Abstract
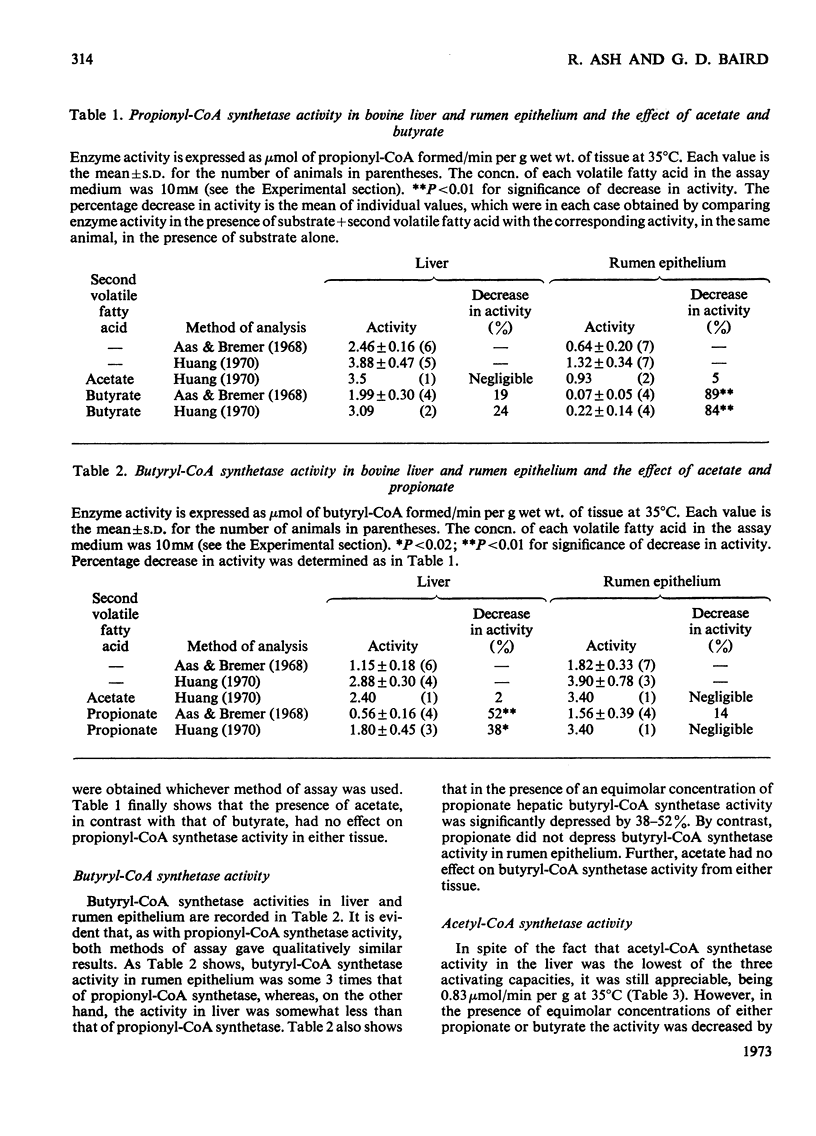
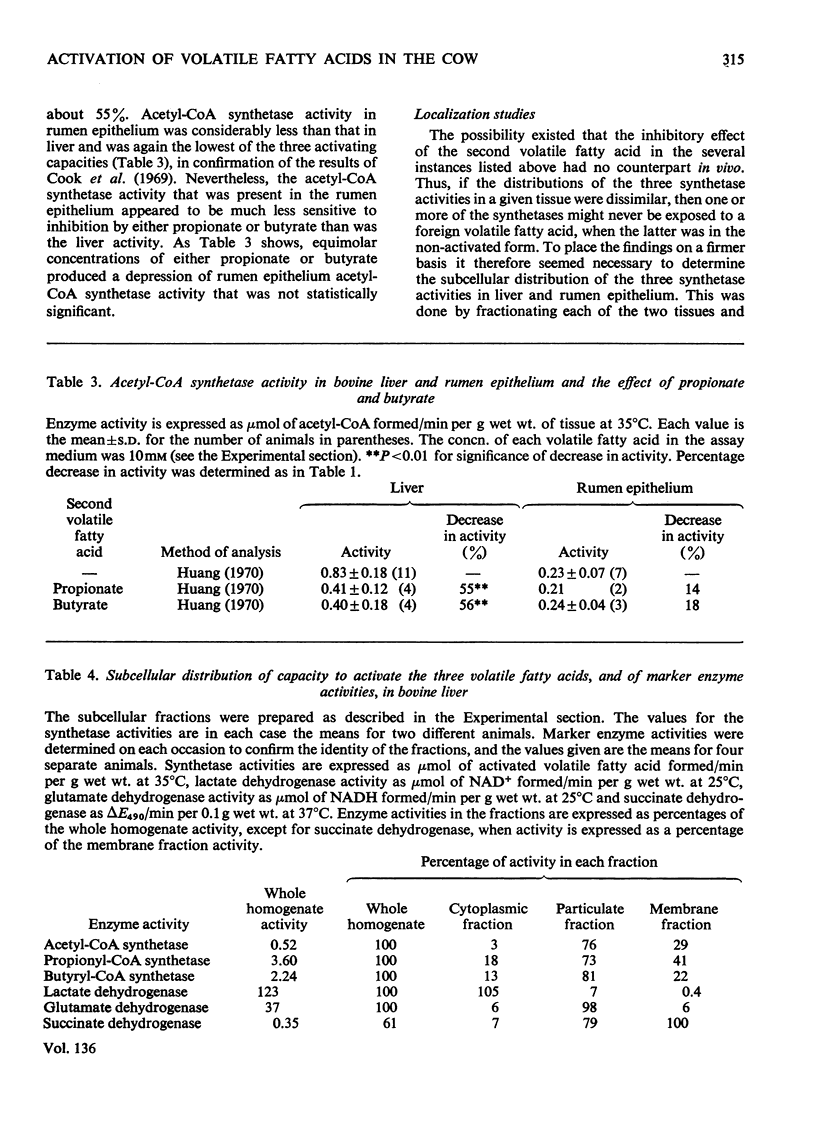
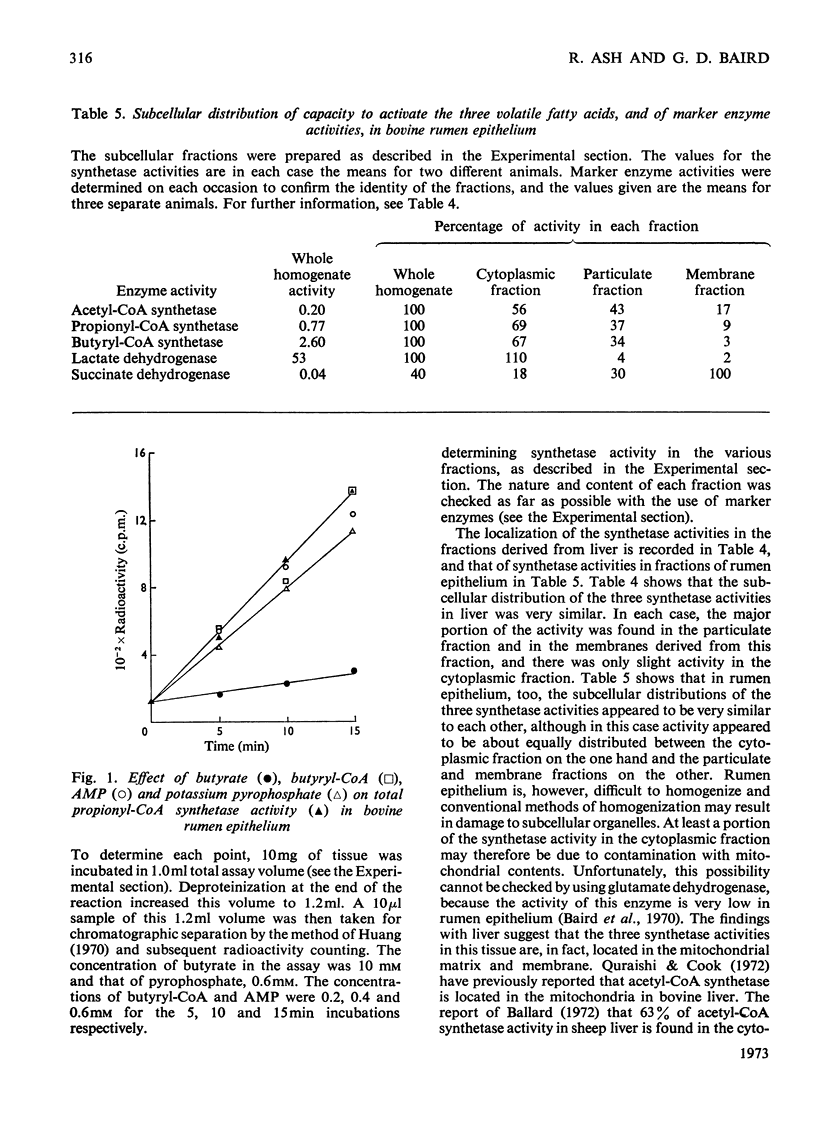
1. The total capacities of homogenates of bovine liver and rumen epithelium to activate acetate, propionate and butyrate were determined. 2. Activating capacities were assayed by measuring the rate of formation of the corresponding CoA esters. The methods used for determining the concentrations of the CoA esters allowed the CoA esters of acetate, propionate and butyrate to be distinguished. It was thus possible to investigate the effect of the presence of a second volatile fatty acid on the rate at which a given volatile fatty acid was activated. 3. The propionate-activating capacity in rumen epithelium was decreased by about 87% in the presence of butyrate, the acetate-activating capacity in liver was decreased by about 55% in the presence of either propionate or butyrate, and the butyrate-activating capacity in liver was decreased by about 40–50% in the presence of propionate. 4. All three activating capacities in liver appeared to be located in the mitochondrial matrix and membrane. The three activating capacities had similar locations to each other in rumen epithelium as well, although in this case activity was more evenly divided between the mitochondria and the cytoplasm. 5. The relative activating capacities towards the volatile fatty acids in the two tissues, together with the ability of one volatile fatty acid to inhibit the activation of another volatile fatty acid, appear to ensure that butyrate is mainly metabolized in the rumen epithelium and that propionate is metabolized in the liver.