Abstract
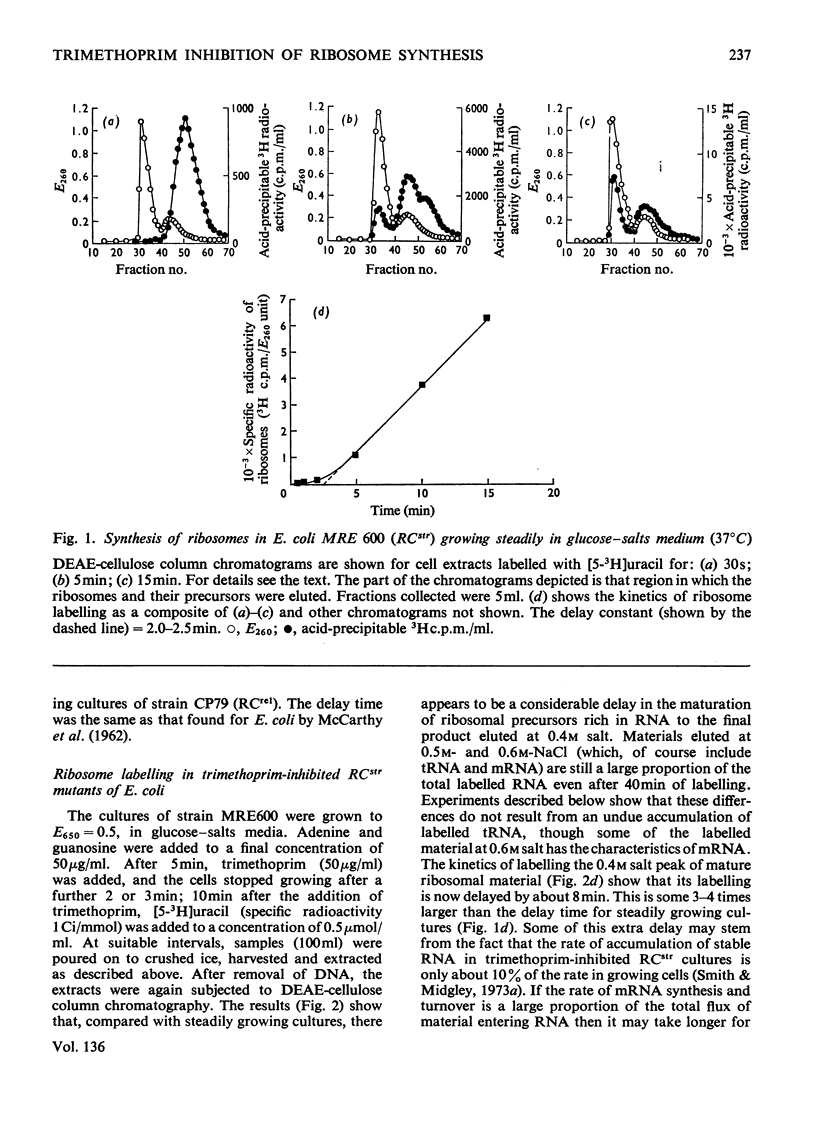
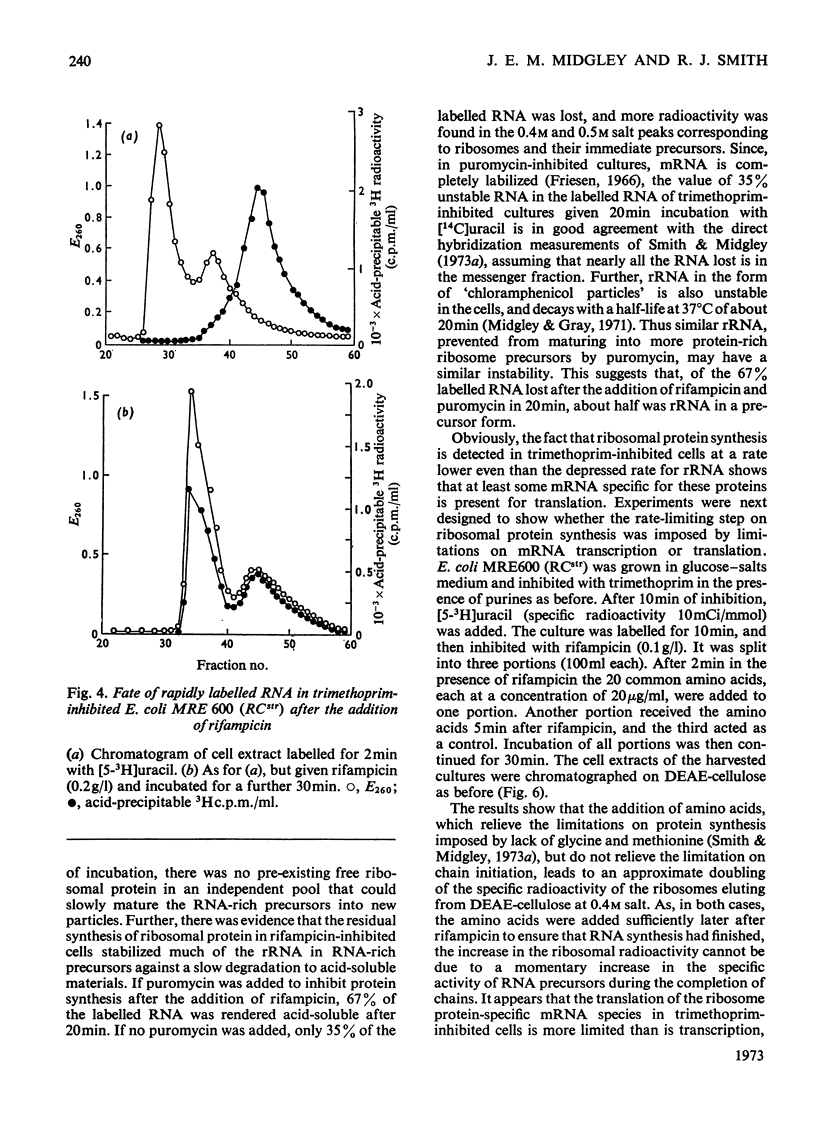
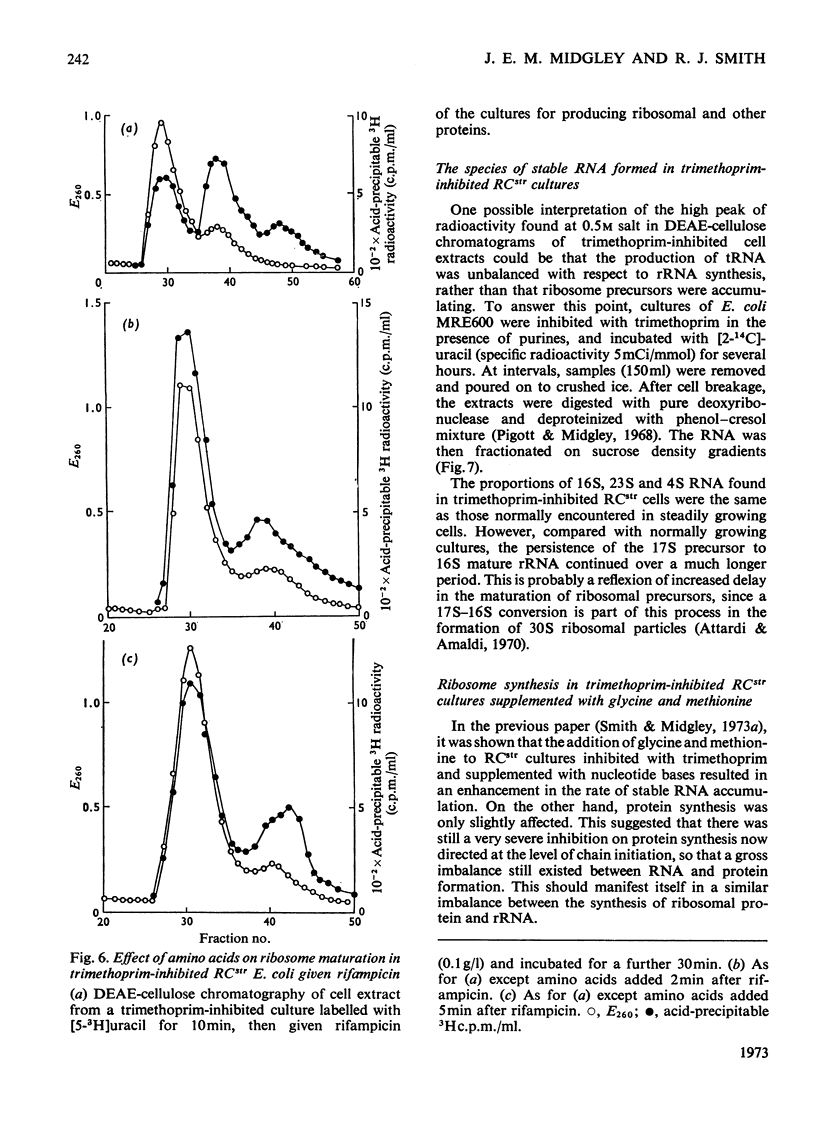
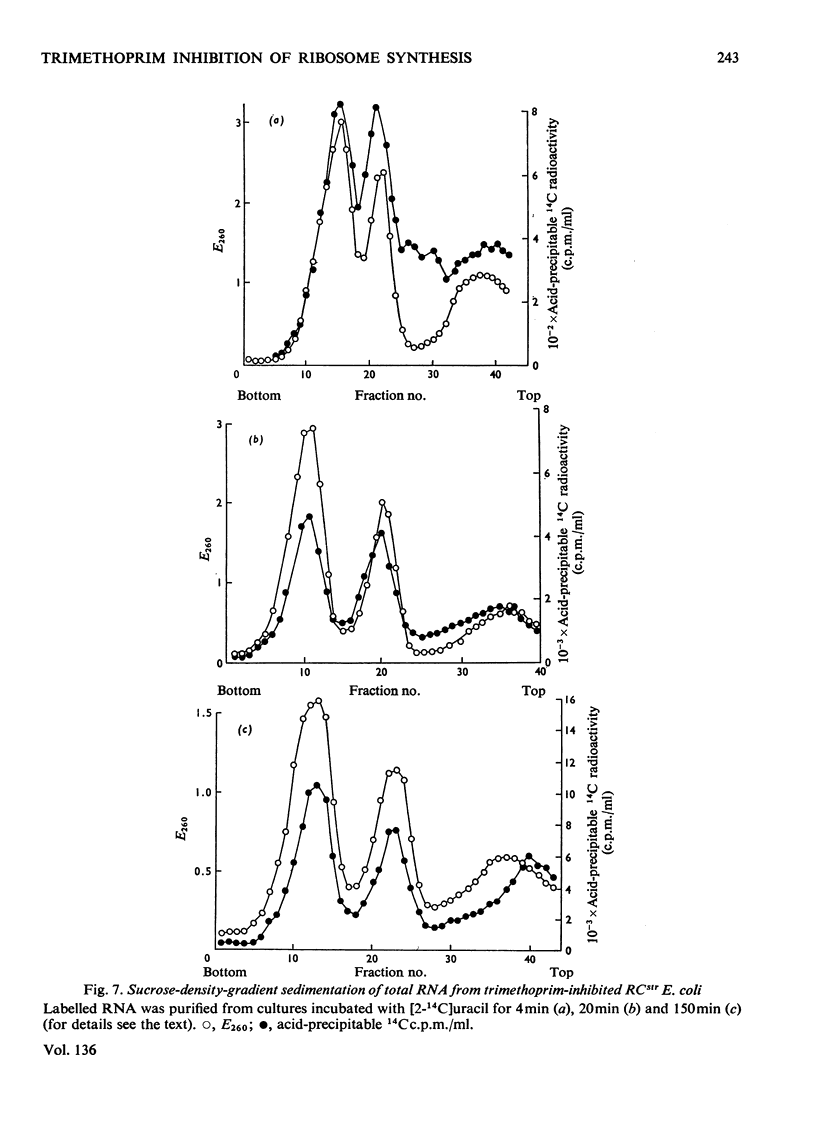
When Escherichia coli was inhibited with trimethoprim in media supplemented with nucleotide bases, glycine and methionine, both RCstr and RCrel strains continued to accumulate RNA at rates very close to those in growing controls. The effects of trimethoprim on protein synthesis were studied by using as an experimental basis the rate of maturation of ribosomal particles from RNA-rich precursors. 1. In RCstr cultures given nucleotide bases but no amino acids, RNA accumulation was inhibited because of amino acid lack. However, maturation of ribosomes from their precursors was more severely inhibited than was the synthesis of rRNA. The restraints on protein synthesis were more severe at the level of translation than the transcription of operons specific for the formation of ribosomal proteins. The kinetic delay time in the passage of rRNA from RNA-rich intermediates to the final ribosome products was therefore increased some three- to four-fold. 2. In RCrel cultures in the same conditions, trimethoprim inhibition stopped ribosomal particle synthesis, but rRNA-rich precursors accumulated. 3. If glycine+methionine were also added to inhibited RCstr cultures, RNA accumulation resumed at a high rate. However, ribosomal maturation was still considerably disturbed because of a disproportionate response of the cells in the formation of protein and RNA. 4. With RCrel cultures, addition of the amino acids caused a large increase in the rate of ribosome maturation, though the degree of disproportionation between the rates of rRNA and ribosomal protein synthesis was now identical with that found in RCstr strains. 