Abstract
Introduction
The haemophilia joint health score (HJHS) is a tool used to assess joint changes in patients with haemophilia. There is lack of consensus on the interpretation of HJHS scores and their clinical relevance.
Aim
To evaluate available literature reporting HJHS changes over time and assess a possible cut‐off value for clinically relevant outcomes and the ideal follow‐up for a meaningful score change.
Methods
We conducted a literature search of studies published between 2011 and 2023 where the HJHS version 2.1 had been adopted to detect changes in joint health in patients with haemophilia. We focused on studies that assessed clinical relevance of HJHS changes, evaluated the use of cut‐off values and reported a follow‐up over time.
Results
Our search identified 213 publications of which 53 (25%) were deemed relevant for this review. Of these, 33 (62%) publications reported the total HJHS score and 20 (38%) reported a single joint HJHS score, while the way of reporting HJHS scores/change was highly variable. Ten publications (19%) assessed clinical relevance, but their methods of calculation differed (defining a cut‐off score, measuring standardised response mean or minimal detectable change). The follow‐up duration varied from 2 weeks to 8 years in these 10 studies.
Conclusions
High variability in assessing HJHS change over time is the primary consequence of its low sensitivity, and the lack of consensus on interpretation and clinical relevance of the score. Therefore, more sensitive tools should be used alongside HJHS to better define the joint health status of patients with haemophilia.
Keywords: clinical relevance, haemophilia, health, joint disease, joints
1. INTRODUCTION
The haemophilia joint health score (HJHS) is a formally validated scoring system to assess changes in joint health in persons with haemophilia (PwH) over time by trained specialists of various disciplines involved in haemophilia care, such as physiotherapists or qualified musculoskeletal health professionals. 1 , 2 HJHS is a standardised and common outcome measurement method, which was originally reported extensively in paediatric patients and young adults. 3
Version 2.1 of the HJHS has been formally validated in juveniles (4–18 years) and adults. 1 , 2 , 4 It comprises scoring the ankle, knee and elbow joints as well as global gait, with a maximum score of 20 for individual joints and 124 for the total score, with a higher score indicating worse joint condition. 1 , 2 , 5 To calculate the total score, first, each of the six individual joints is scored for eight items (swelling 0–3, duration of swelling 0‐1, muscle atrophy 0–2, crepitus on motion 0–2, flexion/extension loss 0–3 each, joint pain 0–2, strength 0–4). The total scores are then added, and together with the gait score (0–4), the total HJHS score is calculated. 2 However, information on the usefulness of scoring the gait is limited and it can be challenging to perform in a clinical setting. Consequently, some studies chose to not include it. 6 , 7 In addition, the single joint scores can also be used to assess individual joint health.
While it is considered as one of the top five most important outcome measures for children and adults, 8 the HJHS has also been criticised for being too time‐consuming to be performed during routine clinical practice. It may also be less reliable when correlating the score to the severity of haemophilia in patients aged >40 years, as demonstrated in a recent study in adults with moderate and mild haemophilia (median HJHS = 13 and 11 for mild and moderate haemophilia in patients aged 41–50 years, respectively). 2
There is currently no uniform definition of what could be considered a clinically relevant change in HJHS, nor the length of follow‐up period needed to detect such changes. A frequently applied concept is that of minimal clinically important difference (MCID), which describes the smallest change in a clinical outcome that is perceived as beneficial by the patient. This is an important distinction from statistical significance, which does not necessarily imply clinical relevance. 9 , 10 In addition, HJHS includes a mixture of reversible/modifiable and irreversible features (in the absence of surgical intervention, e.g., synovectomy, total knee replacement). In this context, clinical interpretation of change versus no‐change in the total score can be misleading and should take the individual items into account.
