Abstract
Aims
Benefits of mineralocorticoid receptor antagonists (MRAs) in heart failure with preserved and mildly reduced ejection fraction (HFpEF/HFmrEF) have not been established. Conventional randomized controlled trials are complex and expensive. The Spironolactone Initiation Registry Randomized Interventional Trial in Heart Failure with Preserved Ejection Fraction (SPIRRIT‐HFpEF) is a unique pragmatic registry‐based randomized controlled trial.
Methods
SPIRRIT‐HFpEF is a multicentre, prospective, randomized, open‐label, blinded endpoint trial conducted on platforms in the Swedish Heart Failure Registry (SwedeHF) and the United States (US) Trial Innovation Network. Patients with HFpEF/HFmrEF are randomized 1:1 to spironolactone (or eplerenone) in addition to usual care, versus usual care alone. The primary outcome is total number of cardiovascular deaths and hospitalizations for heart failure. Outcomes are collected from Swedish administrative complete coverage registries and a US call centre and subsequently adjudicated. Simple eligibility criteria were based on data available in SwedeHF: heart failure as outpatient or at discharge from hospital, left ventricular ejection fraction ≥40%, N‐terminal pro‐B‐type natriuretic peptide >300 ng/L (in sinus rhythm) or >750 ng/L (in atrial fibrillation), with pre‐specified adjustment for elevated body mass index, and chronic loop diuretic use. Power and sample size assessments were based on an event‐driven design allowing enrolment over approximately 6 years, and application of hazard ratios from the TOPCAT trial, Americas subset. The final sample size is expected to be approximately 2400 patients.
Conclusion
SPIRRIT‐HFpEF will be informative on the effectiveness of generic MRAs in HFpEF and HFmrEF, and on the feasibility of conducting pragmatic and registry‐based trials in heart failure and other chronic conditions.
Keywords: Heart failure with preserved ejection fraction, Heart failure with mildly reduced ejection fraction, Mineralocorticoid receptor antagonists, Aldosterone, Spironolactone, Eplerenone, Registry‐based randomized clinical trial, Pragmatic trial
SPIRRIT‐HFpEF design. BNP, B‐type natriuretic peptide; CV, cardiovascular; EF, ejection fraction; eGFR, estimated glomerular filtration rate; EP, endpoint; HF, heart failure; HFpEF, heart failure with preserved ejection fraction; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; SwedeHF, Swedish Heart Failure Registry; US, United States.
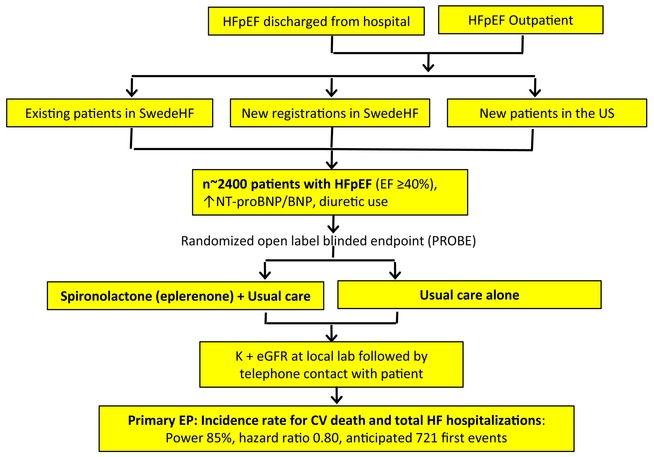
Heart failure with preserved ejection fraction and potential for mineralocorticoid receptor antagonists
Heart failure (HF) with preserved ejection fraction (HFpEF) is a major and growing public health concern with limited treatment options. 1 , 2 , 3 , 4 HFpEF is defined as HF with a left ventricular ejection fraction (LVEF) ≥50%. 1 HF with a reduced ejection fraction has previously been defined as LVEF <40% and more recently ≤40%. Trials in HFpEF have generally included HFpEF but also patients with HF with mid‐range or mildly reduced ejection fraction (HFmrEF), defined as LVEF 40–49% and more recently 41–49%. 1
Heart failure with preserved ejection fraction is thought to be characterized in part by comorbidity‐driven systemic inflammation, microvascular endothelial dysfunction, and fibrosis, leading to increased cardiomyocyte, interstitial and vascular stiffness, resulting in the HF syndrome despite a preserved or normal ejection fraction. 5 , 6 , 7 Renin–angiotensin–aldosterone system inhibitors reduce these maladaptive processes, but five HFpEF randomized controlled trials (RCTs) targeting the renin–angiotensin–aldosterone system did not meet their primary endpoints. 1 As of 2024, only sodium–glucose cotransporter 2 and 1 inhibitors have been shown to reduce the risk of HF hospitalizations or cardiovascular (CV) death in HFpEF. 8 , 9 , 10
In the Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist (TOPCAT) trial, enrolling patients with signs and symptoms of HF, an LVEF ≥45%, and either recent HF hospitalization or elevated natriuretic peptides (NPs), spironolactone did not significantly reduce the composite of death from CV causes, aborted cardiac arrest, or hospitalization for HF. 11 However, post‐hoc analyses of patients randomized in North and South America 12 and of patients included based on NPs 13 suggested that spironolactone may be effective in reducing the composite of HF hospitalization and CV death. Nevertheless, conclusive evidence to recommend mineralocorticoid receptor antagonists (MRAs) in HFpEF is still lacking. A trial with the non‐steroidal MRA finerenone in HFpEF and HFmrEF is currently ongoing. 14 A summary of key completed and ongoing trials in HFpEF/HFmrEF targeting the aldosterone–mineralocorticoid receptor pathway is shown in Table 1 . 11 , 15
Table 1.
