Abstract
Introduction
One of the many challenges in diagnosing bleeding disorders is distinguishing between normal and abnormal bleeding symptoms. Letstalkperiod.ca is an educational website that includes an online self‐administered bleeding assessment tool (Self‐BAT) which is a validated screening tool that enables patients to independently determine their bleeding scores (BS).
Aim
The aim of this study was to evaluate patient outcomes for those referred with an abnormal Self‐BAT BS compared to those referred without the prior use of the Self‐BAT.
Methods
This was a retrospective, observational study. After obtaining REB approval, chart review was performed for patients evaluated for a suspected bleeding disorder in a tertiary care centre between 2016 and 2023.
Results
351 patients (310 female) were identified for inclusion with a mean age of 41 years. Of these patients, 30 were referred for a positive/abnormal Self‐BAT BS and the remainder were referred for other reasons. Patients referred for a positive Self‐BAT BS required interventions for their bleeding symptoms more often (73.3% vs. 36.7%, p ≤ .001). Though they were not diagnosed with an inherited bleeding disorder more often (6.7% vs. 10.7%, p = .754), patients referred for a positive self‐BAT were more likely to be diagnosed with a bleeding disorder when the definition was expanded to include bleeding disorder of unknown cause (56.7% vs. 31.9%, p = .008).
Conclusion
Results of this study suggest that the Self‐BAT at letstalkperiod.ca can be a useful tool for patients and physicians to identify those needing referral to tertiary haematology clinics for evaluation and management of bleeding symptoms.
Keywords: bleeding, coagulopathy, haemorrhage, haemostasis, self‐assessment
1. INTRODUCTION
It is estimated that approximately 1 in 1000 individuals worldwide suffer from a symptomatic inherited bleeding disorder. 1 However, only a minority of these patients are formally diagnosed and receive adequate treatment. 1 , 2 Patients with undiagnosed bleeding disorders are at higher risk of experiencing adverse health outcomes related to uncontrolled and possibly life‐threatening bleeding in the setting of trauma, major surgeries, and obstetrical bleeding. Unrecognized pathological bleeding also poses a greater risk for decreased health‐related quality of life, 3 particularly in women, girls, and people with the potential to menstruate, who at baseline experience increased bleeding symptoms in the form of menorrhagia. 4 , 5
Bleeding disorders can be challenging to formally diagnose. Diagnostic barriers include inconclusive and expensive laboratory tests, limited access to specialist care, and the difficulty, faced by both patients and physicians, in distinguishing between normal and abnormal bleeding symptoms. This particular challenge was first addressed in 2005 when the International Society on Thrombosis and Haemostasis (ISTH) Scientific and Standardization Committee (SSC) on Von Willebrand factor (VWF) established defined thresholds for mucocutaneous bleeding symptoms to be considered significant, as part of a set of criteria for the diagnosis of Von Willebrand disease (VWD) type 1. Additionally, the first quantitative validated bleeding assessment tool (BAT) was published that same year (Vicenza BAT). 6 In 2010 the ISTH developed and endorsed a single BAT to standardize reporting of bleeding symptoms for use in adult and paediatric populations. 7 However, this BAT, and all before it, require expert‐administration. This not only creates a significant barrier to patient accessibility but can be challenging to administer in resource and time‐limited clinical settings. Therefore, in 2015, Deforest et al created and validated the first self‐administered BAT (Self‐BAT), to address the limitations of expert‐administered BATs.8 This tool was found to accurately predict a VWD diagnosis in screened patients, and Self‐BAT bleeding scores (BS) were highly correlated with the expert‐administered ISTH‐BAT. 8 In 2017, an online version of the Self‐BAT was validated and launched on the Let's Talk Period website (http://letstalkperiod.ca), as part of a bleeding awareness knowledge translation project. 9 Along with increasing the awareness of undiagnosed bleeding disorders, the website enabled the general population (particularly women in their reproductive years) to easily access the Self‐BAT, 9 potentially leading to a haematology referral for those with abnormal scores. Individuals who complete the online Self‐BAT with an abnormal bleeding score are provided with this score, along with the normal expected range for their age and gender and are encouraged to discuss this result with their primary care physician.
Since the promotion and launch of the let's talk period website, a project undertaken by a team at Queen's University in Kingston, Ontario, Canada, there has been an increase in referrals for abnormal self‐BAT scores to the haematology clinic at the Kingston General Hospital. The aim of this study was therefore to determine if patients referred to a tertiary care haematology clinic at the Kingston General Hospital, for an abnormal Self‐BAT BS were more likely to be diagnosed with a bleeding disorder and require more clinical intervention compared to those referred without the prior use of the Self‐BAT.
