Abstract
1. The administration of l-tryptophan to fed rats produces a twofold increase in hepatic phosphopyruvate carboxylase activity that represents a comparable increase in enzyme protein. With specific antibody against the enzyme we have shown that the increase in phosphopyruvate carboxylase is partially mediated via an actinomycin D-sensitive increase in enzyme synthesis. 2. In starved animals tryptophan increases the enzyme activity without any change in the relative rate of phosphopyruvate carboxylase synthesis. In this condition degradation of the enzyme is retarded by tryptophan by a mechanism that is not prevented by cycloheximide.
Full text
PDF
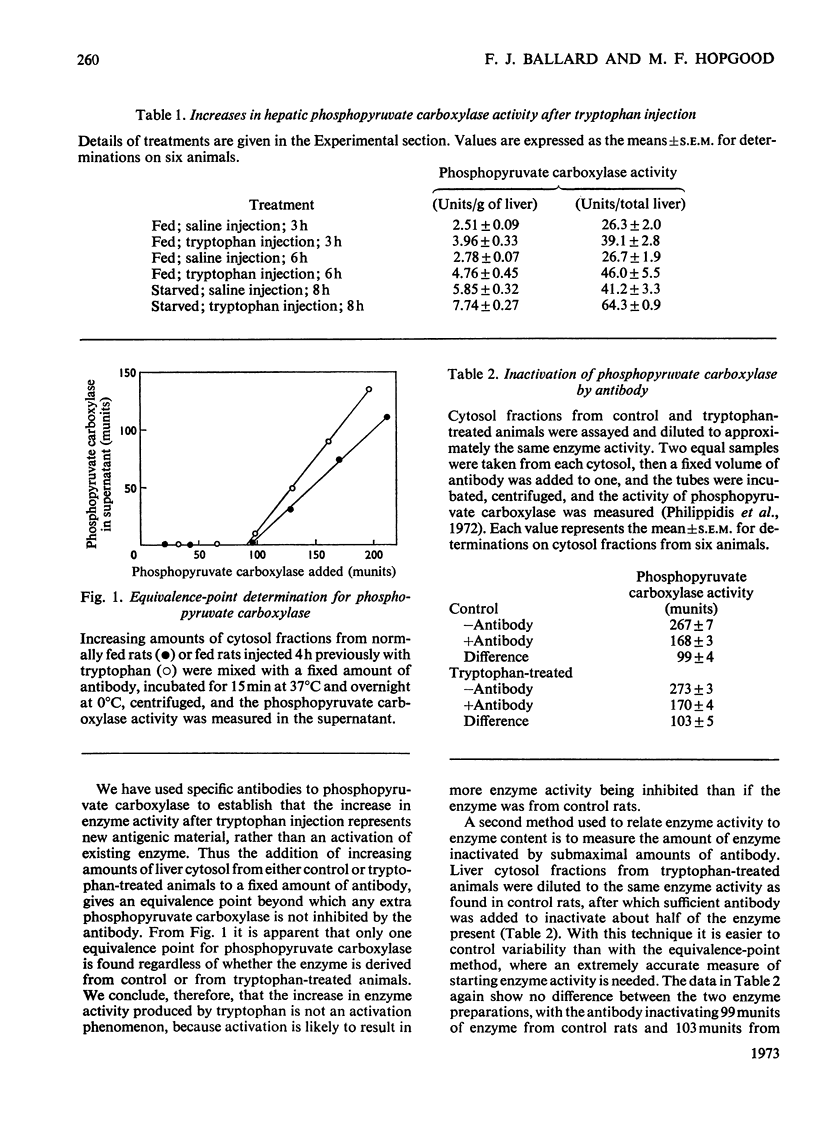

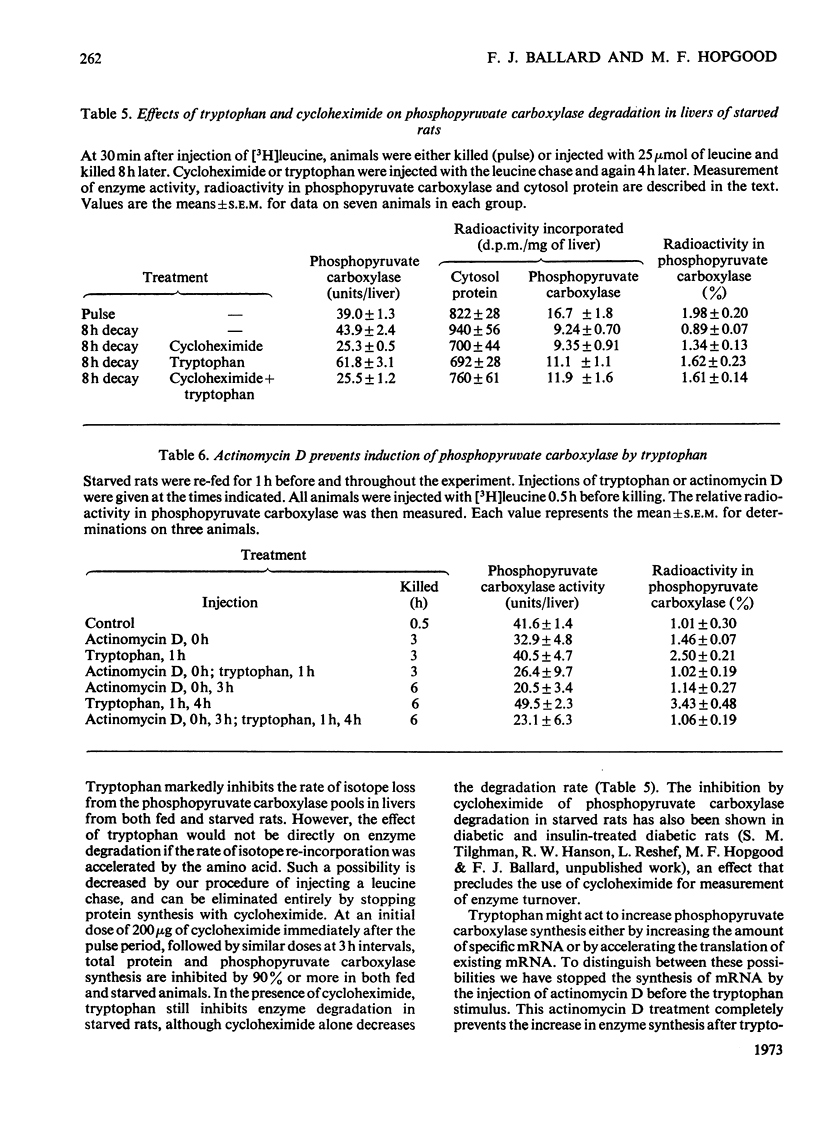


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen R. E., Raines P. L., Regen D. M. Regulatory significance of transfer RNA charging levels. I. Measurements of charging levels in livers of chow-fed rats, fasting rats, and rats fed balanced or imbalanced mixtures of amino acids. Biochim Biophys Acta. 1969 Oct 22;190(2):323–336. doi: 10.1016/0005-2787(69)90083-5. [DOI] [PubMed] [Google Scholar]
- Baliga B. S., Pronczuk A. W., Munro H. N. Regulation of polysome aggregation in a cell-free system through amino acid supply. J Mol Biol. 1968 Jul 14;34(2):199–218. doi: 10.1016/0022-2836(68)90247-7. [DOI] [PubMed] [Google Scholar]
- Ballard F. J., Hanson R. W. Purification of phosphoenolpyruvate carboxykinase from the cytosol fraction of rat liver and the immunochemical demonstration of differences between this enzyme and the mitochondrial phosphoenolpyruvate carboxykinase. J Biol Chem. 1969 Oct 25;244(20):5625–5630. [PubMed] [Google Scholar]
- Chang H. C., Lane M. D. The enzymatic carboxylation of phosphoenolpyruvate. II. Purification and properties of liver mitochondrial phosphoenolpyruvate carboxykinase. J Biol Chem. 1966 May 25;241(10):2413–2420. [PubMed] [Google Scholar]
- Drysdale J. W., Munro H. N. Polysome profiles obtained from mammalian tissues by an improved procedure. Biochim Biophys Acta. 1967 May 30;138(3):616–618. doi: 10.1016/0005-2787(67)90562-x. [DOI] [PubMed] [Google Scholar]
- Fleck A., Shepherd J., Munro H. N. Protein synthesis in rat liver: influence of amino acids in diet on microsomes and polysomes. Science. 1965 Oct 29;150(3696):628–629. doi: 10.1126/science.150.3696.628. [DOI] [PubMed] [Google Scholar]