There is no standardised method to estimate the MCID, but different studies use different methods, thereby leading to difficulties with interpreting treatment effect. 11 One previous study 12 defined a cut‐off score of ≥4 for a clinically relevant change in total HJHS based on expert opinion and on a previous assessment of HJHS in a group of healthy young men, showing that total HJHS scores ranged between 0 and 3. 13 Only few other studies have adapted this cut‐off score. Others have used different definitions, such as calculating a minimum detectable change (MDC). MDC estimates the smallest amount of change that can be considered a real change and is calculated from the standard error of measurement. In the present article, we reviewed the available literature assessing a change in HJHS over time in PwH, focusing on those that defined clinical relevance. Our aim was to provide an overview of the range of HJHS changes observed in clinical practice, the utilisation of cut‐off values to detect a clinically relevant change in HJHS, and the length of follow‐up that may be required to record a meaningful difference.
2. METHODS
2.1. Database search and screening strategy
A PubMed search was conducted in June 2023 using the search string “((“Haemophilia Joint Health Score”[all] OR “Hemophilia Joint Health Score”[all] OR “HJHS”[all]) AND ((“2011”[Date – Publication]: “3000”[Date–Publication]))) AND (“English” [Language])”. Peer‐reviewed publications published between January 2011 (when HJHS version 2.1 was developed) and June 2023 were screened for eligibility. Publications in English using HJHS to detect changes in joint score over time (i.e., providing two or more measurements or a delta value) were included. Publications published before 2011 or reporting use of the HJHS outside of the indication of haemophilia were excluded. Titles and abstracts were first screened for initial eligibility, followed by assessing the full text of publications.
2.2. Data extraction
One reviewer performed the screening and data extraction, with questions and unclarities discussed with two other reviewers when necessary. Data extracted from each publication included: information on publication details, haemophilia severity, number and age of patients, number of patients on prophylaxis, study intervention or assessment, HJHS scores at baseline and post‐trial as well as any reported change, study power, study time interval and any cut‐offs or factors influencing the score.
3. RESULTS
3.1. Screening result
Our search identified 213 potentially relevant publications and the authors added one additional reference to be screened for inclusion. The reason for this reference not having been identified in the original search is most likely that the search term “HJHS” was not mentioned neither in the title nor in the abstract. After removal of one duplicate and other non‐relevant references based on screening of the titles and abstracts, the full text of the remaining publications was screened for inclusion, and data extraction was subsequently performed on 62 publications. Nine further publications were removed after data extraction due to incomplete reporting or the publications being deemed out of scope, leaving 53 (25%) publications for the review (Figure 1). Of these, 33 (62%) reported the total HJHS score and 20 (38%) reported the HJHS scores for single joints only. From those publications reporting the HJHS score of all six joints, two excluded the gait score. 6 , 7 An additional study did not specify its use (implying its exclusion). 14 The full list of publications, including full data extraction results can be found in Table S1.
FIGURE 1.
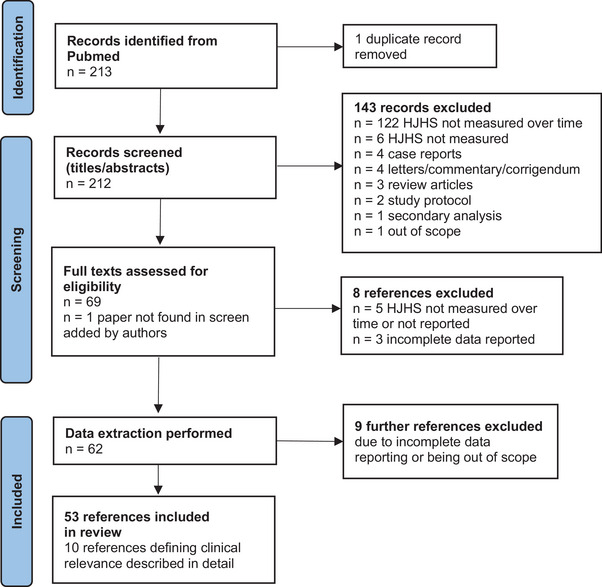
PRISMA flow diagram of the literature search performed for this review. A literature search was conducted on papers published between 2011 and 2023 that used the Haemophilia Joint Health Score (HJHS) in the context of haemophilia.