2. MATERIAL AND METHODS
2.1. Study design and data collection
We performed a retrospective, observational study. Research ethics board approval was obtained from Queen's University. Patients referred for a suspected bleeding disorder (who had never been previously investigated) at our institution's affiliated tertiary care centre, the Kingston General Hospital, between October 2016 and October 2023 were included. Patients whose bleeding was attributed to anticoagulant and/or antiplatelet use (unless a superimposed BD was suspected) or had an acquired cause of bleeding were excluded from our analysis. Referrals were deemed to be for an abnormal self‐BAT bleeding score from the let's talk period website if this was included by the referring physician in the free text of their referral note. The self‐BAT was accessed by patients on the let's talk period website. Charts, including all referral documents, were reviewed.
2.2. Bleeding disorder categories and definitions
We established two categories of bleeding disorder diagnoses, with the first being an inherited bleeding disorder diagnosis, which we defined as a distinct disease entity associated with a unique set of haemostatic and/or genetic abnormalities (e.g., VWD, haemophilia carriers, congenital platelet defect, etc.). 10 This definition was based on one proposed by the European Haematology Association (EHA) in 2019.10 The second diagnostic category, which we termed simply as ‘bleeding disorder diagnosis’, had a broadened definition that included not only inherited bleeding disorders, but also a diagnosis of bleeding disorder of unknown cause (BDUC). This entity, though a relatively recent one, is well‐recognized in the literature. 10 , 11 , 12 , 13 The definition of BDUC in our population consists of a positive expert‐administered bleeding score with normal haemostatic tests including platelet count, PT/aPTT, testing for VWD and platelet aggregation and release 13 , 16 , 17 (Figure 1).
FIGURE 1.
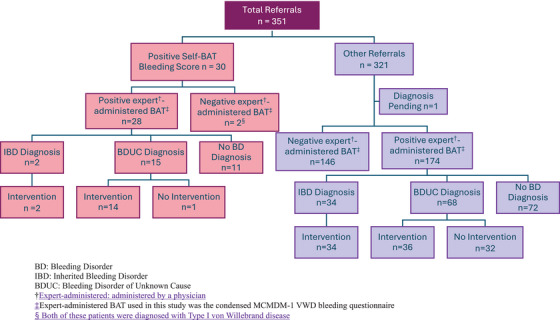
Flow diagram of referrals and results. BD, bleeding disorder; IBD, inherited bleeding disorder; BDUC, bleeding disorder of unknown cause.
†Expert‐administered: administered by a physician. ‡Expert‐administered BAT used in this study was the condensed MCMDM‐1 VWD bleeding questionnaire. § Both of these patients were diagnosed with Type I von Willebrand disease.
2.3. Analysis
Data were deidentified, entered into a spreadsheet (Microsoft Excel®, 2018) and subsequently imported into IBM SPSS (version 29.0 for Windows, Armonk, New York, 2023) for statistical analysis. Categorical data were compared using Pearson Chi‐square tests or the two‐tailed Fisher's Exact test (depending on cell size). Continuous data were compared using the independent samples t‐test. Ferritin and bleeding scores were compared using the Mann−Whitney U, as they were not normally distributed. A p‐value of <.05 was considered statistically significant and no adjustment was made for multiple comparisons.
3. RESULTS
3.1. Cohort characteristics
A total of 351 patients were identified for inclusion in this study, with a mean age of 41 years (range 18–82). 88.3% (310) of the population were female. Of the 351 patients evaluated in our haematology clinic, 30 were specifically referred for an abnormal Self‐BAT BS (i.e., a score of ≥6 for females and ≥4 for males). The remainder of the included patients (321) were referred for other reasons, for example, bleeding/bruising symptoms, abnormal laboratory values, or family history. All patients underwent clinical assessment with the expert‐administered condensed MCMDM‐1VWD bleeding questionnaire at initial visit, with an abnormal/positive score being ≥ 4. Table 1 summarizes the characteristics, laboratory values, and bleeding scores of each cohort as well as their corresponding p values.
TABLE 1.
Patient characteristics, laboratory values and bleeding scores.
| Referred for positive self‐BAT BS | Referred for other reasons | p value | |
|---|---|---|---|
| Female gender (%) | 30 (100) | 280 (87.2) | .035 |
| Mean age years (SD) | 36.1 (±13.1) | 41.9 (±16.1) | .028 |
| Median expert administered BS (IQR) | 7.5 (5–9.25) | 4 (1–7) | <.001 |
| Haemoglobin (g/L), mean (SD a ) | 132 (±11.7) | 136 (±14.7) | .071 |
| Ferritin (µg/L), median (IQR) | 25 (13–57) | 39 (19–75.5) | .069 |
|
Low ferritin (%) ‡Reference range |
53.6 ‡<30 |
40.2 ‡<30 |
.244 |
|
Anaemia (%) ‡Reference range |
16.7 ‡<119 women ‡<136 men |
12.0 ‡<119 women ‡<136 men |
.399 |
SD, standard deviation.