Key heart failure with preserved and mildly reduced ejection fraction randomized trials targeting the aldosterone–mineralocorticoid receptor pathway as of 2024
| Trial | Active treatment | Simplified inclusion criteria | n or target n | Primary outcome | Results/status |
|---|---|---|---|---|---|
| TOPCAT 11 | Spironolactone vs. placebo | LVEF ≥45%; HHF <12 months or ↑NT‐proBNP/BNP | 3445 | CV death + aborted cardiac arrest + HHF | Neutral. Regional differences in outcomes and effect raised questions |
| Aldo‐DHF 15 | Spironolactone vs. placebo | NYHA class II–III; LVEF ≥50%; diastolic dysfunction or atrial fibrillation or flutter; peak VO2 ≤25 ml/kg/min | 422 | Diastolic function and exercise capacity | Improved diastolic function but not exercise capacity |
| FINEARTS‐HF (NCT04435626) | Finerenone vs. placebo | LVEF ≥40%, structural heart disease within 12 months, diuretic need; ↑NT‐proBNP/BNP | ≈6000 | CV death + total HF events | Results presented at the ESC Congress 2024 |
| SPIRRIT‐HFpEF (NCT02901184) | Open label spironolactone/eplerenone vs. usual care | LVEF ≥40%; ↑NT‐proBNP/BNP; regular loop diuretics | ≈2400 | CV death + total HHF | Ongoing |
| SPIRIT‐HF (NCT04727073) | Spironolactone vs. placebo | NYHA class II–IV; LVEF ≥40% and structural heart disease; HHF or IV diuretics within 12 months; ↑NT‐proBNP/BNP | ≈1300 | CV death + total HHF | Ongoing |
|
SOGALDI‐PEF (NCT05676684) |
Open label cross‐over spironolactone + dapagliflozin vs. dapagliflozin | LVEF >40% and structural heart disease; ↑NT‐proBNP/BNP | 108 | NT‐proBNP | Ongoing |
| REDEFINE‐HF (NCT06008197) | Finerenone vs. placebo | NYHA class II–IV; LVEF ≥40%; current/recent HHF, ↑↑NT‐proBNP/BNP | ≈5200 | CV death + total HF events | Ongoing |
| CONFIRMATION‐HF (NCT06024746) | Open‐label finerenone + empagliflozin vs. usual care | Any LVEF; current/recent HHF; ↑NT‐proBNP/BNP | ≈1500 | Death, HF events, KCCQ | Ongoing |
| EASi‐HF (NCT06424288) | Aldosterone synthase inhibitor BI 690517 + empagliflozin vs. placebo + empagliflozin | LVEF ≥40% and structural heart disease; diuretic need or HHF <6 months; or ↑NT‐proBNP | ≈6000 | Time to first event of CV death or HHF | Ongoing |
BNP, B‐type natriuretic peptide; CV, cardiovascular; eGFR, estimated glomerular filtration rate; ESC, European Society of Cardiology; HF, heart failure; HHF, hospitalization for heart failure; IV, intravenous; KCCQ, Kansas City Cardiomyopathy Questionnaire; LVEF, left venticular ejection fraction; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; NYHA, New York Heart Association; VO2, oxygen consumption.
NT‐proBNP cut‐off is generally approximately 3× higher than BNP. NT‐proBNP and BNP cut‐off is generally approximately 3× higher with vs. without atrial fibrillation or flutter. In some studies, NT‐proBNP/BNP may be lower with higher body mass index.
Source: ClinicalTrials.gov.
Thus, based on the mechanisms involved in HFpEF, the fact that MRAs target these mechanisms, their proven efficacy in HF with reduced ejection fraction (HFrEF), and the suggestive but inconclusive findings in TOPCAT post‐hoc analyses, there is a need for an outcome trial of MRAs in HFpEF and also in HFmrEF. Therefore, the first objective of Spironolactone Initiation Registry Randomized Interventional Trial in Heart Failure with Preserved Ejection Fraction (SPIRRIT‐HFpEF) is to assess whether the initiation of spironolactone or eplerenone plus standard care compared to standard care alone improves outcomes in patients with HFpEF.
Methods
Registry‐based pragmatic randomized trial design
Randomization is a primary tool to assess causality and efficacy, but conventional RCTs are often complex and expensive and are unlikely to be conducted for generic and inexpensive treatments such as the MRAs spironolactone and eplerenone. Conventional RCTs have extensive additional limitations that are increasingly burdensome and recognized as major barriers to the future of clinical HF trials 16 (Figure 1 ).
Figure 1.
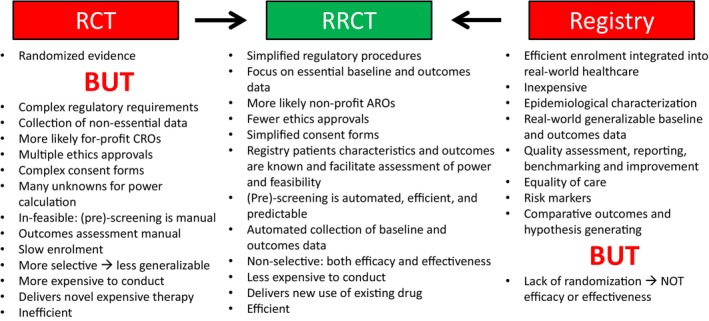
Combining randomization in conventional randomized controlled trials (RCTs) with pragmatic aspects of a registry to create a registry‐based RCT (RRCT). ARO, academic research organization; CRO, contract research organization.
Large simple RCTs, and in particular registry‐based RCTs (RRCTs) are efficient and inexpensive. 16 , 17 Swedish quality registries are known for their many advantageous features such as efficiency and inclusive coverage. However, without randomization, registry data cannot establish causality or efficacy or effectiveness. 16 An RRCT combines randomization with the pragmatic registry concept. Trials may be characterized on the explanatory‐pragmatic spectrum, which can be assessed by the pragmatic‐explanatory continuum indicator summary (PRECIS and PRECIS‐2) tool, 18 where both conventional RCTs and RRCTs have a mix of pragmatic and explanatory components, but where RCTs are generally more explanatory and RRCTs are generally more pragmatic. 17 , 19 The Swedish Heart Failure Registry (SwedeHF) is large, generalizable, and suitable for an efficient RRCT, toward the pragmatic end of the PRECIS spectrum, where screening, enrolment, randomization and baseline data collection occur in the registry, and outcomes are obtained by linkage to mandatory national government registries. 20 , 21 , 22
Pragmatic trials conducted in a registry have previously not been performed in HF. Therefore, a second objective of SPIRRIT‐HFpEF is to assess the feasibility of the pragmatic trial concept for a chronic HF intervention using SwedeHF in collaboration with the United States (US) Trial Innovation Network (TIN) (https://ncats.nih.gov/ctsa/projects/network).
The Swedish Heart Failure Registry
SwedeHF (www.rikssvikt.se) is a national clinical quality registry enrolling patients from a majority of Swedish hospitals (inpatient and outpatient units, including cardiology, internal medicine, and geriatrics; 91% of hospitals in Sweden participating in 2021), and some, but still a minority of, outpatient primary care clinics in Sweden. SwedeHF is managed by the Uppsala Clinical Research centre (UCR, www.ucr.uu.se). SwedeHF was founded in 2000 and is ongoing, with 149 199 registrations from 112 229 unique individuals as of end of 2021. 23 From 2000 to 2017 it included patients with clinician‐judged HF, regardless of ejection fraction, and from 2017, patients with the following HF Internal Classification of Diseases (ICD)‐10 codes: I50.0, I50.1, I50.9, I42.0, I42.6, I42.7, I25.5, I11.0, I13.0, and I13.2. In 2021, approximately 32% of the HF population in Sweden was registered in SwedeHF. 23 Some 80 clinical variables are recorded at each registration, including comorbidities, CV treatments, selected laboratory data, and ejection fraction. From 2000 ejection fraction was categorized as ≥50%, 40–49%, 30–39%, and <30%, and from 2017 also entered with integer digits. In contrast to administrative and claims databases, SwedeHF includes critical numerical non‐categorical values such as blood pressure and heart rate, estimated glomerular filtration rate (eGFR), potassium, N‐terminal pro‐B‐type natriuretic peptide (NT‐proBNP), haemoglobin, and ejection fraction, allowing detailed characterization of the HF syndrome. However, in contrast to many conventional cohorts and trials, it does not require collection of extensive and sometimes cumbersome ancillary data, and does not record echocardiography parameters other than ejection fraction (and valvular disease, as a comorbidity).
