Abstract
Introduction
Despite the progress in gene editing platforms like CRISPR/Cas9 with the potential to transform the standard of care for haemophilia, the language used to explain and discuss gene editing is not aligned across the haemophilia community. Here, we present the objective and rationale for developing a clear, consistent, and globally aligned gene editing lexicon to address these communication gaps.
Methods
Effectively communicating complex gene editing concepts requires a clear and consistent vocabulary. Through collaboration with a diversity of haemophilia stakeholders, our main goal is to develop an accurate, informative lexicon which avoids overpromising or highly technical terminology. Using an innovative process, representatives from several patient and scientific haemophilia organizations and select biotechnology companies will develop and refine language concepts to be tested with approximately seventy participants across the United States of America, United Kingdom, and Germany. Participants will include lived experience experts (LEEs) and haematologists. The process will be overseen by the Lexicon Steering Committee of global experts from leading scientific and patient organizations in the haemophilia and gene editing fields.
Results
Initial feedback provided a robust foundation and rationale for building clear, consistent language around gene editing. This lexicon development framework will allow for increased understanding across the haemophilia community, including the development of valid informed consent and shared decision‐making materials.
Conclusion
Results provide important building blocks for stimuli development and highlight the need for a novel gene editing lexicon. In the next phase, language stimuli will be tested with LEEs and haematologists to better understand audience preferences and help shape the final lexicon.
Keywords: blood coagulation disorders, CRISPR, gene editing, haemophilia, lexicon
1. INTRODUCTION
Haemophilia treatment has progressed rapidly in the last few decades, leading to the wide availability of safe and effective factor replacement therapy. 1 , 2 , 3 , 4 Despite such progress, many people with haemophilia (PWH) face substantial treatment burdens due to breakthrough bleeding, progressive joint disease, mental health issues, and the necessity for chronic, frequent intravenous infusions, resulting in reduced quality of life. 2 , 5 , 6 Advances in gene editing technologies like the clustered regularly interspaced short palindromic repeats (CRISPR)‐CRISPR‐associated protein 9 (Cas9) system show great potential to address these unmet needs of the haemophilia community. 7 , 8
Although the advancements in treatment with gene editing platforms are promising, language used to explain and discuss gene editing is not aligned across key audiences, including lived experience experts (LEEs—people living with haemophilia and their families and caregivers), healthcare professionals (HCPs), advocacy groups, regulatory agencies, and payors. This is due in part to the complexity and evolving nature of gene editing technologies along with the diversity of audiences in the haemophilia community, each with differing levels of awareness, education, and expertise. There is a critical need to establish consistent terminology across the community for fundamental concepts for this rapidly evolving treatment modality. To meet this need for a common language to explain gene editing at a foundational level, a novel gene editing‐focused lexicon is being established. A unified lexicon will help raise awareness, simplify complex concepts, build understanding, and ultimately, facilitate decision‐making.
Here, we present the objective and rationale for developing a clear, consistent, and globally aligned gene editing lexicon for the haemophilia community, including the preliminary insights that helped inform the rationale. Our core objectives include enabling effective communication of complex scientific concepts and treatment considerations, building trust and partnership across stakeholders, creating accuracy and consistency during content development including informed consent forms, resolving conflicts over complex terminology, and reducing misinformation.
To develop a lexicon that achieves these objectives, we will follow a collaborative, multistep approach involving partnerships between representatives from major haemophilia and gene editing organizations, language specialists, haematologists, and LEEs. Rather than develop a lexicon internally, which is then communicated to external stakeholders, we will develop a lexicon in partnership with all major members of the haemophilia community, ensuring that all stakeholders communicate in a unified language. This approach also ensures the lexicon is globally aligned and used by all stakeholders consistently, rather than individual groups developing siloed lexicons independently, which might not include consistent terminology and language (see Figure 1). By collaborating with leading organizations and societies across the haemophilia community to gather the firsthand perspective of those in the community who will be most impacted by this language, we can arrive at a lexicon that is inclusive, scientifically accurate, and informative, while avoiding overpromising or highly technical terminology.