Overall, the cohort of patients referred for an abnormal self‐BAT BS had a higher proportion of abnormal expert‐administered BS than the cohort referred for other reasons (93.3% vs. 54.2%, p ≤ .001). Furthermore, patients referred for an abnormal self‐BAT BS had higher overall expert‐administered BSs than those referred for other reasons, with a median BS of 7.5 seen in self‐BAT referrals versus a median score of four in the other reasons for referral group (p ≤ .001). Mean haemoglobin was found to be 132 (SD ± 11.7) in the self‐BAT group and 136 (SD ± 14.7) in the other reasons for referral group (p = .071). The median ferritin level in the Self‐BAT group was 25 (IQR 13–57), whereas the median ferritin was found to be 39 (IQR 19–75.5) in the other reasons for referral group (p = .069). Low ferritin levels (defined as a ferritin level of <30) were found in 53% of the patients referred for an abnormal Self‐BAT and 40.2% in patients referred for other reasons (p = .244). Finally, anaemia (defined as a haemoglobin of <119 in women and <136 in men) was measured in 16.7% of patients referred for an abnormal Self‐BAT and 12.0% in patients referred for other reasons (p = .399).
3.2. Diagnosis of bleeding disorder and interventions
Ultimately, we found that patients referred for an abnormal self‐BAT BS were not diagnosed with an inherited bleeding disorder more often than those referred for other reasons (6.7% vs. 10.7%, p = .745). However, when using the expanded definition of bleeding disorder diagnosis, we found that patients in the abnormal self‐BAT BS cohort had a statistically significant higher proportion of bleeding disorder diagnoses compared to those in the other reasons for referral cohort (56.7% vs. 31.9%, p = .008). Furthermore, patients referred to our institution for an abnormal Self‐BAT BS underwent interventions regarding their care more frequently compared to those referred for other reasons (73.3% vs. 36.7%, p ≤ .001). These interventions included tranexamic acid, DDAVP, iron supplementation, referral to other specialties including Gynecology and ENT, and/or peri‐procedural haemostatic recommendations (Table 2).
TABLE 2.
Diagnostic and interventional results of referrals.
| Referred for positive self‐BAT BS | Referred for other reasons | p value | |
|---|---|---|---|
| Inherited bleeding disorder diagnosis (%) | 6.7 | 10.7 | .754 |
| Bleeding disorder diagnosis (%) a | 56.7 | 31.9 | .008 |
| Required intervention (%) | 73.3 | 36.7 | <.001 |
| Positive expert administered bleeding score (%) | 93.3 | 54.2 | <.001 |
The ‘Bleeding disorder diagnosis’ category included those diagnosed with an inherited bleeding disorder, as well as those diagnosed with a bleeding disorder of unknown cause.
4. DISCUSSION
Unrecognized abnormal bleeding poses a significant risk to patients’ overall health and their health‐related quality of life. 3 , 4 , 5 The self‐BAT is one of many validated bleeding disorder screening tools that helps to distinguish abnormal versus normal bleeding symptoms. It has the added benefit of being self‐administered and is therefore more accessible to the general public.
The present study has reported the results of the Let's talk period project, which aimed to identify individuals with abnormal bleeding symptoms through the use of a knowledge translation website and easily accessible online self‐BAT. 9 The initial results of the let's talk period project, which included results from the first three months since its launch, showed that the self‐BAT is an effective method of identifying individuals concerned with abnormal bleeding. 9 The website garnered considerable international traffic, and also revealed that a large proportion (45%) of individuals who completed the online self‐BAT (of whom 96% were females) experienced abnormal bleeding. 9
For the present study, we evaluated the effectiveness of the self‐BAT as a screening tool by analysing its impact on referral outcomes at our institution. Data collected in the six years since the inception of the let's talk period project was included. Our study demonstrates that the online Self‐BAT is an effective screening tool for bleeding disorders and does lead to increased interventions in patients which have abnormal Self‐BAT BS.
