Abstract
Introduction
The severity of Von Willebrand disease (VWD) is currently based on laboratory phenotype. However, little is known about the severity of the patient's experience with the disease. The most recent VWD guidelines highlight the need for patient‐reported outcomes (PROs) in VWD.
Aim
The study aimed to investigate the patient‐perspective on VWD severity and to identify key factors that determine the severity of disease experienced by patients.
Materials and methods
Patients participated in a nationwide cross‐sectional study on VWD in the Netherlands (WiN‐study). Patients filled in a questionnaire containing questions on the experienced severity of VWD (4‐point scale), bleeding score (BS) and quality of life (QoL).
Results
We included 736 patients, median age of 41.0 years (IQR 23.0–55.0) and 59.5% were women. A total of 443 had type 1, 269 type 2 and 24 type 3 VWD. Self‐reported severity of VWD was categorized as severe (n = 52), moderate (n = 171), mild (n = 393) or negligible (n = 120). Classification by historically lowest FVIII:C levels < 0.20 IU/mL as a proxy for severe VWD aligned with patient‐reported severity classification with a 72% accuracy. Type 3 VWD (OR = 4.02, 95%CI: 1.72–9.45), higher BS (OR = 1.09, 95%CI: 1.06–1.11), female sex (OR = 1.36, 95%CI: 1.01–1.83), haemostatic treatment in the year preceding study inclusion (OR = 1.53, 95%CI: 1.10–2.13) and historically lowest VWF:Act levels (OR = 0.26, 95%CI: 0.07–1.00) were independent determinants of patient‐reported severity.
Conclusion
This study shows that patient‐reported data provide novel insights into the determinants of experienced disease severity. Our findings highlight the need for studies on PROs with validated questionnaires to assess the burden of VWD.
Keywords: classification, disease severity, patient reported outcome measures, quality of life, Von Willebrand disease
1. INTRODUCTION
Von Willebrand factor (VWF) has an important function in primary haemostasis by mediating the adhesion and aggregation of platelets at the site of injury. 1 Von Willebrand disease (VWD) is caused by a deficiency of VWF and is characterized by a heterogeneous bleeding phenotype. The disease is categorized into three types: VWD with reduced VWF levels (type 1), dysfunctional VWF (type 2) or absence of VWF (type 3). 2
The objective severity classification from a physician's perspective is usually based on VWF antigen and activity levels. 3 , 4 , 5 , 6 A complicating factor is that VWF levels may increase over time in the majority of patients, but the effect of this phenomenon on bleeding and disease severity is unknown. 7 Notably, studies on the correlation of VWF levels and bleeding symptoms show similar bleeding phenotypes across the range of VWF levels, especially between 0.20 and 0.50 IU/mL. 8
Current VWD severity classifications are based on binary evaluations of VWF:Act or FVIII:C levels, with varying cut‐off points. 9 Federici classified VWD more precisely as severe with VWF:RCo levels < 0.10 IU/mL, moderate between 0.10 and 0.30 IU/mL or mild between 0.30 and 0.50 IU/mL. 3 Nonetheless, there are no uniform definitions and cut‐off values for disease severity mentioned across worldwide guidelines on the diagnosis of VWD. 4 , 5 , 6
The need for novel data on severity classification is illustrated by a recent survey that was conducted amongst patients, caregivers and healthcare professionals to prioritize the research topics as input for the latest guidelines on the diagnosis and management of VWD. Both patients/caregivers and health care providers put emphasis on diagnostic criteria and classification. 10 The latest VWD guidelines, therefore, highlight the need for patient‐reported outcomes (PROMs) in VWD. 11 , 12 Health‐related quality of life (HR‐QoL) has been studied previously in VWD, 13 , 14 , 15 but the key factors that contribute to the disease burden remain elusive.
The current study aims to provide more data on the experience of disease severity in VWD patients. First, we compare patient‐reported outcomes (PROs) to the various laboratory‐based severity classifications. Second, we identify factors that contribute to the perceived disease severity of VWD patients using data from the Willebrand in the Netherlands (WiN) study.
2. MATERIALS AND METHODS
2.1. Patient selection
The patients included in this study are from the nationwide cross‐sectional study on VWD in the Netherlands, the WiN study, of which inclusion occurred between 2007 and 2009 in all Hemophilia Treatment Centers in the Netherlands. The study was performed according to the Declaration of Helsinki and has been approved by the Medical Ethical Committees of the participating centres. All study participants signed informed consent at inclusion.