- Foster D. O., Lardy H. A., Ray P. D., Johnston J. B. Alteration of rat liver phosphoenolpyruvate carboxykinase activity by L-tryptophan in vivo and metals in vitro. Biochemistry. 1967 Jul;6(7):2120–2128. doi: 10.1021/bi00859a033. [DOI] [PubMed] [Google Scholar]
- Foster D. O., Ray P. D., Lardy H. A. A paradoxical in vivo effect of L-tryptophan on the phosphoenolpyruvate carboxykinase of rat liver. Biochemistry. 1966 Feb;5(2):563–569. doi: 10.1021/bi00866a023. [DOI] [PubMed] [Google Scholar]
- Hopgood M. F., Ballard F. J. Synthesis and degradation of phosphoenolpyruvate carboxylase in rat liver and adipose tissue. Changes during a starvation-re-feeding cycle. Biochem J. 1973 Jun;134(2):445–453. doi: 10.1042/bj1340445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jost J. P., Khairallah E. A., Pitot H. C. Studies on the induction and repression of enzymes in rat liver. V. Regulation of the rate of synthesis and degradation of serine dehydratase by dietary amino acids and glucose. J Biol Chem. 1968 Jun 10;243(11):3057–3066. [PubMed] [Google Scholar]
- KENNEY F. T., FLORA R. M. Induction of tyrosine-alpha-ketoglutarate transaminase in rat liver. I. Hormonal nature. J Biol Chem. 1961 Oct;236:2699–2702. [PubMed] [Google Scholar]
- Kenney F., Lee K. L., Reel J. R., Hoel D. G. Regulation of tyrosine alpha-ketoglutarate transaminase in rat liver. IX. Studies of the mechanisms of hormonal inductions in cultured hepatoma cells. J Biol Chem. 1970 Nov 10;245(21):5806–5812. [PubMed] [Google Scholar]
- PERAINO C., BLAKE R. L., PITOT H. C. STUDIES ON THE INDUCTION AND REPRESSION OF ENZYMES IN RAT LIVER. 3. INDUCTION OF ORNITHINE DELTA-TRANSAMINASE AND THREONINE DEHYDRASE BY ORAL INTUBATION OF FREE AMINO ACIDS. J Biol Chem. 1965 Jul;240:3039–3043. [PubMed] [Google Scholar]
- Philippidis H., Hanson R. W., Reshef L., Hopgood M. F., Ballard F. J. The initial synthesis of proteins during development. Phosphoenolpyruvate carboxylase in rat liver at birth. Biochem J. 1972 Mar;126(5):1127–1134. doi: 10.1042/bj1261127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray P. D., Foster D. O., Lardy H. A. Paths of carbon in gluconeogenesis and lipogenesis. IV. Inhibition by L-tryptophan of hepatic gluconeogenesis at the level of phosphoenolpyruvate formation. J Biol Chem. 1966 Sep 10;241(17):3904–3908. [PubMed] [Google Scholar]