The majority of studies reported results from a mixed age group, including children and adults (the total age range was 0.8–83 years). Changes in HJHS over time were calculated and reported in 24 publications; the largest decrease in scores reported was −6.14 and the largest increase was +3.77. 15 , 16 Other publications reported only the HJHS scores pre‐ and post‐intervention (e.g., physiotherapy, factor treatment, fascial therapy) rather than a delta value. In addition, the reporting of averages for pre‐ and post‐study HJHS and HJHS change, for example, by mean or median, standard deviation (SD), interquartile range (IQR) or 95% confidence interval (CI), was highly variable among the included studies.
3.2. Clinical significance
Ten out of 53 publications (19%) defined clinical relevance when reporting their results; these studies are listed in Table 1.Three studies defined a clinically meaningful change as ≥4 for total HJHS or ≥2 for individual joint score. 12 , 17 , 18 One study calculated the MCID by multiplying the baseline SD scores by 0.2, 19 and another study used the standardised response mean (SRM) to estimate clinically relevant changes. 20 Five further studies calculated the MDC in their data to assess clinical relevance. 21 , 22 , 23 , 24 , 25
TABLE 1.
Studies published between 2011 and 2023 that assessed the clinical relevance of change in HJHS scores.
| Treatment | HJHS score | HJHS change | Clinically relevant? | Change time interval | Reference | |
|---|---|---|---|---|---|---|
| Pre‐treatment | Post‐treatment | |||||
| Emicizumab |
CG: 4 (0;13) EG: 16 (4; 34) a |
CG: 3 (0; 19) EG: 11 (2; 31) a |
CG: 0 (−2; 3.3) EG: −2 (−9; 1.5) a |
No (HJHS ≥4) |
25 months | Wall et al. 17 |
| Emicizumab | With TJ: 25.6 (20.9) c | – |
All participants: −1.86 (−3.53; −0.20) With TJ: −2.28, (−4.15; −0.42) c |
Yes b (HJHS ≥4) |
49 weeks | Kiialainen et al. 18 |
| No intervention/SoC | 8.5 (3.8; 14.8) a | 11.0 (4.0; 19.0) a | Not reported |
No (HJHS ≥4) |
8 years (median FU) | Kuijlaars et al. 12 |
| Elastic resistance training | – | – |
EG: −2.89 (−5.85; 0.07) CG:.99 (−1.97; 3.95) c |
Yes (baseline SD × 0.2) |
8 weeks | Calatayud et al. 19 |
| Rehabilitation | 11.0 (3.0; 19.0) a | 6.0 (2.0; 11.0) a | −3.3 (3.6) c |
Yes (SRM) |
2 weeks (median FU) | Groen et al. 20 |
| Fascial therapy d |
EG: Left 9 (10) Right 5 (4) CG: Left 5 (7) Right 11 (7) a |
EG: Left 8 (9) Right 4 (5) CG: Left 5 (10) Right 8 (7) a |
EG: Left 1.00 (1.56) Right: 1.14 (1.22) CG: Left −0.14 (2.34) Right: 0.43 (1.27) c |
Yes (MDC) |
3 weeks | Pérez‐Llanes et al. 21 |
| Self‐induced myofascial release d | 5.42 (4.4) c | 4.04 (3.1) c | 1.38 (0.94; 1.81) c |
No (MDC) |
8 weeks | Pérez‐Llanes et al. 25 |
| Immersive VR d | 12.07 (2.65) c | 10.87 (2.28) c | 1.20 (0.82; 1.57) c |
No (MDC) |
4 weeks | Ucero‐Lozano et al. 22 |
| Immersive VR d | 10.77 (3.4) c | 9.92 (3.1) c | −0.84 (−1.16; −0.52) c |
No (MDC) |
4 weeks | Ucero‐Lozano et al. 24 |
| Myofascial release d |
EG: 9.67 (3.87) CG: 8.01 (4.2) c |
EG: 8.14 (3.75) CG: 8.22 (4.29) c |
0.66 (0.45; 0.86) c |
No (MDC) |
3 weeks | Cuesta‐Barriuso et al. 23 |
Note: Ten papers assessed clinical relevance with varying assessment intervals.