2.2. Assessment methods
Patients filled out an extensive questionnaire for inclusion in the WiN study. The questionnaire contained questions on the severity of VWD from patients’ perspective and VWD treatment during their lifetime. These questions were answered mostly by the parents of children below 16 years. Bleeding scores (BS) were assessed using a self‐administered condensed version of the Tosetto BS, with normal scores defined as < 4 for males and < 6 for females. 16 , 17 Blood samples were drawn for the central measurement of VWF and FVIII activity (FVIII:C) levels at study inclusion in the Erasmus MC, and VWF propeptide (VWFpp) levels were measured in the Leiden University Medical Center (LUMC). The assays used for these central measurements have been described previously and are defined as central levels throughout the manuscript. 17 , 18
The Dutch version of the Short Form‐36 (SF‐36) questionnaire, which contains a physical component summary (PCS) and mental component summary (MCS), was used to assess the generic QoL. 19 , 20 , 21 Scores were calculated using standard algorithms, with a score of 50 representing the mean in the US reference population. 22 This instrument proved valid and reliable across multiple patient groups and cultures, except children, and was used in previous studies on inherited bleeding disorders. 23 , 24
2.3. Definitions
In the questionnaire, the patients’ perspective on VWD severity was assessed on a 5‐point scale: negligible, mild, moderate, severe and critical. For analytic purposes, we combined the severe and critical groups due to a relatively small number of patients. The clinical phenotype is primarily defined throughout the manuscript as the cumulative BS.
Patients in the WiN study were included based on the following criteria: haemorrhagic symptoms or a family history of VWD; and historically lowest VWF antigen level (VWF:Ag); and/or VWF activity (VWF:Act) ≤0.30 IU/mL; and/or FVIII:C ≤0.40 IU/mL. Patients were categorized as type 1 (VWF:Act/VWF:Ag > 0.7), type 2 (VWF:Act/VWF:Ag ≤0.7) or type 3 VWD (VWF:Ag, VWF:Act and VWF propeptide (VWFpp) ≤0.05 IU/mL), based on the levels at WiN study inclusion. 1 Laboratory phenotype was categorized according to several literature‐based classifications derived from either historically lowest VWF and/or FVIII:C levels or VWF and/or FVIII:C levels at study inclusion. 3 , 4 , 5 , 6 , 9
2.4. Statistical analysis
Statistical analyses were performed in R version 4.3.1. (2023‐06‐16). The normality of data was assessed visually. Missing data were not replaced. Categorical variables were presented as frequencies and percentages. For normally distributed data, continuous variables were presented as mean and standard deviation (sd). For skewed data, continuous variables were presented as median and interquartile range (IQR).
To compare reported severity groups, p‐values were computed with chi‐square tests for categorical variables and Kruskal–Wallis tests for continuous variables. We assessed differences in BS items between males and females with Mann‐Whitney tests. P‐values below .05 were defined as statistically significant. Multiple testing corrections, using the Bonferroni method, were applied when necessary.
We comprised patient‐reported data into two or three groups to match literature‐derived VWD severity classifications, and computed cross tables to calculate the accuracy of agreement between these observations. For binary assessments, we merged the negligible and mild groups, and the moderate and severe groups. For assessments with three groups, the moderate and severe groups were unified. Accuracy of agreement was calculated by computing a percentage based on the number of concurring observations, represented by the table diagonal, divided by the totality of observations.
In the multivariate analyses on factors associated with patient‐reported severity, we used ordinal logistic regression models with patient‐reported severity on a 4‐point scale as the independent variable. The dependent variables were: sex, BS, VWD type, blood group, central and historically lowest VWF and FVIII activity levels, haemostatic treatment in the year preceding study inclusion, use of haemostatic treatment or other medications, and hospital admission because of bleeding. Haemostatic therapy encompasses at least desmopressin, VWF/FVIII concentrate or cryoprecipitate. Other medications include tranexamic acid, hormonal therapy and iron suppletion. We corrected for age to adjust for the cumulative nature of the BS. We used backward selection to eliminate terms with the highest p‐values from the full model, reducing the model until a stopping rule was met at a p < .05 threshold.
3. RESULTS
From the total study population of 834 patients, 98 patients did not classify the severity of their VWD at inclusion. Therefore, patient‐reported data on disease severity was available for 736 patients. Of these patients, 59.5% (n = 438) were female and 54.8% (n = 403) had blood group O. The median age at inclusion was 41.0 (IQR 23.0–55.0) years and 128 children under 16 years of age were included in the study.
Based on VWF levels at study inclusion, 60.2% (n = 443) had VWD type 1, 36.5% (n = 269) had VWD type 2 and 3.2% (n = 24) had VWD type 3. For patients with available VWF:Act levels at inclusion (n = 572), based on the laboratory severity classification by Federici, 3 we identified 225 (39.3%) mild, 187 (32.7%) moderate and 160 (28.0%) severe VWD patients. For patients with available historically lowest VWF:Act levels (n = 574), based on the same classification method, we noted 44 (7.7%) mild, 341 (59.4%) moderate and 189 (32.9%) severe patients.
Data on disease severity from the patients’ perspective identified four categories of increasing severity (Table 1).
TABLE 1.