Enrolment in Sweden
SwedeHF is used for extensive observational clinical research as described in previous publications, 21 but prior to SPIRRIT‐HFpEF, had never been used as a platform or for pre‐screening for interventional trials. Patients do not provide written informed consent for entry into SwedeHF, but at all clinical encounters, patients in Sweden are informed of data entry into national quality registries and are allowed to opt out. SwedeHF itself does not collect hospitalization or death outcomes, but can be linked to several mandatory complete coverage national administrative registries for additional baseline characteristics and for outcomes and medication adherence (online supplementary Table Appendix S1.). The original establishment of SwedeHF, this study assessing trial feasibility, and the SPIRRIT‐HFpEF trial have national ethics approval. In the SPIRRIT‐HFpEF trial, patients provide written informed consent. In conventional RCTs, pre‐screening (identifying potential patients prior to patient contact and consent) is manual and opportunistic, i.e. occurs as patients are encountered in routine care or by considering selected patient rosters in local investigator clinics. Thus, pre‐screening is inefficient and enrolment unpredictable (Figure 1 ). In the SPIRRIT‐HFpEF RRCT, pre‐screening occurs by conventional opportunistic screening, but also and more importantly, systematically from two sources: (1) ‘retrospective’ pre‐screening of patients who were previously enrolled in SwedeHF and are currently alive and meet eligibility criteria; and (2) prospective pre‐screening of patients enrolled in SwedeHF in routine care during the trial. In conventional RCTs, many eligibility criteria are unknown at pre‐screening and obtained only after patient consent and screening, leading to screen failures, inefficiencies and high costs. In SPIRRIT‐HFpEF, all eligibility criteria are simple and available in SwedeHF prior to patient contact and consent, reducing the risk of screen failures.
Enrolment in the United States
In the US, there is currently no large, longitudinal registry for patients with HF. Instead, patients are recruited to SPIRRIT‐HFpEF from multiple sources including a large national research network, the TIN, which is supported by the National Center for Advancing Translational Sciences, National Institutes of Health. A major aim of SPIRRIT‐HFpEF is to develop experience and infrastructure for future pragmatic clinical trials in the US. Specific pragmatic elements incorporated into the US arm of the trial include limited data collection by sites, with limited in‐person follow‐up, study‐related blood work integrated into routine care, and centralized follow‐up after the first year following enrolment.
SPIRRIT‐HFpEF trial structure and oversight
The design of SPIRRIT‐HFpEF (NCT02901184; EudraCT 2016–002019‐16) is summarized in the Graphical Abstract. The regulatory sponsor for SPIRRIT‐HFpEF is the UCR. The trial is coordinated by UCR in Sweden and Duke Clinical Research Institute (DCRI, dcri.org) in the US. Funding agencies are the Swedish Heart‐Lung Foundation, the Swedish Research Council, and Erling Persson Family Foundation, and the funding sponsor is the National Heart, Lung, and Blood Institute (NHLBI). The study is conducted, and essential documentation archived, in compliance with UCR and DCRI standard operating procedures and standards, respectively, which incorporate the requirements of the ICH Guideline for Good Clinical Practice and the Declaration of Helsinki.
The study was designed by the Principal Investigator together with the Steering Committee, which included academic investigators and representatives from the NHLBI. There is an independent Events Adjudication Committee separately in Sweden and the US, for determining causes of deaths and hospitalizations, and an independent NHLBI‐appointed Data and Safety Monitoring Board.
Due to the COVID‐19 pandemic, enrolment was substantially delayed. It was deemed inappropriate to put patients at risk from extra clinical contacts. Furthermore, in this pragmatic trial, site staff generally have clinical rather than research duties, and many were reassigned to COVID‐19 care during the height of the pandemic and to catching up on elective care once the pandemic subsided. Therefore, to compensate for lower enrolment during the pandemic, follow‐up has been prolonged. The protocol allows the final number of patients and follow‐up time, to obtain the targeted number of patients with an endpoint, to be decided in a pragmatic fashion based on enrolment rates and blinded pooled event rate estimates. As of the second quarter of 2024, approximately 2200 patients were enrolled, with enrolment planned to continue through 2024 and follow‐up until the end of 2025. The final sample size is expected to be approximately 2400 patients.
SPIRRIT‐HFpEF patients and rationale for inclusion and exclusion criteria
Patients eligible for SPIRRIT‐HFpEF are men and women aged ≥50 years who are enrolled in SwedeHF or encountered in the US at discharge from the hospital or as outpatients. In Sweden, patients are enrolled in SwedeHF and pre‐screened for SPIRRIT‐HFpEF in routine care, but provide written informed consent for participation in SPIRRIT‐HFpEF.
For the trial, inclusion criteria were initially a HF diagnosis based on signs and symptoms judged by the investigator, LVEF ≥40% on the most recent LVEF measurement (no time limit), and elevated NT‐proBNP at the most recent measurement (no time limit). To require pre‐randomization screening LVEF and NT‐proBNP would add complexity and reduce enrolment in this pragmatic trial. Potassium must be ≤5.0 mEq/L and eGFR ≥30 ml/min/1.73 m2. In contrast to LVEF and NT‐proBNP, eGFR and potassium must be recent (not older than 30 days), since initiating an MRA with unknown recent eGFR and potassium might expose patients to harm. A blinded interim analysis suggested lower than expected cumulative event rates. Therefore, in a protocol amendment, symptomatic HF (i.e. New York Heart Association class I not allowed) and regular loop diuretic use were added as additional required inclusion criteria. Detailed inclusion and exclusion criteria are listed in Table 2 , 24 together with rationales for their selection. There are only few inclusion and exclusion criteria, in order to keep the trial pragmatic and the results generalizable. The overall rationale for these criteria is to plausibly ensure the presence of HF (e.g. NPs; adjusted for body mass index as shown in online supplementary Table S2 ) and HF severity commensurate with a reasonable risk of hospitalization for HF (e.g. need for loop diuretics), without requiring extensive corroborative information (e.g. SPIRRIT‐HFpEF does not require documented structural or functional heart disease) which may not be readily available, and without excluding too many patients, which could make the results less generalizable and recruitment of more than 2000 patients in only two countries less feasible.