Abbreviations: CG, control group; CI, confidence interval; EG, experimental group; FU, follow up; HJHS, Haemophilia Joint Health Score; IQR, interquartile range; MDC, minimum detectable change; PedHAL, paediatric haemophilia activities list; SD, standard deviation; SRM, standardised response mean; TJ, target joint; VR, virtual reality.
The HJHS scores or the change were reported as median (IQR) in these studies.
A clinically relevant improvement in HJHS was only found in the joint specific domains.
The HJHS scores or the change were reported as mean (SD) or mean (95% CI) in these studies.
Only single joint HJHS scores (e.g., elbow or knee joints) were measured in these studies.
Out of those publications that calculated clinical relevance based on a cut‐off score, Kuijlaars et al. found clinically relevant joint deterioration in 37.1% of participants receiving standard of care after a median follow‐up of 8 years, 12 while two other studies with shorter follow‐up times of up to ∼2 years had mixed results. 17 , 18 Wall et al. reported that while HJHS total scores significantly improved in patients who switched to emicizumab from conventional treatment, this did not reach clinical significance. 17 Another study by Kiialainen et al. evaluating emicizumab prophylaxis found clinically significant improvements after 48 weeks, but only in joint‐specific HJHS in younger patients or those with target joints. 18
Calatayud et al. reported a clinically relevant improvement in total HJHS after an 8‐week long strength training programme, as well as significant reduction in pain and improved muscle strength. 19 Groen et al. calculated the SRM (the mean change divided by the SD) to estimate a clinically relevant change in total HJHS. 20 They reported a SRM of −0.9, corresponding to a −3.3 mean reduction in total HJHS after a 2‐week long physical rehabilitation. Since SRM values of 0.8 represent a large response to therapy, 26 these results suggest a high response to the rehabilitation programme.
The studies that assessed an MDC measured HJHS in single joints rather than total HJHS. Of these studies, only one reported that most (57.1%) patients in the experimental group had a clinically relevant improvement in their elbow joint after a 3‐week long fascial therapy directed to the elbows. 21 In the other four studies using short interventions of myofascial release or immersive virtual reality (VR), the change in single joint HJHS did not reach the calculated MDC in most patients despite a statistically significant change in the score in some of these studies. 22 , 23 , 24 , 25
4. DISCUSSION
We have identified 53 references that assessed changes in HJHS over time in patients with haemophilia in the past 12 years. There was a high variability among these studies in terms of reporting standards, and only a small fraction (n = 10, 18.8%) included an assessment of the potential clinical relevance of their findings. There is currently no uniform definition of what could be considered a clinically relevant change in HJHS.
Some studies in the past calculated an MDC from their results and compared the average change to the MDC, this is however based on measurement error and varies between studies. Some studies used cut‐off points based on available data from healthy patients to assess clinical relevance. Most studies to date, however, did not discuss clinical relevance in relation to their results.
The HJHS has demonstrated very good to excellent reliability (intra‐rate interclass correlation coefficient [ICC] = 0.89, inter‐rate ICC = 0.83), 4 and moderate to strong construct validity. 1 , 2 Although the HJHS is the most psychometrically studied instrument for assessing joint health among patients with haemophilia, 3 there is a lack of guidance for interpreting total HJHS scores. Specifically, total scores on the HJHS cannot be used for the interpretation and prediction of long‐term outcomes for people living with haemophilia.
Two approaches for assessing clinical relevance may be to either define a cut‐off score (e.g., post‐treatment HJHS score < X) that implies preservation of joint function, or to define a specific change in score (e.g., ΔHJHS > X) that reveals an improvement in joint health. Three studies in the current review defined total HJHS score of ≥4 as a cut‐off value based on data from healthy patients. However, it should be noted that HJHS values can be as high as 12 or 19 in healthy adults below or above 50 years, respectively. 2 In addition, the same score might have a significantly different clinical weight and meaning in different patient populations (e.g., children vs. adults; patients who did not receive long‐term prophylaxis vs. those who did). Likewise, a high total score consisting of low scores in each of the assessed joints may imply impaired functional ability in individuals that are actually very able physically. Therefore, besides reporting the total score, studies should also provide the context for the changes in score. For example, after a therapy switch, pain may be the main contributor in the short term, while on the long term, atrophy and/or flexion/extension loss may become more important (this also depends on whether physiotherapy is added to the treatment).