Patient demographics and phenotype per patient‐reported severity category.
| Negligible (n = 120) | Mild (n = 393) | Moderate (n = 171) | Severe (n = 52) | |
|---|---|---|---|---|
| Female sex, n (%) | 64 (53.3) | 226 (57.5) | 114 (66.7) | 34 (65.4) |
| Blood group, n (%) | 60 (50.0) | 221 (56.2) | 98 (57.3) | 24 (46.2) |
| Type of VWD | ||||
| 1, n (%) | 82 (68.3) | 241 (61.3) | 93 (54.4) | 27 (51.9) |
| 2, n (%) | 38 (31.7) | 146 (37.2) | 70 (40.9) | 15 (28.8) |
| 3, n (%) | 0 (0.0) | 6 (1.5) | 8 (4.7) | 10 (19.2) e |
| Age at inclusion, median [IQR] | 39.5 [24.0–55.0] | 40.0 [23.0–53.0] | 43.0 [25.5–58.5] | 45.0 [16.5–55.8] |
| Haemostatic parameters at inclusion | ||||
| VWF:Ag in IU/mL, median [IQR] | 0.35 [0.24–0.45] | 0.29 [0.17–0.45] a | 0.27 [0.16–0.42] a | 0.24 [0.09–0.40] a |
| VWF:Act in IU/mL, median [IQR] | 0.31 [0.16–0.58] | 0.23 [0.08–0.51] a | 0.17 [0.06–0.39] b | 0.12 [0.00–0.44] b |
| VWF:CB in IU/mL, median [IQR] | 0.33 [0.14–0.56] | 0.22 [0.08–0.50] a | 0.14 [0.06–0.40] b | 0.11 [0.02–0.40] b |
| FVIII:C in IU/mL, median [IQR] | 0.56 [0.42–0.75] | 0.50 [0.33–0.73] a | 0.47 [0.29–0.65] a | 0.36 [0.06–0.67] a |
| VWFpp in IU/mL, median [IQR] | 0.95 [0.71–1.18] | 0.93 [0.73–1.19] | 0.97 [0.72–1.25] | 0.82 [0.57–1.11] |
| Phenotypical parameters | ||||
| Bleeding score, median [IQR] | 6 [3–10] | 9 [5–14] a | 14 [9–20] b | 13 [9–21] b |
| n = 102 d | n = 321 d | n = 146 d | n = 39 d | |
| SF‐36 physical (PCS), median [IQR] | 55.4 [48.3–58.5] | 54.2 [47.5–57.3] a | 50.3 [36.6–55.9] b | 39.4 [28.1–50.1] c |
| SF‐36 mental (MCS), median [IQR] | 54.0 [50.4–57.4] | 54.0 [48.9–57.2] | 52.8 [43.1–57.1] | 51.5 [42.8–55.7] |
Significantly different in comparison to the negligible group.
Significantly different in comparison to negligible and mild groups.
Significantly different in comparison to negligible, mild and moderate groups.
Children were excluded from analyses with the SF‐36 questionnaire.
p < .05 shows a significant association between type 3 VWD and the patient‐reported ‘severe’ category.
3.1. Patient‐reported vs. laboratory‐based severity classification
To assess the concordance between patient‐experienced and the laboratory‐based classification by Federici, 3 we twice categorized laboratory phenotype as mild, moderate and severe, based on central and historically lowest VWF:Act levels.
Our analysis revealed that 211 individuals (36.9%) concurred with the severity classification assigned to them based on VWF:Act levels. Notably, for a substantial number of 234 patients (40.9%) we underestimated disease severity, since we assigned them a lower severity category than they would assign themselves. On the other hand, we overestimated disease severity for 127 patients (22.2%), assigning them a higher severity category than they would themselves (Figure 1A). The same analysis with historically lowest VWF:Act levels showed a higher number of 286 patients (49.8%) who reported disease severity in alignment with the laboratory‐based classification. Disease severity was now underestimated for 121 patients (21.1%) and overestimated for 167 patients (29.1%) (Figure 1A).
FIGURE 1.
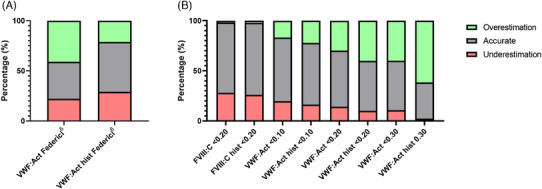
Classification of patient‐reported severity compared to literature classifications. Every column was derived from a cross‐table that compares patient‐reported severity to a single literature‐derived severity classification. To comprise the cross‐tables, patient‐reported data was divided into two or three groups to fit the opposing rating system. The grey fields correspond to the accuracy of the agreement. The green fields represent the proportion of cases in which the reported severity was overestimated by the existing classification. The red fields represent the proportion of cases in which the reported severity was underestimated by the existing classification. All outcomes are represented as proportions of the total amount of patients per cross‐table, expressed in percentages (%). (A) Federici classification. 3 (B) Several other classifications with cutoffs for severity are described on the x‐axis. 4 , 5 , 6
We performed similar comparisons between patient‐reported VWD severity and various binary severity classifications obtained from literature, based on central or historically lowest laboratory levels 4 , 5 , 6 , 9 (Figure 1B). The highest percentage of correct classification between patient‐reported and laboratory‐based severity of VWD was found for historically lowest and central FVIII:C levels < 0.20 IU/mL as a proxy for severe VWD, reaching a 72% accuracy (Figure 1B).