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aas M., Bremer J. Short-chain fatty acid activation in rat liver. A new assay procedure for the enzymes and studies on their intracellular localization. Biochim Biophys Acta. 1968 Oct 22;164(2):157–166. doi: 10.1016/0005-2760(68)90142-2. [DOI] [PubMed] [Google Scholar]
- Baird G. D., Hibbitt K. G., Lee J. Enzymes involved in acetoacetate formation in various bovine tissues. Biochem J. 1970 May;117(4):703–709. doi: 10.1042/bj1170703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard F. J. Supply and utilization of acetate in mammals. Am J Clin Nutr. 1972 Aug;25(8):773–779. doi: 10.1093/ajcn/25.8.773. [DOI] [PubMed] [Google Scholar]
- Bergman E. N., Wolff J. E. Metabolism of volatile fatty acids by liver and portal-drained viscera in sheep. Am J Physiol. 1971 Aug;221(2):586–592. doi: 10.1152/ajplegacy.1971.221.2.586. [DOI] [PubMed] [Google Scholar]
- Chase J. F. The substrate specificity of carnitine acetyltransferase. Biochem J. 1967 Aug;104(2):510–518. doi: 10.1042/bj1040510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook R. M., Liu S. C., Quraishi S. Utilization of volatile fatty acids in ruminants. 3. Comparison of mitochondrial acyl coenzyme A synthetase activity and substrate specificity in different tissues. Biochemistry. 1969 Jul;8(7):2966–2969. doi: 10.1021/bi00835a042. [DOI] [PubMed] [Google Scholar]