FIGURE 1.
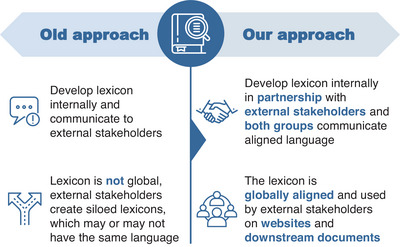
A novel approach was undertaken to develop the lexicon to ensure global alignment and consistency across all stakeholders.
2. MATERIALS AND METHODS
2.1. Study design and setting
The study utilizes a seven‐stage, collaborative, cumulative approach to lexicon development overseen by the Lexicon Steering Committee (Figure 2). The Lexicon Steering Committee is composed of global experts from leading scientific and patient organizations in the haemophilia and gene editing fields. Two of the committee members are PWH and therefore considered LEEs as well. The full list of the steering committee members is shown in Table S1.
FIGURE 2.
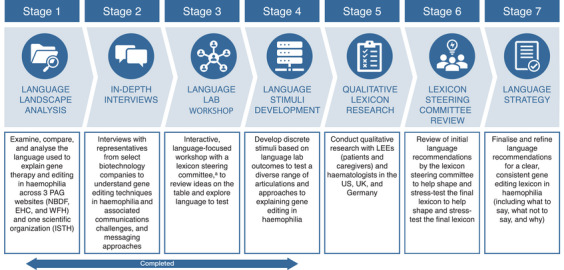
A seven‐stage language strategy process involving collaboration across a diversity of haemophilia stakeholders was adopted for lexicon development. aThe Lexicon Steering Committee is composed of global experts from leading scientific and patient organizations in the haemophilia and gene editing fields.
The seven stages include a language landscape analysis: a comparative audit and analysis of language used to describe gene therapy and editing in haemophilia (stage 1); in‐depth interviews with representatives from select biotechnology companies (stage 2); a language lab: a language‐focused workshop with the Lexicon Steering Committee of global experts from leading scientific and patient organizations in the haemophilia and gene editing fields (stage 3); language stimuli development (stage 4); qualitative lexicon research with stakeholders in the haemophilia community (stage 5); a review and refinement phase with the Lexicon Steering Committee (stage 6), and the finalization of a language strategy: a foundational lexicon for gene editing in haemophilia (stage 7). Of these seven stages, the first four stages have been completed and were considered preliminary phases. The insights from the first three stages led to the development of the language stimuli in stage 4.
Next, qualitative lexicon research (stage 5) will be conducted using outputs from stage 4 with participants from the United States of America, United Kingdom, and Germany. Highly experienced market research professionals with expertise in language research strategies and focus group methodologies will help design and execute the study. The team will apply their linguistic expertise to interpret the outputs at each stage to identify core language building blocks for the lexicon. Then, refinement and finalization of the lexicon will be conducted under the guidance of the Lexicon Steering Committee. The final lexicon will be published in English language and portions of it will be translated to other languages as needed with support from language experts. For further details, refer to the Study Procedures section.
2.2. Study procedure
Through an innovative and iterative process, global experts in haemophilia and gene editing, representatives from several biotechnology companies, and market research professionals first collaborate to develop and refine written language concepts for testing. These concepts will be later evaluated and validated by major stakeholders in the haemophilia community: LEEs and haematologists.
The lexicon development is a concerted effort by members of major stakeholders in the haemophilia community: global experts representing major scientific and patient organizations in haemophilia and gene editing, LEEs, and haematologists; scientific organizations such as the Patient Outreach Program from the American Society of Gene & Cell Therapy (ASGCT), the International Society on Thrombosis and Haemostasis (ISTH), the European Association for Haemophilia and Allied Disorders (EAHAD), and Haemnet; patient advocacy organizations like the National Bleeding Disorders Foundation (NBDF), the European Haemophilia Consortium (EHC), and the World Federation of Hemophilia (WFH), as well as representatives from select biotechnology companies from their medical affairs, patient advocacy, new products, clinical development, scientific and medical publications, research and development, and global trial optimization departments. The details of developing written language concepts for testing are discussed below.