There is some evidence suggesting that severity of haemophilia, 12 prophylaxis, recent joint bleeds, and presence of inhibitors can have an effect on HJHS scores. 27 Some of the publications in this study included only patients on prophylaxis, while others had mixed populations in terms of treatment. Due to the heterogeneity of the publications, it is very difficult to draw any general conclusion on the impact of prophylaxis on HJHS scores. To confirm previously established factors associated with HJHS scores, as well as exploring additional factors (e.g., number of clinic visits, proximity to haemophilia treatment centres, number of surgical interventions, etc.), more research is needed. Additionally, there is presently no evidence supporting the use of total HJHS scores to predict adverse haemophilia‐related outcomes such as annual bleed rate, number of target joints, and number of surgical interventions.
Regarding the observation period needed to capture a relevant change in the joint scores after a shift in therapy, the results in this review were mixed, as some studies reported a significant change after only a few weeks of intervention, while others found comparable scores or smaller changes after several years. The length of follow‐up period required to measure clinically relevant changes is likely to be highly dependent on the type of intervention (e.g., physical interventions such as exercise or fascial therapy vs. pharmacological interventions), and the timing of the assessment following acute interventions (e.g., surgery). Several studies identified age, bleeding rates and haemophilia severity to correlate with changes in the HJHS scores, while the follow‐up duration did not, and severe joint damage can be irreversible regardless of treatment duration. 6 , 12 , 18 , 28 The effect of a longer treatment duration may also be influenced by adherence, which correlates with changes in joint scores. 29 Additionally, it is unknown whether a minimum observation period is required to capture relevant changes in specific age groups, such as children.
There are several limitations that should be considered for the use of the HJHS. Firstly, due to the duration of the assessment (normally 45–60 min) it can be difficult to integrate it into a busy clinic schedule, and there is currently a lack of physiotherapists or qualified musculoskeletal health professionals trained to complete the assessment. In light of this, it has been already proposed to shorten its assessment to include only the most relevant items; however, this approach has not been validated yet. 30
As detailed above, the HJHS achieved high inter‐rater reliability scores in an initial reliability study by Hilliard et al. 4 However, Nijdam et al. reported discrepancies in routine HJHS assessment between physiotherapists. 31 This may hamper comparison of scores, particularly in long‐term and/or multicentre studies.
Based on the currently available evidence, change in HJHS should not be used as a unique outcome for joint health in the current treatment landscape and it should ideally be accompanied by more sensitive approaches. 32 In addition, more focus should be given to the very early signs of joint damage. While HJHS is a valid and reliable tool to follow patient joint health over time, its usefulness may be limited for describing the correlation between joint involvement, functional impairment, and the potential impact on quality of life. For example, a lower score on the scale due to low ratings of certain items for a specific joint might imply good overall joint health, while functionally it might be a more significant problem for the patient.
There is still a need to clarify questions such as what a clinically relevant change means in this context, the length of follow‐up period sufficient to detect these changes, and what can be considered as evidence of joint health being sustained. Future studies, such as the currently ongoing pathfinderReal study designed to assess whether joint health is maintained in patients with haemophilia A after switching to an extended half‐life prophylactic treatment, could help evaluate these questions. 33
Importantly, a holistic approach should be considered when evaluating patients’ joint health as the HJHS alone gives little to no information on patients’ ability to perform everyday activities. Therefore, measures such as the HJHS should be used in conjunction with performance‐based measures of physical functioning. 34
5. CONCLUSION
In conclusion, the available data on changes in HJHS are highly heterogenous and changes in joint scores over time are influenced by several factors, which should be considered when planning follow‐up duration for a given treatment. Based on the available literature, a cut‐off score of 4 for change in total HJHS and 2 for individual joints could be accepted to assess clinical relevance, but the real relevance of these cut‐off scores should be further evaluated in future studies.