The most common final diagnosis of patients referred to a haematologist for a possible bleeding tendency is that of BDUC (also known as unclassified bleeding disorder/UBD). 11 − 14 In recent years, there has been a marked increase in the diagnosis of BDUC, particularly in women. 11 , 13 , 15 In many cases there is a positive family history of bleeding, and it is suspected that the underlying problem is heritable, although any objective genetic or haemostatic abnormality has yet to be discovered/proven. 13 Accordingly, we chose to create a separate diagnostic category to include patients with BDUC, labelled as simply ‘bleeding disorder diagnosis’, as opposed to ‘inherited bleeding disorder diagnosis’. The rationale for the inclusion of BDUC patients in our analysis stems from their tendency to experience adverse health outcomes related to their bleeding symptoms, as well as a demonstrable benefit to interventions such as tranexamic acid and DDAVP. 15 , 16 Ultimately, we have demonstrated that a statistically significant higher proportion of patients referred to our haematology clinic for an abnormal self‐BAT were diagnosed with BDUC, compared to those referred for other reasons.
Although it was initially conceived and validated as a screening tool for VWD, 8 two studies have shown the effectiveness of the self‐BAT as a screening tool for other inherited bleeding disorders, for example, congenital platelet defects, 18 and haemophilia carriers. 19 In their study, Punt et al. 18 found that the Self‐BAT had a higher sensitivity in diagnosis of congenital platelet defect, referring to its ability in detecting a bleeding tendency, instead of detecting a particular inherited bleeding disorder. Their findings are consistent with our results, therefore highlighting the self‐BAT's use in recognizing BDUC, and potentially leading to impactful interventions in patients, depending on clinical context.
Indeed, the most evident limitation to our study is the significantly smaller size of the abnormal Self‐BAT cohort in comparison to the other reason for referral cohort. A potential cause of this small cohort size is a possible limited awareness of the let's talk period website, which was initially launched and promoted over six years ago. Perhaps this indicates that the let's talk period website requires further promotion, to both the general population and medical professionals. Furthermore, the small number of referrals for abnormal Self‐BATs could also reflect the limited access to primary care physicians that exists in our Canadian health care system, where patients must first be referred by another physician (most commonly their general practitioner/family doctor) in order to access specialist care.
Finally, the obvious gender skewing observed in our population is to be expected, given that the intended target population of the Let's Talk Period project was women and that men, comparatively, do not face as many haemostatic challenges in their lifetimes. Although this limits our ability to derive any conclusions regarding abnormal bleeding symptoms in males, it is a limitation inevitably faced when studying all BATs, for the reason mentioned above.
5. CONCLUSION
In summary, our study demonstrates that the online Self‐BAT is a reliable screening tool for bleeding disorders, and an abnormal BS in a patient should prompt a referral to specialized haematology care. Further promotion and awareness of the online self‐BAT at http://letstalkperiod.ca could lead to increased diagnosis of bleeding disorders and meaningful interventions for patients.
AUTHOR CONTRIBUTIONS
Laura McDonald analysed the data and wrote the manuscript. Julie Grabell and Jennifer Leung assisted in performing the research and revising the manuscript. Wilma Hopman performed statistical analysis. Paula James designed the research study, supervised research, analysed and interpreted the data and revised the manuscript. All authors have reviewed and approved the final version of the manuscript.
ETHICS STATEMENT
All studies referred to on this review article had been conducted with appropriate ethics approval.
ACKNOWLEDGEMENT
We acknowledge the contributions of Kingston General Hospital and Hotel Dieu Hospital. P.J. has received research funding from Bayer and has provided consultancy services to Band/Guardian Therapeutics, Star/Vega Therapeutics, Roche and BioMarin. J.L. has received honoraria from CSL Behring.