2.2.1. Stage 1 (Language landscape analysis)
An in‐depth audit and comparative analysis were conducted on currently existing public‐facing language used to describe gene therapy and gene editing in haemophilia across three patient advocacy groups (NBDF, EHC, WFH) and one scientific organization (ISTH). By analyzing what is being said, and looking for patterns, inspiration, and white space, we can better understand topics that are critical or difficult to communicate, and create impactful frequently asked questions and communication needs that must be included in a globally aligned lexicon.
2.2.2. Stage 2 (In‐depth interviews with biotechnology company partners)
Five one‐hour in‐depth interviews (N = 10) were conducted with representatives from select biotechnology companies to understand gene editing techniques in haemophilia and associated communications challenges to uncover key anticipated questions and identify potential framing and messaging approaches. Refer to the Participant Selection section for further details regarding selection criteria for this step.
2.2.3. Stage 3 (Language lab)
We define language lab as an interactive language‐focused workshop. It was a three‐hour immersive brainstorming workshop held with the Lexicon Steering Committee to review key communications topics and explore promising language to bring into research. Representatives also completed questionnaires before the workshop to gather more input. Findings are used as a basis for the development of a comprehensive discussion guide containing discrete language stimuli described in stage 4 below.
2.2.4. Stage 4 (Language stimuli development)
The culmination of initial language refinement stages will result in discrete language stimuli, designed to assess a diverse range of articulations and approaches to identify critical elements of gene editing in haemophilia.
In addition, stimuli will include articulation exercises (polling questions) to quantify reactions to specific pieces of language across audiences and markets. When developing these stimuli, priority will be given to insights that ensure consistency and appropriateness for all audiences alike: haematologists and LEEs.
2.2.5. Stage 5 (Qualitative lexicon research)
Eleven qualitative research sessions will be held with sixty to seventy participants across three markets (USA, UK, and Germany) comprising haematologists and LEEs to test, vet, and validate language concepts for clarity, comprehension, and credibility (Table 1). Detailed discussion guides will be used to collect, refine, and test language. Please refer to the Participant Selection section for details regarding inclusion criteria for this stage.
TABLE 1.
Planned qualitative research participants representing LEEs and haematologist.
| Country | USA | UK | Germany |
|---|---|---|---|
| Audiences |
LEEs: people living with haemophilia A or B, families, and loved ones caring for them Haematologists: Treating people with moderate‐to‐severe haemophilia |
||
| Sessions |
5 × 2 hr Qualitative listening Sessions (N∼25) 2 × with haematologists (n∼10) 2 × with patients (n∼10) 1 × with caregivers (n∼5) |
3 × 2 hr Qualitative listening Sessions (N∼15–20) 1 × with haematologists (n∼5–8) 1 × with patients (n∼5–8) 1 × with caregivers (n∼5) |
3 × 2 hr Qualitative listening Sessions (N∼15–20) 1 × with haematologists (n∼5–8) 1 × with patients (n∼5–8) 1 × with caregivers (n∼5) |
| Total | N = 60–70 | ||
Abbreviation: LEE, lived experience expert.
2.2.6. Stage 6 (Lexicon steering committee review)
To further refine and validate findings from qualitative research, the Lexicon Steering Committee will review and share input on preliminary findings and initial language recommendations to finalize the lexicon.
2.2.7. Stage 7 (Language strategy)
Finally, optimised language recommendations for a clear, consistent gene editing lexicon in haemophilia will be built based on an analysis of the findings across each stage. It will include recommendations on what to say, what not to say, and why, along with country‐ and audience‐specific language recommendations. The language strategy will include insights on audience mindsets, communications context and guardrails, and will provide considerations for adapting language based on technological evolution and audience literacy levels.
2.3. Participant selection
Participants for in‐depth interviews (stage 2)—which shaped the preliminary language framework for the study—included representatives from select biotechnology companies. These participants were not part of the Lexicon Steering Committee but were chosen for their deep knowledge of current and novel genetic editing techniques and technologies, involvement, and experience in developing and trialling such technologies, and knowledge of the haemophilia community, including PWH and patient advocates.
