M. R., Mandal, C., Pothen, A. & Maass, C. DigiLoCS: A leap forward in predictive organ-on-chip simulations. bioRxiv : the preprint server for biology, 2024.2003.2028.587123, 10.1101/2024.03.28.587123 (2024).
- 35.digipredict.eu. Edge AI-deployed DIGItal Twins for PREDICTing disease progression and need for early intervention in infectious and cardiovascular diseases beyond COVID-19.https://www.digipredict.eu/about/ (2021).
- 36.Abdollahi, H. et al. Radiopharmaceutical therapy on-a-chip: a perspective on microfluidic-driven digital twins towards personalized cancer therapies. Sci. Bull.68, 1983–1988 (2023). [DOI] [PubMed] [Google Scholar]
- 37.Trasta, A. Personalized medicine and proper dosage: Over- and undertreatment of chronic diseases endanger patients’ health and strain public health systems. EMBO Rep.10.15252/embr.201845957 (2018). [DOI] [PMC free article] [PubMed]
- 38.Swen, J. J. et al. A 12-gene pharmacogenetic panel to prevent adverse drug reactions: an open-label, multicentre, controlled, cluster-randomised crossover implementation study. Lancet401, 347–356 (2023). [DOI] [PubMed] [Google Scholar]
- 39.Venkatesh, K. P., Raza, M. M. & Kvedar, J. C. Health digital twins as tools for precision medicine: Considerations for computation, implementation, and regulation. npj Digital Med.5, 150 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]