3.2. Determinants of patient‐reported severity
To identify the key factors contributing to higher patient‐reported disease severity, we assessed the clinical phenotype of the included patients (Table 1). We found a significant association between type 3 VWD and the self‐reported ‘severe’ category (p < .01). This was illustrated by a high proportion of type 3 patients (41.7%) considering their disease as severe, compared to type 1 (6.1%) and type 2 (5.6%) patients (p < .05, Figure 2). None of the type 3 patients reported negligible severity. Notably, the reported severity of type 1 and type 2 patients was similarly distributed (p = .16, Figure 2).
FIGURE 2.
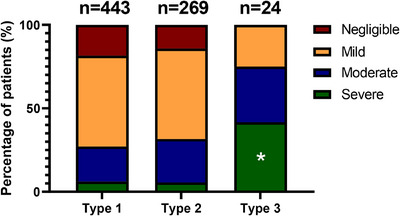
Patient‐reported severity and VWD type. Patient‐reported severity was assessed by means of a 5‐point scale: negligible, mild, moderate, severe and critical. The critical and severe groups were then combined.
3.3. Patient‐reported severity and VWF laboratory levels
Patients who reported mild, moderate or severe disease had similar VWF:Ag, VWF:Act, VWF:CB and FVIII:C levels(Figure 3). Patients who reported their disease as negligible had higher VWF:Ag, VWF:Act, VWF:CB and FVIII:C levels than moderate and severe patients (p < .01 for all, Figure 3).
FIGURE 3.
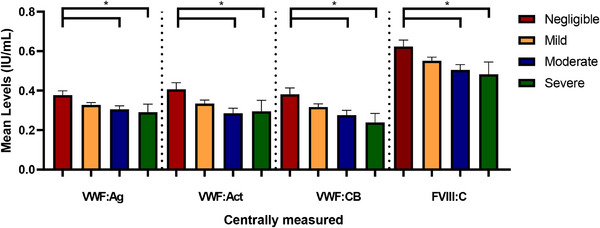
Patient‐reported severity and VWF levels. Patient‐reported severity on a four‐point scale in relation to VWF and FVIII: C levels. *Significant result after correction for multiple tests.
3.4. Patient‐reported severity and bleeding score
Of all patients with normal BS, only a small proportion (2.1%) reported VWD as severe. In contrast, 15.3% of patients with BS > 20 reported VWD as severe (p < .001).
For each level of reported disease severity, the overall BS seemingly increased, indicating a correlation between higher reported severity categories and higher BS (p < .01, Figure 4A). Subgroup analysis revealed that the negligible category was associated with lower BS than all other categories (p < .01 for all). Additionally, patients with mild reported severity had lower BS than those in the moderate and severe groups (p < .01 for both). Patients who reported moderate and severe disease had similar BS (p = 1.00).
FIGURE 4.
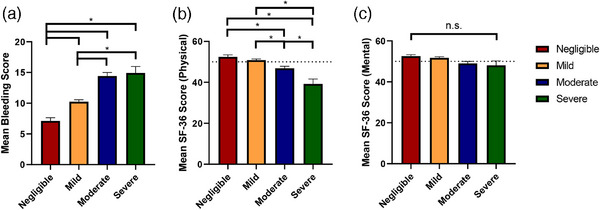
Patient‐reported severity and clinical phenotype. Patient‐reported severity on a four‐point scale in relation to (A) the Tosetto VWD bleeding score, (B) the SF‐36 physical domain (normal mean score 50 depicted by horizontal dashed line), and (C) the SF‐36 mental domain (normal mean score 50 depicted by horizontal dashed line). *Significant result after correction for multiple tests.
The BS encompasses multiple bleeding items, almost all of which contributed to these findings. Exclusively, postpartum haemorrhage (PPH) and central nervous system (CNS) bleeding did not show significance for this comparison (Figure S1). Furthermore, when comparing males and females with severe VWD, there was similarity across most bleeding items (Figure S2A–D). Females had higher mean scores for cutaneous bleeds (0.77 ± 0.65 vs. 1.24 ± 0.43, p < .01), males had higher mean scores for muscle bleeds (0.88 ± 1.49 vs. 0.23 ± 0.78, p = .03).
3.5. Patient‐reported severity and quality of life
Results from the SF‐36 showed that increased reported severity corresponded with lower HR‐QoL scores for the physical domain (p < .01, Figure 4B). Subgroup analysis showed that patients who reported severe VWD had SF‐36 PCS scores below the reference population mean (Table 1). The MCS scores were similar for each severity category (p = .125, Figure 4C) and above the reference mean for each category (Table 1).
3.6. Multivariate analysis of patient‐reported severity
From an ordinal logistic regression model, we derived that there were several significant contributors to patient‐reported disease severity (Table 2). Patients with type 3 VWD were more probable to report higher disease severity compared to patients with type 1 VWD (OR 4.02, 95%CI: 1.72–9.45), whereas this was not found for type 2 VWD patients (OR 0.91, 95%CI: 0.66–1.26). Females were more probable to report higher severity of VWD compared to males (OR 1.36, 95%CI: 1.01–1.83).
TABLE 2.