AUTHOR CONTRIBUTIONS
All authors conceived the study, reviewed the literature, contributed to drafting and review of the manuscript and approved the final version for submission.
CONFLICT OF INTEREST STATEMENT
Cihan Ay received personal fees for lectures and/or participation in advisory boards from Bayer, Biomarin, Biotest, CSL Behring, Novo Nordisk, Pfizer, Roche, LFB, Takeda, and SOBI. Maria Elisa Mancuso has acted as paid consultant, speaker and or advisor for Bayer, Biomarin, CSL Behring, Kedrion, LFB, Novo Nordisk, Octapharma, Pfizer, Roche, Sanofi, Sobi, and Takeda. Davide Matino reports research grants paid directly to the Institution (McMaster University) from Bayer, Pfizer, Novo Nordisk, Sanofi, Spark, Octapharma, Roche; personal fees/honoraria from Sanofi, Sobi, Novo Nordisk, Bayer, Pfizer, Octapharma, and Roche for participation in advisory boards, lectures and preparation of educational material. Karen Strike received research support from Hamilton Health Sciences Health Professional Clinical Research Award, Health Professional Investigator Award, McMaster Children's Hospital Foundation, Pfizer Canada, and Bayer; travel support from Pfizer, Bayer, Novo Nordisk; and consultation fees from Sanofi, Bayer, Hemalytic, Takeda, Pfizer, Biogen, Novo Nordisk, Roche, Baxalta, and Octapharma. Gianluigi Pasta received reimbursement for attending symposia/congresses and/or honoraria for speaking and/or consulting from Bayer, Kedrion, Novo Nordisk, Octapharma, Pfizer, Roche, Sobi, and Takeda.
ETHICS STATEMENT
Not applicable.
Supporting information
Supporting Information
ACKNOWLEDGEMENTS
Medical writing support was provided by Ashfield MedComms GmbH (Mannheim, Germany), an Inizio company, funded by Novo Nordisk.
Ay C, Mancuso ME, Matino D, Strike K, Pasta G. The haemophilia joint health score for the assessment of joint health in patients with haemophilia. Haemophilia. 2024;30:1265–1271. 10.1111/hae.15116
DATA AVAILABILITY STATEMENT
The data that supports the findings of this study are available in the supplementary material of this article.
REFERENCES
- 1. Feldman BM, Funk SM, Bergstrom BM, et al. Validation of a new pediatric joint scoring system from the International Hemophilia Prophylaxis Study Group: validity of the hemophilia joint health score. Arthritis Care Res (Hoboken). 2011;63(2):223‐230. [DOI] [PubMed] [Google Scholar]
- 2. St‐Louis J, Abad A, Funk S, et al. The hemophilia joint health score version 2.1 validation in adult patients study: a multicenter international study. Res Pract Thromb Haemost. 2022;6(2):e12690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gouw SC, Timmer MA, Srivastava A, et al. Measurement of joint health in persons with haemophilia: a systematic review of the measurement properties of haemophilia‐specific instruments. Haemophilia. 2019;25(1):e1‐e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hilliard P, Funk S, Zourikian N, et al. Hemophilia joint health score reliability study. Haemophilia. 2006;12(5):518‐525. [DOI] [PubMed] [Google Scholar]
- 5. Feldman BM, Funk S, Hilliard P, et al. Hemophilia Joint Health Score (HJHS) 2.1. 2011; Accessed 15 January, 2024. https://elearning.wfh.org/resource/hemophilia‐joint‐health‐score‐hjhs/
- 6. Lambert C, Meite N, Sanogo I, Lobet S, Hermans C. Feasibility and outcomes of low‐dose and low‐frequency prophylaxis with recombinant extended half‐life products (Fc‐rFVIII and Fc‐rFIX) in Ivorian children with hemophilia: two‐year experience in the setting of World Federation of Haemophilia humanitarian aid programme. Haemophilia. 2021;27(1):33‐40. [DOI] [PubMed] [Google Scholar]