Participants for qualitative research (stage 5) will include representatives from major stakeholders in the haemophilia community: LEEs and haematologists. Eligible participants in this stage are intended to be representative of the general haemophilia community. Suitable participants will be identified from market research panels. Structured screener interviews will be used to confirm eligibility to ensure a diverse set of participants from across the United States of America, United Kingdom, and Germany as detailed in Table 2.
TABLE 2.
Eligibility criteria for qualitative research for stage 5 (qualitative lexicon research).
Patients
|
Caregivers
|
| Haematologists |
Moderate defined as factor levels 1–5%, and severe defined as factor levels < 1%. 18
Defined as treating at least 25% of the total number of patients treated.
Defined as compromising at least 50% of their working hours.
2.4. Analyses
Results will be derived qualitatively, through in‐depth analysis and synthesis of respondents’ reactions to language, based on clarity, comprehension, and credibility, and forced choice polling exercises for specific lexicon elements. To develop a lexicon with the broadest application, priority will be given to findings relevant across most audiences and markets.
3. RESULTS
The findings from the preliminary phases of insight gathering (stages 1–3) established the rationale and a framework for an easily understood, clear, and consistent lexicon to explain gene editing in haemophilia.
Feedback from stages 1 to 2 indicates the need for clearer explanations and simple, approachable language. These findings reflect the insights gathered from an audit of language currently used by patient advocacy groups and scientific organizations (NBDF, EHC, WFH, ISTH) and input from representatives across select biotechnology companies. Critical analysis of the current state of knowledge within the haemophilia community when it comes to risks, benefits, and considerations of treatment, particularly regarding gene therapy and gene editing‐related topics, are detailed below.
3.1. Descriptions of the gene therapy and editing technologies
Descriptions of gene therapy and gene editing concepts currently used by leading patient advocacy groups and scientific organizations are varied and imprecise, and the community's understanding is limited. The distinctions between gene therapy and related approaches, like gene editing and gene transfer, are not common knowledge, and ‘gene therapy’ is often used as an umbrella term.
“Patients see gene therapies emerging and aren't always understanding they're not all the same.” — Biotechnology Company Partner
3.2. Novelty
Gene editing's novelty—and the current language describing it—sparks confusion and concern. Patient advocacy groups and scientific organizations often use terms like ‘experimental' given gene editing therapies are not yet approved, which may further heighten those concerns.
“One of the dynamics we anticipate is that right now it's a little bit science fiction.” — Biotechnology Company Partner
“They ask us questions like, where are you inserting that, and why do you use delivery, and do you change me?” — Biotechnology Company Partner
3.3. Irreversibility of treatment
The current irreversibility of treatment raises safety questions and concerns and is a key topic for which aligned language is needed. The language used to define it varies across patient advocacy groups and scientific organizations, with some using terms like ‘one‐time’ and others using terms including ‘irreversible’, ‘one‐off’, and ‘can’t be undone'.
“One of the common questions I get asked is ‘Can you reverse it?’ or ‘If something goes wrong, what are you going to do about it?’” — Biotechnology Company Partner
3.4. Community affinity
Strong haemophilia community bonds may pose emotional barriers against undertaking gene editing. These emotional implications of treatment are not adequately addressed by patient advocacy groups and scientific organizations. Consequently, the nonphysical implications of treatment are key topics that could benefit from the development of new language.
“The idea of getting rid of haemophilia…it touches on identity crisis.” — Biotechnology Company Partner.
Feedback from stage 3—a language‐focused workshop with the Lexicon Steering Committee—solidified key topics worth communicating, provided inspiration and guardrails for visual and written language to communicate them, and identified areas with high potential for misunderstanding. Key insights are discussed below.
3.5. Metaphors for gene editing
Metaphors can help simplify and alleviate fear around gene editing but may come at the cost of nuance. While metaphors are a key way to increase patient understanding, effort is needed to ensure they adequately encompass the nuances of novel technologies, techniques, and of the irreversibility that is characteristic of gene editing.