Factors contributing towards patient‐reported severity of VWD.
| OR | 95% CI | p‐value | |
|---|---|---|---|
| Female sex | 1.36 | 1.01–1.83 | .04 |
| Age at inclusion | 0.99 | 0.99–1.00 | .05 |
| Type 3 a | 4.02 | 1.72–9.45 | <.01 |
| Bleeding score | 1.09 | 1.06–1.11 | <.01 |
| Haemostatic treatment in the year preceding inclusion | 1.53 | 1.10–2.13 | .01 |
| Historical VWF:Act in IU/mL | 0.26 | 0.07–1.00 | .05 |
Reference category is type 1.
Higher historically lowest VWF:Act levels correlated significantly with lower reported disease severity by patients in our cohort (OR 0.26, 95%CI: 0.07–1.00), indicating that for each IU/mL increase in historically lowest VWF:Act levels, the odds of reporting greater severity decreased. Central VWF:Ag, VWF:Act and FVIII:C levels and historically lowest VWF:Ag and FVIII:C levels were not independent determinants of patient‐reported severity.
From a clinical perspective, a higher BS was a significant determinant of patient‐reported severity (OR 1.09, 95%CI: 01.06–1.11), indicating that for each point increase in the BS, the odds of reporting greater severity increased. Additionally, patients who received haemostatic treatment with desmopressin or VWF/FVIII concentrate due to bleeding within 1‐year preceding inclusion were more probable to report higher VWD severity (OR 1.53, 95%CI: 1.10–2.13).
4. DISCUSSION
We found that PROs provided novel insights into the determinants of VWD severity, beyond the established severity classifications based solely on VWF levels. 4 , 5 , 6 The highest concordance with patient‐reported disease severity was found when FVIII:C levels < 0.20 IU/mL were used as a proxy for severe disease. However, we identified multiple additional independent determinants that influence patient‐reported disease severity, such as higher BS and type 3 VWD.
Disease severity is a crucial concept in clinical medicine, playing a vital role in the diagnostic process and the development of treatment plans. The exclusion or devaluation of the patients’ perspective during diagnosis may negatively impact HR‐QoL, since a strong patient‐physician partnership is known to reduce disease burden. 25 Understanding the timing and reasons behind patients' perceptions of disease severity is essential for creating effective and informed treatment plans, such as determining the frequency of hospital visits, and prioritizing treatment elements. Given the growing emphasis on shared decision‐making in healthcare, patient input is expected to become increasingly valuable. To our knowledge, this study represents the first large cohort analysis comparing PROs on disease severity with clinical and laboratory parameters. Despite data collection occurring 15 years ago, the findings remain relevant today as the laboratory‐based severity classification criteria have also been used since 2004. 3
Although VWF and FVIII activity levels seem to univariately reflect patient‐reported severity in the majority of cases in this study, multivariate analysis revealed that only historically lowest VWF:Act levels were independently associated with patient‐reported severity. Given that VWF levels can increase over time in the majority of patients, 7 relying solely on laboratory levels to classify VWD severity has limitations and severity classifications may have to be reconsidered periodically.
Disease severity may be underestimated in patients with high laboratory levels who report severe disease. They can experience bleeding that is not properly assessed in a BS, such as recurring bleeds. Also, the negative effects of bleeding sequelae, such as joint bleeds or bleeds with emotional or social effects, can be underestimated in bleeding assessment tools. 26 , 27 Conversely, patients with low laboratory levels who report mild disease may perceive VWD as less severe due to a lack of haemostatic challenges, normalizing bleeding perception over time or because of family members, or specific pathophysiology. VWF levels in type 1C patients are particularly low due to high clearance of VWF, but they experience limited bleeding, possibly due to normal production and secretion of VWF. 28 , 29
Although the clinical phenotype, particularly bleeding, was identified as an important determinant of patient‐reported VWD severity, we were unable to precisely discover which type of bleeding contributes most significantly. Bleeding assessment tools, though not designed for self‐administration, have proven reliable and feasible compared to physician‐administered scores. 17 , 30 Our validation method involved comparing randomly selected patients with matched controls, showing comparability between self‐completed and expert‐administered scores. 17 However, the cumulative nature of the BS may obscure the effects of recent and recurring bleeding episodes, as a single treatment with VWF concentrates can saturate the scoring system for that particular bleeding item.
The suggestion that recent bleeding weighs more heavily for patients than past bleeding episodes is in line with studies that show increased underreporting of healthcare service utilization over time. 31 , 32 , 33 Additionally, we found that haemostatic treatment in the year preceding study inclusion independently influenced patient‐perceived disease severity, but treatment or hospital admission in the past did not.
Recurring bleeding, like heavy menstrual bleeding (HMB) is reported in over 80% of females with VWD, 17 , 34 and may explain why we found that females are more probable to report higher severity than males. Women may classify their VWD as more severe based on these recurrent bleedings, and consequently, it is probable that women‐specific bleeding is underestimated in clinical practice. Future studies should periodically assess PROs to mitigate this type of bias.