McDonald L, Grabell J, Leung J, Hopman W, James P. Evaluating the impact of the self‐BAT screening tool on patient outcomes: Results of the let's talk period project. Haemophilia. 2024;30:1400–1405. 10.1111/hae.15113
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Skinner MW. WFH: closing the global gap–achieving optimal care. Haemophilia. 2012;18(4):1‐12. doi: 10.1111/j.1365-2516.2012.02822. [DOI] [PubMed] [Google Scholar]
- 2. Stonebraker JS, Bolton‐Maggs PHB, Brooker M, et al. The world federation of hemophilia annual global survey 1999–2018. Haemophilia. 2020;26(4):591‐600. doi: 10.1111/hae.14012 [DOI] [PubMed] [Google Scholar]
- 3. Lippi G, Pasalic L, Favaloro EJ. Detection of mild inherited disorders of blood coagulation: current options and personal recommendations. Expert Rev Hematol. 2015;8(4):527‐542. doi: 10.1586/17474086.2015.1039978 [DOI] [PubMed] [Google Scholar]
- 4. Rae C, Furlong W, Horsman J, et al. Bleeding disorders, menorrhagia and iron deficiency: impacts on health‐related quality of life. Haemophilia. 2013;19(3):385‐391. doi: 10.1111/hae.12014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Presky KO, Kadir RA. Women with inherited bleeding disorders—challenges and strategies for improved care. Thromb Res. 2020;196:569‐578. doi: 10.1016/j.thromres.2019.07.004 [DOI] [PubMed] [Google Scholar]
- 6. Rodeghiero F, Castaman G, Tosetto A, et al. The discriminant power of bleeding history for the diagnosis of type 1 von Willebrand disease: an international, multicenter study. J Thromb Haemost. 2005;3(12):2619‐2626. [DOI] [PubMed] [Google Scholar]
- 7. Rodeghiero F, Tosetto A, Abshire T, et al. ISTH/SSC bleeding assessment tool: a standardized questionnaire and a proposal for a new bleeding score for inherited bleeding disorders. J Thromb Haemost. 2010;8(9):2063‐2065. doi: 10.1111/j.1538-7836.2010.03975.x [DOI] [PubMed] [Google Scholar]
- 8. Deforest M, Grabell J, Albert S, et al. Generation and optimization of the self‐administered bleeding assessment tool and its validation as a screening test for von Willebrand disease. Haemophilia. 2015;21(5):e384‐e388. doi: 10.1111/hae.12747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Reynen E, Grabell J, Ellis AK, James P. Let's talk period! Preliminary results of an online bleeding awareness knowledge translation project and bleeding assessment tool promoted on social media. Haemophilia. 2017;23(4):e282‐e286. doi: 10.1111/hae.13271 [DOI] [PubMed] [Google Scholar]
- 10. Rodeghiero F, Pabinger I, Ragni M, et al. Fundamentals for a systematic approach to mild and moderate inherited bleeding disorders: an EHA consensus report. Hemasphere. 2019;3(4):e286. doi: 10.1097/HS9.0000000000000286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Quiroga T, Goycoolea M, Panes O, et al. High prevalence of bleeders of unknown cause among patients with inherited mucocutaneous bleeding. A prospective study of 280 patients and 299 controls. Haematologica. 2007;92(3):357‐365. doi: 10.3324/haematol.10816 [DOI] [PubMed] [Google Scholar]
- 12. Mezzano D, Quiroga T. Diagnostic challenges of inherited mild bleeding disorders: a bait for poorly explored clinical and basic research. J Thromb Haemost. 2019;17(2):257‐270. doi: 10.1111/jth.14363 [DOI] [PubMed] [Google Scholar]
- 13. Thomas W, Downes K, Desborough MJR. Bleeding of unknown cause and unclassified bleeding disorders; diagnosis, pathophysiology and management. Haemophilia. 2020;26(6):946‐957. doi: 10.1111/hae.14174 [DOI] [PubMed] [Google Scholar]
- 14. Baker RI, O'Donnell JS. How I treat bleeding disorder of unknown cause. Blood. 2021;138(19):1795‐1804. doi: 10.1182/blood.2020010038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. MacDonald S, Wright A, Beuche F, et al. Characterization of a large cohort of patients with unclassified bleeding disorder; clinical features, management of haemostatic challenges and use of global haemostatic assessment with proposed recommendations for diagnosis and treatment. Int J Lab Hematol. 2020;42(2):116‐125. doi: 10.1111/ijlh.13124 [DOI] [PubMed] [Google Scholar]
- 16. Obaji S, Alikhan R, Rayment R, Carter P, Macartney N, Collins P. Unclassified bleeding disorders: outcome of haemostatic challenges following tranexamic acid and/or desmopressin. Haemophilia. 2016;22(2):285‐291. doi: 10.1111/hae.12811 [DOI] [PubMed] [Google Scholar]
- 17. Zegers SAM, Smit Y, Saes JL, et al. Diagnostic work up of patients with increased bleeding tendency. Haemophilia. 2020;26(2):269‐277. doi: 10.1111/hae.13922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Punt MC, Blaauwgeers MW, Timmer MA, Welsing PMJ, Schutgens REG, van Galen KPM. Reliability and feasibility of the self‐administered ISTH‐bleeding assessment tool. TH Open. 2019;3(4):e350‐e355. doi: 10.1055/s-0039-3400483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Young JE, Grabell J, Tuttle A, et al. Evaluation of the self‐administered bleeding assessment tool (self‐BAT) in haemophilia carriers and correlations with quality of life. Haemophilia. 2017;23(6):e536‐e538. doi: 10.1111/hae.13354 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