- Rhoads R. E., McKnight G. S., Schimke R. T. Quantitative measurement of ovalbumin messenger ribonucleic acid activity. Localization in polysomes, induction by estrogen, and effect of actinomycin D. J Biol Chem. 1973 Mar 25;248(6):2031–2039. [PubMed] [Google Scholar]
- Schimke R. T., Sweeney E. W., Berlin C. M. Studies of the stability in vivo and in vitro of rat liver tryptophan pyrrolase. J Biol Chem. 1965 Dec;240(12):4609–4620. [PubMed] [Google Scholar]
- Snoke R. E., Johnston J. B., Lardy H. A. Response of phosphopyruvate carboxylase to tryptophan metabolites and metal ions. Eur J Biochem. 1971 Dec;24(2):342–346. doi: 10.1111/j.1432-1033.1971.tb19692.x. [DOI] [PubMed] [Google Scholar]
- Tomkins G. M., Gelehrter T. D., Granner D., Martin D., Jr, Samuels H. H., Thompson E. B. Control of specific gene expression in higher organisms. Expression of mammalian genes may be controlled by repressors acting on the translation of messenger RNA. Science. 1969 Dec 19;166(3912):1474–1480. doi: 10.1126/science.166.3912.1474. [DOI] [PubMed] [Google Scholar]
- Treadow B. R., Khairallah E. A. Regulation of phospho-enol-pyruvate carboxykinase during starvation and glucose repression. Nat New Biol. 1972 Oct 4;239(92):131–133. doi: 10.1038/newbio239131a0. [DOI] [PubMed] [Google Scholar]
- Wicks W. D., McKibbin J. B. Evidence for translational regulation of specific enzyme synthesis by N 6 , O 2' -dibutyryl cyclic AMP in hepatoma cell cultures. Biochem Biophys Res Commun. 1972 Jul 11;48(1):205–211. doi: 10.1016/0006-291x(72)90364-6. [DOI] [PubMed] [Google Scholar]
- Wunner W. H., Bell J., Munro H. N. The effect of feeding with a tryptophan-free amino acid mixture on rat-liver polysomes and ribosomal ribonucleic acid. Biochem J. 1966 Nov;101(2):417–428. doi: 10.1042/bj1010417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yatvin M. B., Pitot H. C. Action of actinomycin D on tryptophan uptake in the adrenalectomized rat and its effect on induction of tryptophan pyrrolase and tyrosine transaminase in liver. J Biol Chem. 1970 Sep 25;245(18):4673–4676. [PubMed] [Google Scholar]