Table 2.
Inclusion and exclusion criteria
| Inclusion criteria | Exclusion criteria |
|---|---|
|
1) Written informed consent. 2) Age ≥50 years. HFpEF may be present in patients younger than 50. But the risk of events is low. 3) Stable HF defined by symptoms and signs of HF as judged by local investigator. Patients may be enrolled as an outpatient or in‐hospital at, or close to, the time of hospital discharge. Most previous HFpEF trials have enrolled outpatients only. But in the real‐world, patients are encountered, and drug initiation is considered, both after stabilization in acute HF and in the outpatient setting. Recent HFrEF and HFpEF trials and post‐hoc analyses of past HFrEF trials also suggest that treatment benefit occurs early. Recent HFrEF trials have included both inpatients and outpatients. Guidelines now emphasize early and rapid initiation of drugs in HFrEF. 4) Most recent left ventricular EF ≥40%. In this pragmatic trial, there is no time limit for how recent this EF must be, and there is no adjudication of the inclusion EF. 5) Elevated natriuretic peptide levels, as defined by any of the following (natriuretic peptides are lower in obesity; therefore, the criteria are progressively lower with higher BMI, according to online supplementary Table
S2
):
Early HFpEF trials did not require natriuretic peptides and there is concern that many patients may not have had HF. Natriuretic peptides are now universally available and therefore considered appropriate in SPIRRIT‐HFpEF despite the pragmatic design. In contrast, recent HFpEF trials have required natriuretic peptides but also some evidence of structural or functional heart disease. SPIRRIT‐HFpEF does not require evidence of structural or function heart disease because this may not be universally available on existing echocardiograms (and would therefore require obtaining a study‐specific echocardiogram which is not pragmatic), and because in patients with signs and symptoms of HF and elevated natriuretic peptides, structural and/or functional heart disease is nearly universally present. 24 6) Regular use of loop diuretics, defined as daily or most days of the week. Most (but not all) HF outcome trials require loop diuretics for enrichment. SPIRRIT‐HFpEF initially did not (to increase generalizability and feasibility of enrolment), but early blinded review of events suggested a lower than expected event rate, and the protocol was amended to require loop diuretics. 7) NYHA class II–IV Patients with NYHA class I HFpEF do exist but have exceedingly low event rates. |
1) Previously enrolled in this study. 2) Known EF <40% ever. Most (but not all) HFpEF trials have excluded patients with previous reduced EF and thus now improved EF in order to ensure exclusion of the HFrEF ‘phenotype’. We elected to also do so. 3) Current absolute indication or contraindication for MRA in judgement of investigator. In the absence of absolute indication, patients currently treated with an MRA may have the MRA discontinued and then be included in the trial, according to investigator judgement. TOPCAT suggested that spironolactone may be effective in HFpEF in the lower EF range and in some patients, clinicians may consider spironolactone to be indicated. However, this was a post‐hoc analysis and the 2021 ESC HF guidelines provided a recommendation of only IIb‐C for spironolactone and only in HFmrEF. 4) Known chronic liver disease. To maintain the pragmatic design, screening liver function tests were not required, as they would not be obtained prior to initiation of spironolactone in routine clinical care. 5) Probable alternative explanations for symptoms such as:
6) Heart transplant or LVAD recipient. 7) Presence of cardiac resynchronization therapy device. 8) Systolic blood pressure <90 mmHg or >160 mmHg at baseline (assessments documented in medical records within 30 days of baseline accepted). 9) K >5.0 mmol/L (non‐haemolysis sample a ; most recent, not older than 30 days). 10) eGFR by MDRD <30 ml/min/1.73 m2 (most recent, not older than 30 days). 11) Current dialysis. 12) Current lithium use. 13) Actual or potential for pregnancy. 14) Participation in another interventional clinical trial where an MRA is studied. Co‐enrolment in trials and observational studies of other medical and device interventions is permitted. 15) Not suitable in the opinion of the investigator due to severe or terminal comorbidity with poor prognosis, or characteristics that may interfere with adherence to trial protocol. |
The table lists complete inclusion and exclusion criteria and for select criteria, also the rationale in italics.
BNP, B‐type natriuretic peptide; EF, ejection fraction; eGFR, estimated glomerular filtration rate; ESC, European Society of Cardiology; HF, heart failure; HFmrEF, heart failure with mildly reduced ejection fraction; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; LVAD, left ventricular assist device; MDRD, Modification of Diet in Renal Disease; MRA, mineralocorticoid receptor antagonist; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; NYHA, New York Heart Association.
All K values in the trial refer to non‐haemolysis samples. If haemolysis, blood test needs to be repeated.
The eligibility criteria were designed to exclude patients with the worst health status and greatest competing risk. However, even among eligible patients, those that agree to participate in the trials may have lower event rates than the overall anticipated eligible population. One principle of an RRCT is that results should be broadly generalizable. Therefore, enrichment strategies may not be consistent with the RRCT concept. However, we also did not consider it appropriate to include patients with characteristics such as terminal cancer, in whom adherence would be limited, competing events would dominate, and randomization to active treatment would not be expected to have a major effect.
In Sweden, trial patient characteristics are entered into the online SwedeHF (registry) case report form and automatically exported to a specific electronic data capture database. There is no separate case report form for baseline characteristics for Swedish patients in the trial. In the US, sites enter data in a traditional electronic case report form, although data collection is streamlined to minimize participant and clinician burden compared with traditional clinical trials.
SPIRRIT‐HFpEF intervention, study visits and follow‐up
Patients are randomized 1:1 to open‐label MRA (either spironolactone or eplerenone) plus usual care versus usual care alone. Patients randomized to MRA receive a prescription to be filled and paid for out of pocket. (In both Sweden and the US, spironolactone can be obtained for approximately 10 US cents per day. In Sweden, patients pay out‐of‐pocket for prescription medications up to an annual maximum of approximately 250 Euro, after which additional medication costs are covered by the government). Spironolactone is more potent than eplerenone, but the initial dose in guidelines and clinical practice is generally 25 mg/day for both drugs and is the dose recommended in SPIRRIT‐HFpEF. A dose of 25 mg every other day or 25 mg, half tablet every day, may be prescribed at investigator discretion for patients judged to be at higher risk of hyperkalaemia or kidney dysfunction. Patients who at baseline are known not to tolerate spironolactone due to gynecomastia or who prefer eplerenone will be prescribed eplerenone. Eplerenone is relatively inexpensive, although more expensive than spironolactone. Spironolactone (or eplerenone) is recommended to be increased to a target dose of 50 mg daily if tolerated.