- 7. Lobet S, Meité N, Koninckx MI, et al. Implementation and assessment of a self‐ and community‐based rehabilitation programme in patients with haemophilia from Côte d'Ivoire. Haemophilia. 2019;25(5):859‐866. [DOI] [PubMed] [Google Scholar]
- 8. Dover S, Blanchette VS, Srivastava A, Fischer K, Abad A, Feldman BM. Clinical outcomes in hemophilia: towards development of a core set of standardized outcome measures for research. Res Pract Thromb Haemost. 2020;4(4):652‐658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wu X, Liu J, Tanadini LG, et al. Challenges for defining minimal clinically important difference (MCID) after spinal cord injury. Spinal Cord. 2015;53(2):84‐91. [DOI] [PubMed] [Google Scholar]
- 10. Cook CE. Clinimetrics corner: the minimal clinically important change score (MCID): a necessary pretense. J Man Manip Ther. 2008;16(4):E82‐E83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Falissard B, Sapin C, Loze JY, Landsberg W, Hansen K. Defining the minimal clinically important difference (MCID) of the Heinrichs‐carpenter quality of life scale (QLS). Int J Methods Psychiatr Res. 2016;25(2):101‐111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kuijlaars IAR, Timmer MA, de Kleijn P, Pisters MF, Fischer K. Monitoring joint health in haemophilia: factors associated with deterioration. Haemophilia. 2017;23(6):934‐940. [DOI] [PubMed] [Google Scholar]
- 13. Sluiter D, Foppen W, de Kleijn P, Fischer K. Haemophilia Joint Health Score in healthy adults playing sports. Haemophilia. 2014;20(2):282‐286. [DOI] [PubMed] [Google Scholar]
- 14. Seuser A, Navarrete‐Duran M, Auerswald G, Mancuso ME. Muscle function deterioration in patients with haemophilia: prospective experience from Costa Rica. Haemophilia. 2018;24(4):e230‐e241. [DOI] [PubMed] [Google Scholar]
- 15. Crivianu‐Gaita V, Rivard GE, Carcao M, et al. Pilot study of once‐a‐day prophylaxis for youth and young adults with severe haemophilia A. Haemophilia. 2016;22(5):e401‐405. [DOI] [PubMed] [Google Scholar]
- 16. Liu Y, Chen L, Li K, Shi M, Poon MC. Severe haemophilia A children on low‐dose tertiary prophylaxis showed less joint deterioration and better maintenance of functional independence than children on on‐demand treatment: a 6‐year follow‐up study. Haemophilia. 2020;26(5):779‐785. [DOI] [PubMed] [Google Scholar]
- 17. Wall C, Xiang H, Palmer B, et al. Emicizumab prophylaxis in haemophilia A with inhibitors: three years follow‐up from the UK Haemophilia Centre Doctors' Organisation (UKHCDO). Haemophilia. 2023;29(3):743‐752. [DOI] [PubMed] [Google Scholar]
- 18. Kiialainen A, Niggli M, Kempton CL, et al. Effect of emicizumab prophylaxis on bone and joint health markers in people with haemophilia A without factor VIII inhibitors in the HAVEN 3 study. Haemophilia. 2022;28(6):1033‐1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Calatayud J, Pérez‐Alenda S, Carrasco JJ, et al. Safety and effectiveness of progressive moderate‐to‐vigorous intensity elastic resistance training on physical function and pain in people with hemophilia. Phys Therapy. 2020;100(9):1632‐1644. [DOI] [PubMed] [Google Scholar]
- 20. Groen W, van der Net J, Lacatusu AM, Serban M, Helders PJ, Fischer K. Functional limitations in Romanian children with haemophilia: further testing of psychometric properties of the paediatric haemophilia activities list. Haemophilia. 2013;19(3):e116‐e125. [DOI] [PubMed] [Google Scholar]
- 21. Perez‐Llanes R, Merono‐Gallut J, Donoso‐Ubeda E, Lopez‐Pina J, Cuesta‐Barriuso R. Safety and effectiveness of fascial therapy in the treatment of adult patients with hemophilic elbow arthropathy: a pilot study. Physiother Theory Pract. 2022;38(2):276‐285. [DOI] [PubMed] [Google Scholar]