“The complexity here is to be accurate to what we're doing, while also keeping the metaphor understandable for everyone”. — Lexicon Steering Committee Member
Promising territories to explore include metaphors that draw comparisons with editing pieces of language in a Word document or book.
“Something that came to my mind is the ‘search and replace’ function in a Word document.” — Lexicon Steering Committee Member
“Using the book chapter analogy is in my view much better, with two options: correction of the mistake or adding a corrigendum.” — Lexicon Steering Committee Member
3.6. Definitions of the treatment class
Descriptors for gene editing (such as ‘treatment’ vs. ‘technology’ vs. ‘medicine’ vs. ‘drug’) are inconsistent across the academic and scientific communities, indicating a lack of alignment on accurate definitions that set adequate expectations.
“I think of it as a medical technology, in a cellular therapy category.” — Lexicon Steering Committee Member
“It's a new drug. Or you could call it a new molecular or genetic medicine.” — Lexicon Steering Committee Member
“This is an infusion… we need some nouns or definitions that include physically what this medical therapy is.” — Lexicon Steering Committee Member
3.7. Misconceptions about gene editing
The biggest misconception related to gene editing is that its effects are inheritable. This is a major patient concern, meaning effort is required to distinguish between somatic and germline therapies clearly and definitively.
“It's so important that we distinguish between germline and somatic gene editing…people don't understand.” — Lexicon Steering Committee Member
“It's like genetically modified food. People react to it so strongly, but they don't really understand it.” — Lexicon Steering Committee Member
3.8. Alignment with describing the goal of gene editing
Representatives use a diverse set of language to describe the goal of gene editing in haemophilia, indicating a need to align on consistent language. Currently used language varies in defining haemophilia as caused by a ‘mistake’ or ‘missing’ genes with gene editing offering a ‘correction’. There is a need to ensure language on these topics is crafted with care and sensitivity so that it does not place undue blame on patients.
“It's supposed to provide durable correction of a defective gene, resulting in endogenous production of factor 8 or factor 9.” — Lexicon Steering Committee Member
“I'd be careful on this….the goal is to reduce or eliminate the need for clotting factor infusions.” — Lexicon Steering Committee Member
“If I were a person with haemophilia…wouldn't I be more worried about whether I'd bleed or not? So, is it not something to stop or reduce bleeding?” — Lexicon Steering Committee Member
Initial insights as reported above from stages 1 to 3 helped establish key topic areas for language stimuli development (see Table 3). The next step will involve fully developing and finalizing the discrete language stimuli, designed to test a diverse range of articulations (a series of forced‐choice polling questions designed to quantify reactions to, and preferences towards, specific pieces of language) and approaches to explain critical elements of gene editing in haemophilia (stage 4).
TABLE 3.
Key topics for language stimuli development as identified through initial insights from stages 1–3 of the language strategy process.
|
Abbreviation: CRISPR, clustered regularly interspaced short palindromic repeats.
4. DISCUSSION
There is a critical need for dependable and consistent sources of information that can help better understand the risks and benefits of potential new gene therapies designed with the haemophilia community members in mind. 2 , 3 , 9 , 10 , 11 , 12 , 13 Past efforts have identified the need for shared decision‐making tools as new therapies for haemophilia continue to increase in complexity. 14 , 15 In alignment with previous findings, the current study and similar work 16 suggest that consistent use of a community‐informed lexicon aims to minimize miscommunication and facilitate informed decision‐making regarding treatment opportunities and choices. Therefore, it is critical to create an inclusive, scientifically accurate, and understandable lexicon that facilitates informative communication among all major stakeholders and balances existing knowledge with sensitivities across the community along with the levels of existing doubt and scepticism.