Our study was limited by the absence of validated and VWD‐specific questionnaires with a severity scale for assessing HR‐QoL, 35 which left room for interpatient interpretation of response options regarding disease severity. Disease‐specific PROMs exist for haemophilia, but were not validated for VWD and other inherited bleeding disorders. 36 , 37 Recently, we studied PROs measurement information systems (PROMIS) in non‐severe VWD and showed that they are a feasible, valid, and reliable alternative for the SF‐36v2. 38 Additionally, our study was unable to explore the perspectives of elderly patients, a growing population of VWD patients who face unique haemostatic challenges, such as falls and surgery. 39 , 40 The patients’ perspective on VWD among elderly individuals could be a valuable avenue for future research.
5. CONCLUSION
To summarize, patient‐experienced severity is affected by a complex interplay of demographics, laboratory parameters, bleeding episodes, physical discomfort and the timing of consultation. It, therefore, seems too straightforward to base our classification of VWD severity in guidelines solely on laboratory parameters. We showed that, for a variety of laboratory cut‐off levels, the patients’ experiences deviate from the current severity classification. We also pitched multiple determinants that influence disease severity from the patients’ perspective, of which type 3 and higher BS were the most significant. The integration of disease‐specific PROMs may enhance our understanding of disease perception, identify more determinants of disease severity and improve patient‐centred care in VWD diagnosis and management.
AUTHOR CONTRIBUTIONS
Calvin B. van Kwawegen and Ferdows Atiq designed the study. Calvin B. van Kwawegen collected the data, performed statistical analyses interpreted the data and wrote the manuscript. Frank W.G. Leebeek conceived of and designed the study, interpreted data and critically revised the manuscript. All authors critically revised and gave their consent to the final version of the manuscript.
CONFLICT OF INTEREST STATEMENT
Ferdows Atiq is supported by a Rubicon grant (452022310) from the Netherlands Organization for Health Research and Development (ZonMw). Ferdows Atiq received research support from CSL Behring, Takeda, Octapharma and Sobi. Marjon H. Cnossen has received grants from governmental research institutes, such as the Dutch Research Institute (NWO), ZonMW, Innovation Fund, NWO‐Dutch Research Agenda, and unrestricted investigator‐initiated research grants as well as educational and travel funding from various companies over the years (Pfizer, Baxter/Baxalta/Shire, Bayer Schering Pharma, CSL Behring, Sobi, Novo Nordisk, Novartis, and Nordic Pharma); she served as a member on steering boards of Roche and Bayer. All grants, awards, and fees go to the Erasmus MC. Karina Meijer reports speaker fees from Alexion, Bayer and CSL Behring, participation in trial steering committees for Bayer and Astra Zeneca, consulting fees from Uniqure and Therini, participation in data monitoring and endpoint adjudication committee for Octapharma. Marieke J.H.A. Kruip received grants from governmental research institutes, such as the Dutch Research Institute (ZonMW/NWO), Dutch Thrombosis Foundation, Innovation fund, unrestricted grants from Bayer, Pfizer, Daiichi Sankyo, Sobi and Boehringer Ingelheim and speakers fee from Bayer. Jeroen Eikenboom received research support from CSL Behring. Karin P.M. van Galen received unrestricted research support from Octapharma. Frank W.G. Leebeek received unrestricted research support from CSL Behring and Takeda for performing the Willebrand in the Netherlands (WiN) study and uniQure and SOBI for studies not related to this article, and he is a consultant for uniQure, CSL Behring, BioMarin, and Takeda, of which the fees go to the institution. Calvin B. van Kwawegen, Karin Fijnvandraat, Saskia E.M. Schols, Joke de Meris and Johanna G. van der Bom declare no conflicts of interest.
ETHICS STATEMENT
The study has been performed according to the Declaration of Helsinki and has been approved by the Medical Ethical Committees of the participating centres. All study participants signed informed consent at inclusion.
Supporting information
Supporting Information
ACKNOWLEDGEMENTS
The WiN study was supported (in part) by research funding from the Dutch Hemophilia Foundation (Stichting Haemophilia), and CSL Behring (unrestricted grant) and was founded by multiple members of the European Reference Network on Rare Hematological Diseases (ERN‐EuroBloodNet). A complete list of the WiN study members appears in the Appendix.
1. LIST OF THE MEMBERS OF THE WIN STUDY
Academic Medical Centre, Amsterdam; K. Fijnvandraat, M. Coppens. VU University Medical Centre, Amsterdam; A. Kors, S. Zweegman. The Netherlands Haemophilia Society: J. de Meris. Amphia Hospital, Breda: G.J. Goverde, M.H. Jonkers. Catharina Hospital, Eindhoven: N. Dors, M.R. Nijziel. Maxima Medical Centre, Eindhoven: L. Nieuwenhuizen. University Medical Centre Groningen, Groningen: K. Meijer, R.Y.J. Tamminga. Kennemer Gasthuis, Haarlem: P.W. van der Linden. HagaZiekenhuis, The Hague: P.F. Ypma. Leiden University Medical Centre, Leiden: H.C.J. Eikenboom, J.G. van der Bom, F.J.W. Smiers. Maastricht University Medical Centre, Maastricht: B. Granzen, K. Hamulyák. Radboud University Medical Centre, Nijmegen: P. Brons, B.A.P. Laros‐van Gorkom, S.E.M. Schols. Erasmus University Medical Centre, Rotterdam: F.W.G. Leebeek (principal investigator), M.H. Cnossen, J. Boender, F. Atiq, C.B. van Kwawegen. Van Creveld Clinic, University Medical Centre Utrecht, Utrecht: E.P. Mauser‐Bunschoten, K.P.M. van Galen.