Most previous RRCTs in Sweden have involved one‐time interventions (i.e. not chronic therapy) without the need for monitoring during follow‐up. 25 , 26 Spironolactone is familiar to all clinicians but is associated with a risk of hyperkalaemia and worsening kidney function. Therefore, there are no required in‐person follow‐up visits in the trial, but potassium and eGFR are measured for all patients in both groups at local laboratories at weeks 1, 4, 26, and 52 after randomization, at end of study, 7–14 days after any dose changes, and as needed at the discretion of the investigator. Local sites order blood tests, instruct patients to have blood drawn at their local clinic, and these laboratory results are then available to investigators in Sweden from regional electronic health records, and in the US laboratory data are acquired from site investigators from individual testing sites and/or the electronic medical record. After obtaining the laboratory result, the investigator or study coordinator has telephone contact with the patient and provides instruction on continued treatment and records any events, laboratory values, and treatment decisions in a separate case report form.
SPIRRIT‐HFpEF outcomes and central adjudication
The primary outcome is the total number of CV deaths and HF hospitalizations. The original primary outcome was time to CV death or first HF hospitalization, but in a protocol amendment, this was modified to total number of HF hospitalizations or CV deaths. Repeated hospitalizations represent clinically highly relevant events. Furthermore, the statistical power for total number of composite events is typically greater than for time to first composite event. 27 Secondary outcomes include time to CV death or first HF hospitalization and its individual components and others presented in online supplementary Table S3 .
SPIRRIT‐HFpEF has a prospective, randomized, open‐label, blinded endpoint (PROBE) design, where treatment is randomized and open‐label, but central outcome adjudication is blinded to treatment assignment. In Sweden, all deaths, HF hospitalizations, and new comorbidity diagnoses (hospitalized or as outpatients) are first centrally identified based on ICD codes from national registries. For CV death and HF hospitalization, these centrally obtained events then trigger adjudication based on relevant source documents. This is a unique pragmatic feature, where no follow‐up for or collection of events is required by sites. In the US, sites collect data including safety monitoring for hyperkalaemia and reductions in eGFR during the first year of enrolment. After the first year post‐enrolment, clinical events are collected from a centralized call centre. Scheduled and unscheduled local follow‐up contacts including eGFR, potassium, and adverse events are unblinded to the local investigator. All detected CV deaths and HF hospitalizations are adjudicated by separate blinded central adjudication committees in Sweden and the US according to a joint pre‐specified Central Events Committee Charter. All adverse events and serious adverse events that are study endpoints will be collected as study endpoints only. Adverse events and serious adverse events previously known to be related to study drug or that are caused by or expected in patients with the illness (HFpEF) will not be reported. Figure 2A shows flow of data in Sweden and Figure 2B shows flow of data in the US.
Figure 2.
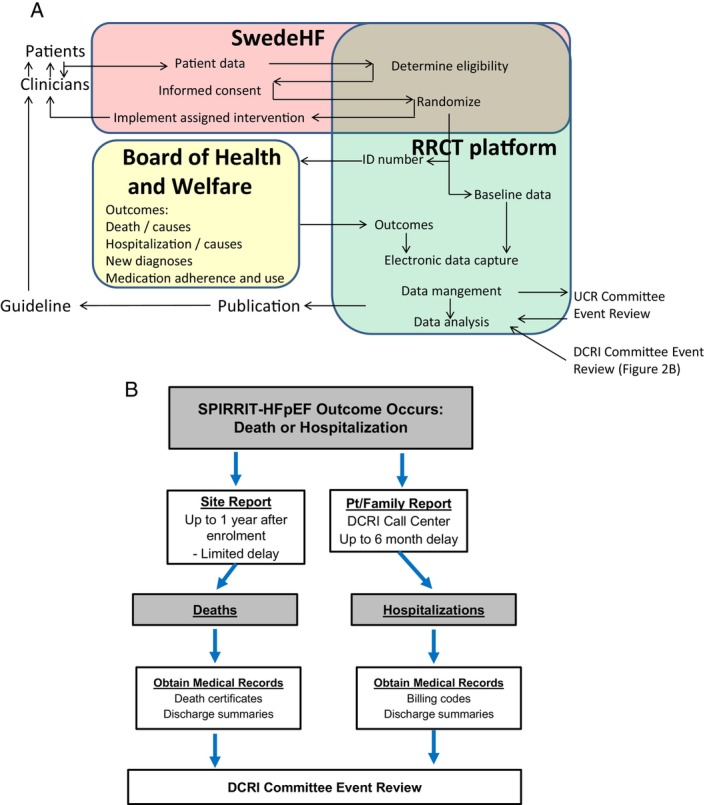
Flow of data in SPIRRIT‐HFpEF in Sweden (A) and the US (B). DCRI, Duke Clinical Research Institute; RRCT, registry‐based randomized controlled trial; SwedeHF, Swedish Heart Failure Registry; UCR, Uppsala Clinical Research centre.
SPIRRIT‐HFpEF sub‐groups
There are a few sub‐groups with an a priori clinical rationale for a potential interaction between randomized treatment arm and sub‐group. Ejection fraction 40–49% (HFmrEF) is not normal but has previously been included in HFpEF trials (generally enrolling patients with LVEF ≥45% or >40%). Atrial fibrillation may confound interpretation of signs and symptoms of HF and of NPs. Patients in Sweden and the US may differ with regard to race, ethnicity, and clinical characteristics. Of the approximate planned sample size of 2400, a majority (approximately 80%) is planned to be recruited in Sweden where the registry infrastructure and feasibility of large enrolment is in place, and a minority (approximately 20%) are planned to be enrolled in the US, where the pragmatic trial concept is emerging but the trial platform, the TIN, is not yet mature for all of the pragmatic trial features.
Power calculation and estimated sample size in SPIRRIT‐HFpEF
The primary outcome is the total number of CV deaths and HF hospitalizations. Study drug discontinuation is expected to be high since monitoring is minimal. Early permanent discontinuation of study drug in TOPCAT was as high as 34% in the spironolactone group and 31% in the placebo group. 11 We assumed that these rates would not be higher in SPIRRIT‐HFpEF. Therefore, no sample size adjustment was made for study drug discontinuation or crossover. Event rates in the more generalizable SwedeHF population are higher than in more selected trial populations. The randomized treatment effect may be smaller in SPIRRIT‐HFpEF than in TOPCAT Americas due to greater competing non‐CV risk in SPIRRIT‐HFpEF.