Given the need for such a lexicon is global, there are some key perspectives future studies may look to incorporate to ensure it evolves to be more inclusive and impactful. For instance, future studies should prioritize a more diverse set of participants inclusive of stakeholders outside of the United States of America and Europe, to account for a broader range of country‐specific language nuances and audience mindsets. A survey of gene therapy participants at a UK haemophilia centre indicated that preferences and attitudes towards novel therapies like gene therapy may vary by participant age as well as by caregiver type; siblings and parents of PWH were found to be more open to gene therapy than spouses or partners. 17 In addition, including additional members of the care team such as nurses and nurse practitioners as well as HCPs of different specialties will help reach audiences with a wider variety of knowledge and awareness levels. Future studies may also benefit by taking a more quantitative approach to lexicon preferences, given this study's qualitative focus. Similarly, addressing the differences in attitudes and preferences between people with moderate versus severe haemophilia, and PWH A versus haemophilia B are important future considerations as well.
Another important consideration is regarding the perception of this lexicon by those involved in the application of gene editing in other disease states besides haemophilia. Although as of the writing of this manuscript, authors are not aware of other gene editing lexicons for haemophilia or other diseases, it would be critical to collaborate with future developers of such lexicons for wider adoption and applicability of the current lexicon more generally. Incorporating these perspectives may allow for the lexicon to be more inclusive of perspectives and preferences across the wider haemophilia community and beyond, including women and PWH from across the globe.
5. CONCLUSION
The results from this study provide important building blocks for the development of a clear and consistent gene editing lexicon. The next stage will involve the testing of novel language (i.e., a diverse set of articulations explaining key elements of gene editing), which will be developed based on the analysis of insights from members of the haemophilia community. The resulting initial draft of the lexicon will be tested with LEEs and haematologists to understand what resonates most across audiences and to help build the lexicon which will be validated, refined, and finalised with the Lexicon Steering Committee.
Given this lexicon is being developed with input from a multidisciplinary group of experts within the haemophilia community, it is well‐positioned to have a meaningful impact on this community in forming the basis for the development of educational materials, informed consent forms, and shared decision‐making tools.
AUTHOR CONTRIBUTIONS
Conceptualization: Cedric Hermans, Micheala Jones, and Craig Kessler. Writing—review and editing: Cedric Hermans, Leonard A. Valentino, Courtney D. Thornburg, Carmen Unzu, Mark A. Kay, Flora Peyvandi, Penni Smith, Wolfgang Miesbach, William McKeown, Glenn F. Pierce, Kate Khair, Steven W. Pipe, Katarina Starcevic, Monisha Pillai, Micheala Jones, Megan Chiao, Ilia Antonino, and Craig Kessler. Methodology: Katarina Starcevic, Monisha Pillai, and Micheala Jones.
CONFLICT OF INTEREST STATEMENT
C.H. has received research funding from Bayer, CSL Behring, Novo Nordisk, Pfizer, Shire/Takeda, and Sobi; honoraria and speaker's bureau from Bayer, CAF‐DCF, CSL Behring, Hoffmann‐La Roche, LFB, Novo Nordisk, Octapharma, Pfizer, Shire/Takeda, Sobi, BioMarin Pharmaceutical, Regeneron Pharmaceuticals Inc., and UniQure. L.A.V. is former president and chief executive officer of the National Bleeding Disorders Foundation (NBDF). C.D.T. received grants/research funding from BioMarin Pharmaceutical, Novo Nordisk; and honoraria/consultancy fees from Bluebird Bio, CSL Behring, Genentech/Roche, Pfizer, Regeneron Pharmaceuticals Inc., Sanofi Genzyme, and Spark Therapeutics. C.U. has received grants/research funding from Pfizer, and consultancy fees from Regeneron Pharmaceuticals, Inc. M.A.K. is inventor of a filed patent held by Stanford University; and cofounder of, serves on the board of directors and scientific advisory board