van Kwawegen CB, Fijnvandraat K, Kruip MJHA, et al. Patient‐reported data on the severity of Von Willebrand disease. Haemophilia. 2024;30:1348–1356. 10.1111/hae.15103
DATA AVAILABILITY STATEMENT
Upon reasonable request, original data can be reviewed by sending an email to the corresponding author.
REFERENCES
- 1. Leebeek FW, Eikenboom JC. Von Willebrand's Disease. N Engl J Med. 2016;375(21):2067‐2080. [DOI] [PubMed] [Google Scholar]
- 2. Sadler JE, Budde U, Eikenboom JC, et al. Update on the pathophysiology and classification of Von Willebrand disease: a report of the Subcommittee on Von Willebrand Factor. J Thromb Haemost. 2006;4(10):2103‐2114. [DOI] [PubMed] [Google Scholar]
- 3. Federici AB. Clinical diagnosis of Von Willebrand disease. Haemophilia. 2004;10(4):169‐176. [DOI] [PubMed] [Google Scholar]
- 4. Nichols WL, Hultin MB, James AH, et al. Von Willebrand disease (VWD): evidence‐based diagnosis and management guidelines, the National Heart, Lung, and Blood Institute (NHLBI) Expert Panel report (USA). Haemophilia. 2008;14(2):171‐232. [DOI] [PubMed] [Google Scholar]
- 5. Castaman G, Goodeve A, Eikenboom J. European Group on Von Willebrand D. Principles of care for the diagnosis and treatment of Von Willebrand disease. Haematologica. 2013;98(5):667‐674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Laffan MA, Lester W, O'Donnell JS, et al. The diagnosis and management of Von Willebrand disease: a United Kingdom Haemophilia Centre Doctors Organization guideline approved by the British Committee for Standards in Haematology. Br J Haematol. 2014;167(4):453‐465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Atiq F, Blok R, van Kwawegen CB, et al. Type 1 VWD classification revisited: novel insights from combined analysis of the LoVIC and WiN studies. Blood. 2024;143(14):1414‐1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lavin M, Aguila S, Schneppenheim S, et al. Novel insights into the clinical phenotype and pathophysiology underlying low VWF levels. Blood. 2017;130(21):2344‐2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Leebeek FWG, Atiq F. How I manage severe Von Willebrand disease. Br J Haematol. 2019;187(4):418‐430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kalot MA, Al‐Khatib M, Connell NT, et al. An international survey to inform priorities for new guidelines on Von Willebrand disease. Haemophilia. 2020;26(1):106‐116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. James PD, Connell NT, Ameer B, et al. ASH ISTH NHF WFH 2021 guidelines on the diagnosis of Von Willebrand disease. Blood Adv. 2021;5(1):280‐300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Connell NT, Flood VH, Brignardello‐Petersen R, et al. ASH ISTH NHF WFH 2021 guidelines on the management of Von Willebrand disease. Blood Adv. 2021;5(1):301‐325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. de Wee EM, Fijnvandraat K, de Goede‐Bolder A, et al. Impact of Von Willebrand disease on health‐related quality of life in a pediatric population. J Thromb Haemost. 2011;9(3):502‐509. [DOI] [PubMed] [Google Scholar]
- 14. de Wee EM, Mauser‐Bunschoten EP, Van Der Bom JG, et al. Health‐related quality of life among adult patients with moderate and severe Von Willebrand disease. J Thromb Haemost. 2010;8(7):1492‐1499. [DOI] [PubMed] [Google Scholar]
- 15. Atiq F, Mauser‐Bunschoten EP, Eikenboom J, et al. Sports participation and physical activity in patients with Von Willebrand disease. Haemophilia. 2019;25(1):101‐108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tosetto A, Castaman G, Rodeghiero F. Assessing bleeding in Von Willebrand disease with bleeding score. Blood Rev. 2007;21(2):89‐97. [DOI] [PubMed] [Google Scholar]
- 17. de Wee EM, Sanders YV, Mauser‐Bunschoten EP, et al. Determinants of bleeding phenotype in adult patients with moderate or severe Von Willebrand disease. Thromb Haemost. 2012;108(4):683‐692. [DOI] [PubMed] [Google Scholar]
- 18. Sanders YV, Groeneveld D, Meijer K, et al. Von Willebrand factor propeptide and the phenotypic classification of Von Willebrand disease. Blood. 2015;125(19):3006‐3013. [DOI] [PubMed] [Google Scholar]
- 19. Hays RD, Morales LS. The RAND‐36 measure of health‐related quality of life. Ann Med. 2001;33(5):350‐357. [DOI] [PubMed] [Google Scholar]
- 20. Van der Zee KI, Sanderman R. Het meten van de algemene gezondheidstoestand met de RAND‐36: een handleiding. Noordelijk centrum voor gezondheidsvraagstukken; 1993:1‐23. [Google Scholar]
- 21. Ware JE Jr, Sherbourne CD. The MOS 36‐item short‐form health survey (SF‐36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473‐483. [PubMed] [Google Scholar]