Although the primary outcome is total number of CV death and HF hospitalization events, the trial was initially powered (and remains powered) for time to CV death or first HHF. Originally, the expected sample size was 3200 patients. The sample size calculation is event‐based, and follow‐up is planned to continue until the accrual of at least 721 CV deaths or first (not recurrent) HF hospitalizations, i.e. 721 patients with a composite event. This gives 85% power to detect a hazard ratio of 0.80 for time to first composite event, with a two‐sided significance of 0.05, using Schoenfeld's formula. TOPCAT Americas had 1796 patients and a hazard ratio 0.74 for CV death and 0.82 for its primary endpoint. The primary endpoint in SPIRRIT‐HFpEF was originally all‐cause death, but the protocol was amended with the primary endpoint changed to time to CV death or first hospitalization for HF, and then amended again to total number of CV deaths and hospitalization for HF (i.e. including recurrent events). We anticipate that counting recurrent events will further increase power, although this remains controversial, and overdispersion of events (i.e. many repeat HF hospitalizations in a small group of patients) may conceivably reduce power. 28 Table 3 shows the number of events to maintain 75%, 80%, 85%, and 90% power. A blinded interim analysis performed in 2022 suggested that fewer than 3200 patients and instead approximately 2400 or less would be needed to collect 721 first composite events.
Table 3.
Required number of events for the composite of time to cardiovascular death or first heart failure hospitalization
| Hazard ratio 0.80 | Hazard ratio 0.81 | Hazard ratio 0.82 | |
|---|---|---|---|
| 75% Power | 558 | 625 | 705 |
| 80% Power | 632 | 707 | 797 |
| 85% Power | 721 | 809 | 913 |
| 90% Power | 844 | 947 | 1067 |
The primary endpoint is incidence rate for total number of heart failure hospitalizations and cardiovascular death, but power is based on time to first cardiovascular death or heart failure hospitalization. The sample size is based on 85% power for a hazard ratio of 0.80, requiring 721 events.
Calculations are from the log‐rank test using nQuery.
Discussion
The SPIRRIT‐HFpEF trial is to our knowledge the first RRCT in HF and one of the first for a chronic condition and chronic intervention. The trial will address an important clinical question, the efficacy and safety of MRAs in routine practice for patients with HFpEF/HFmrEF. This trial also provides important insights for the future of pragmatic clinical trial design for patients with chronic conditions and interventions, using a registry to plan and conduct a trial and to facilitate recruitment.
Since the trial began before but continued during the COVID‐19 pandemic, it is difficult to draw conclusions on efficiency for enrolment and follow‐up. During the pandemic, staff were often reassigned and patients were recommended to avoid unnecessary contact with the healthcare system. However, the RRCT design afforded some flexibility in identifying patients remotely and allowing for trial follow‐up with limited in‐person contact. As such, the trial has been able to continue enrolment and follow‐up in 2020 and 2021 during the most challenging times of the pandemic, but at dramatically reduced rates.
While the RRCT design is unique and allows for efficient enrolment, we have still identified that enrolment remains limited by availability of study personnel. For example, in the US, enrolment occurred at traditional clinical sites with established clinical research teams for HF and many other conditions. In Sweden, a combination of sites, some with established clinical research teams, but other sites that participated primarily in clinical care and the SwedeHF registry were selected for the study. At many sites, study personnel were deployed to pandemic‐related duties, thereby limiting enrolment by reduced clinical research staff availability.
Limitations
While the RRCT concept used in SPIRRIT‐HFpEF offers many advantages, there are also potential challenges, disadvantages and limitations. Monitoring during follow‐up is less frequent than in a conventional trial, which could conceivably expose patients to greater risk. There was consensus in the Steering Committee that spironolactone and eplerenone are familiar drugs and that the monitoring with blood draws and remote contacts, albeit limited, is adequate to limit risk. Simple and pragmatic eligibility criteria may result in enrolment of more frail patients with greater competing risk and potentially smaller relative treatment benefit. However, with less strict eligibility criteria, the trial results will also be more generalizable. The absence of physical follow‐up visits, placebo, and pill counting precludes monitoring of adherence to treatment assignment, and the risk of non‐adherence and cross‐over is likely higher than in a conventional RRCT. However, treatment discontinuation commonly occurs also in conventional RCTs, also with placebo, and the current sample size includes margins to account for some anticipated cross‐over. The open‐label design entails a risk of a placebo effect in patients randomized to MRA. To ameliorate this concern, there are no subjective outcomes such as quality of life, and none that are easily affected by a placebo effect, such as functional capacity. We cannot exclude that awareness of treatment assignment may affect patients' decisions to seek unplanned care. It is also possible that providers' awareness of treatment assignment may affect decisions regarding hospitalization. However, in the SPIRRIT‐HFpEF trial setting, most providers encountering patients with acute HF will not be aware of their participation in this trial. Furthermore, all outcomes are adjudicated blinded to randomized treatment (PROBE design). SwedeHF is an existing registry integrated into both routine care and into SPIRRIT‐HFpEF. By including US sites and the TIN in the trial, experiences in pragmatic HF trials are shared between Sweden and the US, but differences in patient identification and outcomes collection also add complexity to trial operations.
Conclusion
Mineralocorticoid receptor antagonists may be effective in HFpEF and/or HFmrEF but this has not been conclusively determined. RRCTs are promising but have never been performed in HF. SPIRRIT‐HFpEF is a pragmatic RRCT utilizing SwedeHF and the US TIN. SPIRRIT‐HFpEF has dual aim: (i) assessing the efficacy of MRAs in HFpEF/HFmrEF, and (ii) assessing the feasibility of the pragmatic trial concept for a chronic HF intervention. SPIRRIT‐HFpEF has the potential to improve care of patients with HFpEF and/or HFmrEF and to transform the conduct of clinical trials in HF.
Supporting information
Appendix S1. Supporting Information.
Acknowledgements
The SPIRRIT‐HFpEF Steering Committee thanks Mark Pfeffer, Scott Solomon, Faiez Zannad, Adrian Hernandez, Bodil Svennblad, Eugene Braunwald, Javed Butler, and Monica Shah for input during early phases of the trial design. The SwedeHF Steering Committee thanks all staff members at all care units in Sweden for their contribution to the SwedeHF Registry.
Funding
SPIRRIT‐HFpEF is funded by the Swedish Heart‐Lung Foundation (grant no. 20150063, 20160562); the Swedish Research Council (grant no. 2017‐00521); the Erling Persson Foundation (grant no.20160928); National Heart, Lung, and Blood Institute (NHLBI), National Institutes of Health (NIH) (grant no. U01HL134679, U01HL134694, U24HL173568, and UG3HL173571); and National Institutes of Health Center for Advancing Translational Science (NCATS), National Institutes of Health (NIH) awards U24TR001608 and U24TR001579. The views expressed in this manuscript are those of the authors and do not necessarily represent official views of the National Heart, Lung, and Blood Institute, National Institutes of Health, or the United States Department of Health and Human Services.