5. When inhibited RCrel cultures were supplemented, there was still a severe inhibition of protein synthesis at the level of chain initiation, but inaccuracies in the process of polypeptide chain elongation were greatly decreased. This suggests that the effects of the RCrel mutation on the fidelity of protein synthesis in bacteria are not directed at the point of chain initiation.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Attardi G., Amaldi F. Structure and synthesis of ribosomal RNA. Annu Rev Biochem. 1970;39:183–226. doi: 10.1146/annurev.bi.39.070170.001151. [DOI] [PubMed] [Google Scholar]
- Dale B. A., Greenberg G. R. Effect of the folic acid analogue, trimethoprim, on growth, macromolecular synthesis, and incorporation of exogenous thymine in Escherichia coli. J Bacteriol. 1972 Jun;110(3):905–916. doi: 10.1128/jb.110.3.905-916.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenstadt J., Lengyel P. Formylmethionyl-tRNA dependence of amino acid incorporation in extracts of trimethoprim-treated Escherichia coli. Science. 1966 Oct 28;154(3748):524–527. [PubMed] [Google Scholar]
- Friesen J. D. Control of messenger RNA synthesis and decay in Escherichia coli. J Mol Biol. 1966 Oct;20(3):559–573. doi: 10.1016/0022-2836(66)90011-8. [DOI] [PubMed] [Google Scholar]
- Hall B. G., Gallant J. A. Effect of the RC gene product on constitutive enzyme synthesis. J Mol Biol. 1971 Oct 14;61(1):271–273. doi: 10.1016/0022-2836(71)90225-7. [DOI] [PubMed] [Google Scholar]