for, and is a consultant for, LogicBio Therapeutics. F.P. reports consulting fees from BioMarin Pharmaceutical, CSL Behring, Grifols, Roche, Sanofi, and Sobi; and honoraria from Spark Therapeutics and Takeda. P.S. is on the steering committee for Regeneron Pharmaceuticals, Inc. W.Mi. reports consultancy for Bayer, BioMarin Pharmaceutical, Biotest, Chugai, CSL Behring, Novo Nordisk, Pfizer, and Sanofi. W.Mc. reports no conflicts of interest. G.F.P. reports membership of the board of directors of the World Federation of Hemophilia and Voyager Therapeutics, and scientific advisory board for Be Bio; and consultancy for BioMarin Pharmaceutical, Decibel, Frontera, Metagenomi, and Regeneron Pharmaceuticals, Inc. K.K. reports consultancy fees from Sobi; is co‐owner/founder of, and director of research for, Haemnet Ltd.; and honorary vice president for UK Haemophilia Society. S.W.P. reports consultancy for Apcintex, ASC Therapeutic, Bayer, BioMarin Pharmaceutical, CSL Behring, HEMA Biologics, LFB, Novo Nordisk, Pfizer, Precision Biosciences, Roche/Genentech, Sanofi, Spark Therapeutics, Takeda, and UniQure; and grants/research funding from BioMarin Pharmaceutical, Freeline, Genentech/Roche, Sanofi, and UniQure. K.S. and M.P. report consultancy fees from Regeneron Pharmaceuticals, Inc. M.J. and M.C. hold stock or stock options for, and are employees of, Regeneron Pharmaceuticals, Inc. I.A. is an employee of Intellia Therapeutics, Inc., with compensation and stock options. C.K. reports grants/research support from Bayer, Genentech, Octapharma, and Regeneron Pharmaceuticals, Inc.; consultancy fees/honoraria from Bayer, Biogen, BioMarin Pharmaceutical, CSL, Genentech, Novo Nordisk, Octapharma, Pfizer, and Takeda; and participation in a data and safety monitoring board for Bayer and Octapharma.
ETHICS STATEMENT
The methodology was approved by the Lexicon Steering Committee. All participants provided written informed consent before participating. The study was conducted in accordance with the principles of the Declaration of Helsinki and the International Conference on Harmonization Good Clinical Practice guidelines.
Supporting information
Supporting Information
ACKNOWLEDGEMENTS
The authors thank the patients, caregivers, and their family members involved in this study. The authors would also like to thank Amy L. Dunn, MD, for her contributions to the initial stages of lexicon development. The sponsor was involved in the study design and data collection, analysis, and interpretation as well as data checking of results described in the manuscript. We acknowledge assistance with writing and developing the manuscript by Anil Sindhurakar, PhD, of Regeneron Pharmaceuticals, Inc. Editorial and figure support was provided by Dan Smethurst, PhD, of Oberon, OPEN Health Communications (London, UK), and funded by Regeneron, in accordance with Good Publication Practice (GPP). The authors had unrestricted access to study data, were responsible for all content and editorial decisions and received no honoraria related to the development of this publication. This study was funded by Regeneron Pharmaceuticals, Inc. and Intellia Therapeutics, Inc.
Hermans C, Valentino LA, Thornburg CD, et al. A novel gene editing lexicon strategy for the haemophilia community: Research plan for development and preliminary results. Haemophilia. 2024;30:1272–1280. 10.1111/hae.15108
DATA AVAILABILITY STATEMENT
Qualified researchers may request access to study documents (including the lexicon development framework and operationalisation survey) that support the methods and findings reported in this manuscript. Individual anonymized participant data will be considered for sharing 1) once the product and indication has been approved by major health authorities (e.g., FDA, EMA, PMDA, etc) or development of the product has been discontinued globally for all indications on or after April 2020 and there are no plans for future development 2) if there is legal authority to share the data and 3) there is not a reasonable likelihood of participant re‐identification. Submit requests to https://vivli.org/
REFERENCES
- 1. Marchesini E, Morfini M, Valentino L. Recent advances in the treatment of hemophilia: a review. Biologics. 2021;15:221‐235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Weyand AC, Pipe SW. New therapies for hemophilia. Blood. 2019;133(5):389‐398. [DOI] [PubMed] [Google Scholar]