Conflict of interest: There are no conflicts of interest related to this manuscript or the SPIRRIT‐HFPEF trial. Outside of the submitted work, there are the following disclosures: L.H.L.: supported by Karolinska Institutet, the Swedish Research Council (grant 523‐2014‐2336), the Swedish Heart Lung Foundation (grants 20150557, 20190310), and the Stockholm County Council (grants 20170112, 20190525); advisor/consultant: AstraZeneca, Novartis, Bayer, Vifor Pharma, Pharmacosmos, Edwards, Merck/MSD, Medscape; honoraria/lecture fees: AstraZeneca, Novartis, Vifor Pharma, Bayer, Medscape, Radcliffe Cardiology; research grants: AstraZeneca, Novartis, Boehringer Ingelheim, Vifor Pharma, Pharmacosmos; Stock ownership: AnaCardio. S.J.: research grants to his institution from AstraZeneca, NovoNordisk, Bayer, Jansen. A.D.D.: research funding through his institution from the American Heart Association, Biofourmis, Bodyport, Cytokinetics, American Regent, Inc, the NHLBI, Novartis, and Story Health; consulting services for and/or receives honoraria from Abiomed, AstraZeneca, Cardionomic, InnaMed, LivaNova, Natera, Novartis, Procyrion, Story Health, Vifor, and Zoll; non‐financial support from Abbott for educational and research activities. K.J.A.: research grants from NIH, PCORI, Merck, Bayer. M.F.: consulting fee from Novartis, Boehringer Ingelheim, Vifor Pharma, and AstraZeneca; supported by the National Heart, Lung, and Blood Institute (NHLBI) (K23HL151744), the American Heart Association (20IPA35310955), Bayer, Bodyport, BTG Specialty Pharmaceuticals and Verily; consulting fees from Abbott, Alleviant, Audicor, AxonTherapies, Bayer, Bodyguide, Bodyport, Boston Scientific, Cadence, Coridea, CVRx, Daxor, Deerfield Catalyst, Edwards LifeSciences, Feldschuh Foundation, Fire1, Gradient, Intershunt, Medtronic, NXT Biomedical, Pharmacosmos, PreHealth, Shifamed, Splendo, Sumacor, Vironix, Viscardia, Zoll. K.D.A.: consultant: Medtronic, Abbott, and Procyrion; stock options: Procyrion. U.D.: research grants from AstraZeneca, Pfizer, Vifor Pharma, Boehringer Ingelheim, Boston Scientific, Roche Diagnostics; and consultancies/honoraria from AstraZeneca, Amgen and Pfizer. P.D.N.: employee of Division of Cardiovascular Sciences, National Heart, Lung, and Blood Institute, Bethesda, MD, USA. J.L.F.: employee of Division of Cardiovascular Sciences, National Heart, Lung, and Blood Institute, Bethesda, MD, USA. S.Y.: employee of NHLBI. Ca.H.: consulting fees from Novartis, Roche Diagnostics and AnaCardio; research grants from Bayer and speaker honoraria from MSD and Novartis; supported by the Swedish Research Council (grant 20180899). Cl.H.: consultant: AstraZeneca, NovoNordisk, Bayer, Coala Life; honoraria/lecture fees: Boehringer Ingelheim, Bayer, Amarin; research grants: Pfizer. P.K.: fee for a lecture from Vifor Pharma, AstraZeneca, and Boehringer Ingelheim and is a member of the Advisory Board from Pharmacosmos, Novartis and AstraZeneca. M.N.: lecture fees: Novartis, AstraZeneca, Vifor Pharma, Orion Pharma. E.D.P.: research funding from Amgen, Janssen, BMS, Esperion; advisory boards for Novartis, Novo Nordisk, Pfizer, Bayer, and Cerner. T.P.: honoraria/lecture fees from Pfizer. J.S.: direct or indirect stock ownership in companies (Anagram kommunikation AB, Sence Research AB, Symptoms Europe AB, MinForskning AB) providing services to companies and authorities in the health sector including Amgen, AstraZeneca, Bayer, Boehringer, Eli Lilly, Gilead, GSK, Göteborg University, Itrim, Ipsen, Janssen, Karolinska Institutet, LIF, Linköping University, Novo Nordisk, Parexel, Pfizer, Region Stockholm, Region Uppsala, Sanofi, STRAMA, Takeda, TLV, Uppsala University, Vifor Pharma, WeMind. J.O.: fees to his institution for consultant/advisory boards (study steering committees) and lectures from Amgen, AstraZeneca, Bayer, Novartis, Pfizer, and Roche Diagnostics. B.P.: consulting fees from Bayer, Astra Zeneca, Boehringer Ingelheim/Lilly, and Phase‐bio; consulting fees and stock options from SCPharmaceuticals, SQinnovations, G3pharmaceuticals, Relypsa/Vifor, Cereno scientific, KBP Pharmaceuticals, Sarfez, Tricida, Proton Intel, and Brain‐storm Medical. All other authors have nothing to disclose.