- Lancini G. C., Sartori G. Rifamycins LXI: in vivo inhibition of RNA synthesis of rifamycins. Experientia. 1968 Nov 15;24(11):1105–1106. doi: 10.1007/BF02147783. [DOI] [PubMed] [Google Scholar]
- Lazzarini R. A., Dahlberg A. E. The control of ribonucleic acid synthesis during amino acid deprivation in Escherichia coli. J Biol Chem. 1971 Jan 25;246(2):420–429. [PubMed] [Google Scholar]
- McCarthy B. J., Britten R. J., Roberts R. B. The Synthesis of Ribosomes in E. coli: III. Synthesis of Ribosomal RNA. Biophys J. 1962 Jan;2(1):57–82. doi: 10.1016/s0006-3495(62)86841-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midgley J. E., Gray W. J. The control of ribonucleic acid synthesis in bacteria. The synthesis and stability of ribonucleic acid in chloramphenicol-inhibited cultures of Escherichia coli. Biochem J. 1971 Apr;122(2):149–159. doi: 10.1042/bj1220149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miovic M., Pizer L. I. Effect of trimethoprim on macromolecular synthesis in Escherichia coli. J Bacteriol. 1971 Jun;106(3):856–862. doi: 10.1128/jb.106.3.856-862.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips L. A., Franklin R. M. The in vivo distribution of bacterial polysomes, ribosomes, and ribosomal subunits. Cold Spring Harb Symp Quant Biol. 1969;34:243–253. doi: 10.1101/sqb.1969.034.01.030. [DOI] [PubMed] [Google Scholar]