- 22. Ware JE. SF‐36 physical and mental health summary scales: a user's manual. Health Assessment Lab; 1994. [Google Scholar]
- 23. McHorney CA, Ware JE Jr, Lu JF, Sherbourne CD. The MOS 36‐item Short‐Form Health Survey (SF‐36): III. Tests of data quality, scaling assumptions, and reliability across diverse patient groups. Med Care. 1994;32(1):40‐66. [DOI] [PubMed] [Google Scholar]
- 24. McHorney CA, Ware JE Jr, Raczek AE. The MOS 36‐Item Short‐Form Health Survey (SF‐36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care. 1993;31(3):247‐263. [DOI] [PubMed] [Google Scholar]
- 25. U.S. Department of Health and Human Services FDA Center for Drug Evaluation and Research; U.S. Department of Health and Human Services FDA Center for Biologics Evaluation and Research; U.S. Department of Health and Human Services FDA Center for Devices and Radiological Health . Guidance for industry: patient‐reported outcome measures: use in medical product development to support labeling claims: draft guidance. Health Qual Life Outcomes. 2006;4:79. doi: 10.1186/1477-7525-4-79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kempers EK, van Kwawegen CB, de Meris J, et al. Social participation is reduced in type 3 Von Willebrand disease patients and in patients with a severe bleeding phenotype. Haemophilia. 2022;28(2):278‐285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kempers EK, van Kwawegen CB, de Meris J, et al. Colorectal cancer screening in patients with inherited bleeding disorders: high cancer detection rate in hemophilia patients. J Thromb Haemost. 2023;21(5):1177‐1188. [DOI] [PubMed] [Google Scholar]
- 28. Castaman G, Tosetto A, Rodeghiero F. Reduced Von Willebrand factor survival in Von Willebrand disease: pathophysiologic and clinical relevance. J Thromb Haemost. 2009;7(1):71‐74. [DOI] [PubMed] [Google Scholar]
- 29. Gézsi A, Budde U, Deák I, et al. Accelerated clearance alone explains ultra‐large multimers in Von Willebrand disease Vicenza. J Thromb Haemost. 2010;8(6):1273‐1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Punt MC, Blaauwgeers MW, Timmer MA, Welsing PMJ, Schutgens REG, van Galen KPM. Reliability and feasibility of the self‐administered ISTH‐bleeding assessment tool. TH Open. 2019;3(4):e350‐e355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Grimmer K, Bowman P. The effect of age and chronicity on patient recall of public hospital outpatient clinic use. Aust Health Rev. 1997;20(1):78‐87. [DOI] [PubMed] [Google Scholar]
- 32. Wallihan DB, Stump TE, Callahan CM. Accuracy of self‐reported health services use and patterns of care among urban older adults. Med Care. 1999;37(7):662‐670. [DOI] [PubMed] [Google Scholar]
- 33. Jenkins P, Earle‐Richardson G, Slingerland DT, May J. Time dependent memory decay. Am J Ind Med. 2002;41(2):98‐101. [DOI] [PubMed] [Google Scholar]
- 34. De Wee EM, Knol HM, Mauser‐Bunschoten EP, et al. Gynaecological and obstetric bleeding in moderate and severe Von Willebrand disease. Thromb Haemost. 2011;106(5):885‐892. [DOI] [PubMed] [Google Scholar]
- 35. van Hoorn ES, Houwing ME, Al Arashi W, et al. Patient‐reported outcomes in autosomal inherited bleeding disorders: a systematic literature review. Haemophilia. 2022;28(2):197‐214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Teela L, Luijten MAJ, Kuijlaars IAR, et al. Psychometrics of the patient‐reported outcomes measurement information system measures in hemophilia: the applicability of the pediatric item banks. Res Pract Thromb Haemost. 2023;7(6):102159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. van Balen EC, Haverman L, Hassan S, et al. Validation of PROMIS Profile‐29 in adults with hemophilia in the Netherlands. J Thromb Haemost. 2021;19(11):2687‐26701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. van Hoorn ES, Willems SPE, Al Arashi W, et al. Psychometrics of patient‐reported outcomes measurement information system in Von Willebrand disease, inherited platelet function disorders, and rare bleeding disorders. Res Pract Thromb Haemost. 2024;8(4):102474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cuevas‐Trisan R. Balance Problems and Fall Risks in the Elderly. Clin Geriatr Med. 2019;35(2):173‐183. [DOI] [PubMed] [Google Scholar]
- 40. Pofahl WE, Pories WJ. Current status and future directions of geriatric general surgery. J Am Geriatr Soc. 2003;51(7):S351‐S354. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Data Availability Statement
Upon reasonable request, original data can be reviewed by sending an email to the corresponding author.