- 3. Mannucci PM. Hemophilia treatment innovation: 50 years of progress and more to come. J Thromb Haemost. 2023;21(3):403‐412. [DOI] [PubMed] [Google Scholar]
- 4. Gogia P, Tarantino M, Schramm W, Aledort L. New directions to develop therapies for people with hemophilia. Expert Rev Hematol. 2023;16(6):417‐433. [DOI] [PubMed] [Google Scholar]
- 5. Iorio A, Skinner MW, Clearfield E, et al. Core outcome set for gene therapy in haemophilia: results of the coreHEM multistakeholder project. Haemophilia. 2018;24(4):e167‐e172. [DOI] [PubMed] [Google Scholar]
- 6. Brod M, Bushnell DM, Neergaard JS, Waldman LT, Busk AK. Understanding treatment burden in hemophilia: development and validation of the Hemophilia Treatment Experience Measure (Hemo‐TEM). J Patient Rep Outcomes. 2023;7(1):17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhang H, McCarty N. CRISPR‐Cas9 technology and its application in haematological disorders. Br J Haematol. 2016;175(2):208‐225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. De Wolf D, Singh K, Chuah MK, VandenDriessche T. Hemophilia gene therapy: the end of the beginning?. Hum Gene Ther. 2023;34(17‐18):782‐792. [DOI] [PubMed] [Google Scholar]
- 9. Astermark J, Blatný J, Königs C, Hermans C, Jiménez‐Yuste V, Hart DP. Considerations for shared decision management in previously untreated patients with hemophilia A or B. Ther Adv Hematol. 2023;14:20406207231165857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pierce GF, Pasi KJ, Coffin D, et al. Towards a global multidisciplinary consensus framework on haemophilia gene therapy: report of the 2nd World Federation of Haemophilia Gene Therapy Round Table. Haemophilia. 2020;26(3):443‐449. [DOI] [PubMed] [Google Scholar]
- 11. Khair K, Steadman L, Chaplin S, Holland M, Jenner K, Fletcher S. Parental perspectives on gene therapy for children with haemophilia: the Exigency study. Haemophilia. 2021;27(1):120‐128. [DOI] [PubMed] [Google Scholar]
- 12. Fletcher S, Jenner K, Pembroke L, Holland M, Khair K. The experiences of people with haemophilia and their families of gene therapy in a clinical trial setting: regaining control, the Exigency study. Orphanet J Rare Dis. 2022;17(1):155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pietu G, Giraud N, Chamouard V, Duport G, Lienhart A, Dargaud Y. Perspectives and perception of haemophilia gene therapy by French patients. Haemophilia. 2024;30(1):68‐74. [DOI] [PubMed] [Google Scholar]
- 14. Limjoco J, Calatroni A, Aristizabal P, Thornburg CD. Gene therapy preferences and informed decision‐making: results from a National Hemophilia Foundation Community Voices in research survey. Haemophilia. 2023;29(1):51‐60. [DOI] [PubMed] [Google Scholar]
- 15. Limjoco J, Thornburg CD. Development of a haemophilia A gene therapy shared decision‐making tool for clinicians. Haemophilia. 2023;29(5):1184‐1190. [DOI] [PubMed] [Google Scholar]
- 16. Hart DP, Branchford BR, Hendry S, et al. Optimizing language for effective communication of gene therapy concepts with hemophilia patients: a qualitative study. Orphanet J Rare Dis. 2021;16(1):189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Aradom E, Gomez K. The patient gene therapy journey: findings from qualitative interviews with trial participants at one UK haemophilia centre. J Haem Pract. 2021;8(1):32‐44. [Google Scholar]
- 18. Centers for Disease Control and Prevention . Diagnosing Hemophilia. 2023. Accessed January 29, 2024. https://www.cdc.gov/hemophilia/testing/?CDC_AAref_Val [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Data Availability Statement
Qualified researchers may request access to study documents (including the lexicon development framework and operationalisation survey) that support the methods and findings reported in this manuscript. Individual anonymized participant data will be considered for sharing 1) once the product and indication has been approved by major health authorities (e.g., FDA, EMA, PMDA, etc) or development of the product has been discontinued globally for all indications on or after April 2020 and there are no plans for future development 2) if there is legal authority to share the data and 3) there is not a reasonable likelihood of participant re‐identification. Submit requests to https://vivli.org/