- Pigott G. H., Midgley J. E. Characterization of rapidly labelled ribonucleic acid in Escherichia coli by deoxyribonucleic acid-ribonucleic acid hybridization. Biochem J. 1968 Nov;110(2):251–263. doi: 10.1042/bj1100251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih A. Y., Eisenstadt J., Lengyel P. On the relation between ribonucleic acid synthesis and peptide chain initiation in E. coli. Proc Natl Acad Sci U S A. 1966 Nov;56(5):1599–1605. doi: 10.1073/pnas.56.5.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sippel A., Hartmann G. Mode of action of rafamycin on the RNA polymerase reaction. Biochim Biophys Acta. 1968 Mar 18;157(1):218–219. doi: 10.1016/0005-2787(68)90286-4. [DOI] [PubMed] [Google Scholar]
- Smith R. J., Midgley J. E. The effect of trimethoprim on macromolecular synthesis in Escherichia coli. Regulation of ribonucleic acid synthesis by 'Magic Spot' nucleotides. Biochem J. 1973 Oct;136(2):249–257. doi: 10.1042/bj1360249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. J., Midgley J. E. The effect of trimethoprim on macromolecular synthesis in Escherichia coli. Biochem J. 1973 Oct;136(2):225–234. doi: 10.1042/bj1360225a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TURNOCK G., WILD D. G. THE SYNTHESIS OF RIBOSOMES BY A MUTANT OF ESCHERICHIA COLI. Biochem J. 1965 Jun;95:597–607. doi: 10.1042/bj0950597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travers A. Control of transcription in bacteria. Nat New Biol. 1971 Jan 20;229(3):69–74. doi: 10.1038/newbio229069a0. [DOI] [PubMed] [Google Scholar]
- Yoshida K., Osawa S. Origin of the protein component of chlormaphenicaol particles in Escherichia coli. J Mol Biol. 1968 May 14;33(3):559–569. doi: 10.1016/0022-2836(68)90306-9. [DOI] [PubMed] [Google Scholar]