References
- 1. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Bohm M, et al.; ESC Scientific Document Group . 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2022;24:4–131. 10.1002/ejhf.2333 [DOI] [PubMed] [Google Scholar]
- 2. Savarese G, Lund LH. Global public health burden of heart failure. Card Fail Rev 2017;3:7–11. 10.15420/cfr.2016:25:2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Savarese G, Stolfo D, Sinagra G, Lund LH. Heart failure with mid‐range or mildly reduced ejection fraction. Nat Rev Cardiol 2022;19:100–116. 10.1038/s41569-021-00605-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Becher PM, Lund LH, Coats AJS, Savarese G. An update on global epidemiology in heart failure. Eur Heart J 2022;43:3005–3007. 10.1093/eurheartj/ehac248 [DOI] [PubMed] [Google Scholar]
- 5. Paulus WJ, Tschope C. A novel paradigm for heart failure with preserved ejection fraction: Comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol 2013;62:263–271. 10.1016/j.jacc.2013.02.092 [DOI] [PubMed] [Google Scholar]
- 6. Shah SJ, Lam CSP, Svedlund S, Saraste A, Hage C, Tan RS, et al. Prevalence and correlates of coronary microvascular dysfunction in heart failure with preserved ejection fraction: PROMIS‐HFpEF. Eur Heart J 2018;39:3439–3450. 10.1093/eurheartj/ehy531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hamo CE, DeJong C, Hartshorne‐Evans N, Lund LH, Shah SJ, Solomon S, et al. Heart failure with preserved ejection fraction. Nat Rev Dis Primers 2024;10:55. 10.1038/s41572-024-00540-y [DOI] [PubMed] [Google Scholar]
- 8. Bhatt DL, Szarek M, Steg PG, Cannon CP, Leiter LA, McGuire DK, Lewis JB, et al.; SOLOIST‐WHF Trial Investigators. Sotagliflozin in patients with diabetes and recent worsening heart failure. N Engl J Med 2021. 384(2):117–128. 10.1056/NEJMoa2030183 [DOI] [PubMed] [Google Scholar]
- 9. Anker SD, Butler J, Filippatos G, Ferreira JP, Bocchi E, Bohm M, et al.; EMPEROR‐Preserved Trial Investigators. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med 2021. 385(16):1451–1461. 10.1056/NEJMoa2107038 [DOI] [PubMed] [Google Scholar]
- 10. Solomon SD, McMurray JJV, Claggett B, de Boer RA, de Mets D, Hernandez AF, et al.; DELIVER Trial Committees and Investigators. Dapagliflozin in heart failure with mildly reduced or preserved ejection fraction. N Engl J Med 2022; 387(12):1089–1098. 10.1056/NEJMoa2206286 [DOI] [PubMed] [Google Scholar]
- 11. Pitt B, Pfeffer MA, Assmann SF, Boineau R, Anand IS, Claggett B, et al.; TOPCAT Investigators. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med 2014. 370(15):1383–1392. 10.1056/NEJMoa1313731 [DOI] [PubMed] [Google Scholar]
- 12. Pfeffer MA, Claggett B, Assmann SF, Boineau R, Anand IS, Clausell N, et al. Regional variation in patients and outcomes in the Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist (TOPCAT) trial. Circulation 2015;131:34–42. 10.1161/CIRCULATIONAHA.114.013255 [DOI] [PubMed] [Google Scholar]
- 13. Girerd N, Ferreira JP, Rossignol P, Zannad F. A tentative interpretation of the TOPCAT trial based on randomized evidence from the brain natriuretic peptide stratum analysis. Eur J Heart Fail 2016;18:1411–1414. 10.1002/ejhf.621 [DOI] [PubMed] [Google Scholar]
- 14. Vaduganathan M, Claggett BL, Lam CSP, Pitt B, Senni M, Shah SJ, et al. Finerenone in patients with heart failure with mildly reduced or preserved ejection fraction: Rationale and design of the FINEARTS‐HF trial. Eur J Heart Fail 2024;26:1324–1333. 10.1002/ejhf.3253 [DOI] [PubMed] [Google Scholar]
- 15. Edelmann F, Wachter R, Schmidt AG, Kraigher‐Krainer E, Colantonio C, Kamke W, et al. Aldo‐DHF Investigators. Effect of spironolactone on diastolic function and exercise capacity in patients with heart failure with preserved ejection fraction: the Aldo‐DHF randomized controlled trial. JAMA 2013;309:781–791. 10.1001/jama.2013.905 [DOI] [PubMed] [Google Scholar]
- 16. Lund LH, Oldgren J, James S. Registry‐based pragmatic trials in heart failure: Current experience and future directions. Curr Heart Fail Rep 2017;14(2):59–70. 10.1007/s11897-017-0325-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Usman MS, Butler J, Khan MS. Pragmatism in clinical trials: Essential, not optional. Eur Heart J 2022;43:3285–3287. 10.1093/eurheartj/ehac400s [DOI] [PubMed] [Google Scholar]
- 18. Thorpe KE, Zwarenstein M, Oxman AD, Treweek S, Furberg CD, Altman DG, et al. A pragmatic‐explanatory continuum indicator summary (PRECIS): A tool to help trial designers. J Clin Epidemiol 2009;62:464–475. 10.1016/j.jclinepi.2008.12.011 [DOI] [PubMed] [Google Scholar]
- 19. Silverman ME. A view from the millennium: The practice of cardiology circa 1950 and thereafter. J Am Coll Cardiol 1999;33:1141–1151. 10.1016/s0735-1097(99)00027-3 [DOI] [PubMed] [Google Scholar]
- 20. Becher PM, Schrage B, Benson L, Fudim M, Corovic Cabrera C, Dahlstrom U, et al. Phenotyping heart failure patients for iron deficiency and use of intravenous iron therapy: Data from the Swedish Heart Failure Registry. Eur J Heart Fail 2021;23:1844–1854. 10.1002/ejhf.2338 [DOI] [PubMed] [Google Scholar]
- 21. Savarese G, Vasko P, Jonsson A, Edner M, Dahlstrom U, Lund LH. The Swedish Heart Failure Registry: A living, ongoing quality assurance and research in heart failure. Ups J Med Sci 2019;124:65–69. 10.1080/03009734.2018.1490831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Savarese G, Dahlstrom U, Vasko P, Pitt B, Lund LH. Association between renin‐angiotensin system inhibitor use and mortality/morbidity in elderly patients with heart failure with reduced ejection fraction: A prospective propensity score‐matched cohort study. Eur Heart J 2018;39:4257–4265. 10.1093/eurheartj/ehy621 [DOI] [PubMed] [Google Scholar]
- 23. Swedish Heart Failure Registry . SWEDEHEART Annual Report 2021. 2022. https://www.ucr.uu.se/swedeheart/dokument‐sh/arsrapporter‐sh/1‐swedeheart‐annual‐report‐2021‐english‐2/viewdocument/3384 (Accessed 17 August 2024).
- 24. Hage C, LÖfstrÖm U, Donal E, Oger E, KapLon‐Cie Slicka A, Daubert JC, et al. Do patients with acute heart failure and preserved ejection fraction have heart failure at follow‐up: Implications of the Framingham criteria. J Card Fail 2020;26:673–684. 10.1016/j.cardfail.2019.04.013 [DOI] [PubMed] [Google Scholar]
- 25. Hofmann R, Svensson L, James SK. Oxygen therapy in suspected acute myocardial infarction. N Engl J Med 2018;378:201–202. 10.1056/NEJMc1714937 [DOI] [PubMed] [Google Scholar]
- 26. Erlinge D, James S. Bivalirudin versus heparin monotherapy in myocardial infarction. N Engl J Med 2018;378(3):300. 10.1056/NEJMc1714520 [DOI] [PubMed] [Google Scholar]
- 27. Rogers JK, Pocock SJ, McMurray JJ, Granger CB, Michelson EL, Ostergren J, et al. Analysing recurrent hospitalizations in heart failure: A review of statistical methodology, with application to CHARM‐Preserved. Eur J Heart Fail 2014;16:33–40. 10.1002/ejhf.29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Claggett B, Pocock S, Wei LJ, Pfeffer MA, McMurray JJV, Solomon SD. Comparison of time‐to‐first event and recurrent‐event methods in randomized clinical trials. Circulation 2018;138:570–577. 10.1161/CIRCULATIONAHA.117.033065 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supporting Information.