- 22. Ucero‐Lozano R, Perez‐Llanes R, Lopez‐Pina JA, Cuesta‐Barriuso R. 180‐degree immersive VR motion visualization in the treatment of haemophilic ankle arthropathy. Haemophilia. 2023;29(1):282‐289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cuesta‐Barriuso R, Perez‐Llanes R, Donoso‐Ubeda E, Lopez‐Pina JA, Merono‐Gallut J. Effects of myofascial release on frequency of joint bleedings, joint status, and joint pain in patients with hemophilic elbow arthropathy: a randomized, single‐blind clinical trial. Medicine (Baltimore). 2021;100(20):e26025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ucero‐Lozano R, Perez‐Llanes R, Lopez‐Pina JA, Cuesta‐Barriuso R. Approach to knee arthropathy through 180‐degree immersive VR movement visualization in adult patients with severe hemophilia: a pilot study. J Clin Med. 2022;11(20). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Perez‐Llanes R, Donoso‐Ubeda E, Merono‐Gallut J, Ucero‐Lozano R, Cuesta‐Barriuso R. Safety and efficacy of a self‐induced myofascial release protocol using a foam roller in patients with haemophilic knee arthropathy. Haemophilia. 2022;28(2):326‐333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Husted JA, Cook RJ, Farewell VT, Gladman DD. Methods for assessing responsiveness: a critical review and recommendations. J Clin Epidemiol. 2000;53(5):459‐468. [DOI] [PubMed] [Google Scholar]
- 27. Bladen M, Main E, Hubert N, Koutoumanou E, Liesner R, Khair K. Factors affecting the Haemophilia Joint Health Score in children with severe haemophilia. Haemophilia. 2013;19(4):626‐631. [DOI] [PubMed] [Google Scholar]
- 28. Ribeiro AJT, Amorim FF, Soares BMD, Santana LA, Imoto AM. Functional and joint evaluation in a prospective cohort of patients with severe haemophilia. Haemophilia. 2021;27(2):314‐320. [DOI] [PubMed] [Google Scholar]
- 29. Zanon E, Tagliaferri A, Pasca S, et al. Physical activity improved by adherence to prophylaxis in an Italian population of children, adolescents and adults with severe haemophilia A: the SHAPE Study. Blood Transfus. 2020;18(2):152‐158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kuijlaars IAR, van der Net J, Feldman BM, et al. Evaluating international Haemophilia Joint Health Score (HJHS) results combined with expert opinion: options for a shorter HJHS. Haemophilia. 2020;26(6):1072‐1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nijdam A, Bladen M, Hubert N, et al. Using routine Haemophilia Joint Health Score for international comparisons of haemophilia outcome: standardization is needed. Haemophilia. 2016;22(1):142‐147. [DOI] [PubMed] [Google Scholar]
- 32. Li Y, Wang F, Pan C, et al. Comparison of joint status using ultrasound assessments and Haemophilia Joint Health Score 2.1 in children with haemophilia. Front Med (Lausanne). 2023;10:1193830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ay C, Benitez‐Hidalgo O, Gidley G, et al. Noninterventional study assessing joint health in persons with hemophilia A after switching to turoctocog alpha pegol: design of pathfinderReal. Res Pract Thromb Haemost. 2024;8(2):102363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bladen M, Harbidge H, Drechsler W, et al. Identifying performance‐based outcome measures of physical function in people with haemophilia (IPOP). Haemophilia. 2023;29(6):1611‐1620. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Data Availability Statement
The data that supports the findings of this study are available in the supplementary material of this article.