- Cook R. M., Miller L. D. Utilization of volatile fatty acids in ruminants. I. Removal of them from portal blood by the liver. J Dairy Sci. 1965 Oct;48(10):1339–1345. doi: 10.3168/jds.S0022-0302(65)88460-0. [DOI] [PubMed] [Google Scholar]
- HOLDSWORTH E. S., NEVILLE E., NADER C. THE METABOLISM OF ACETATE IN THE SHEEP. Biochim Biophys Acta. 1964 May 11;86:240–249. doi: 10.1016/0304-4165(64)90049-2. [DOI] [PubMed] [Google Scholar]
- Huang K. P. A sensitive assay method of acetyl CoA synthetase. Anal Biochem. 1970 Sep;37(1):98–104. doi: 10.1016/0003-2697(70)90263-0. [DOI] [PubMed] [Google Scholar]
- LENG R. A., ANNISON E. F. Metabolism of acetate, propionate and butyrate by sheep-liver slices. Biochem J. 1963 Feb;86:319–327. doi: 10.1042/bj0860319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng R. A., Steel J. W., Luick J. R. Contribution of propionate to glucose synthesis in sheep. Biochem J. 1967 Jun;103(3):785–790. doi: 10.1042/bj1030785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MASORO E. J., FELTS J. M., PANAGOS S. S., RAPPORT D. The regulation of hepatic acetate-1-C14 metabolism by short-chain fatty acids. Arch Biochem Biophys. 1957 Jun;68(2):270–283. doi: 10.1016/0003-9861(57)90359-4. [DOI] [PubMed] [Google Scholar]
- PENNINGTON R. J., APPLETON J. M. Further studies on the inhibition of acetate metabolism by propionate. Biochem J. 1958 May;69(1):119–125. doi: 10.1042/bj0690119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PENNINGTON R. J. Biochemistry of dystrophic muscle. Mitochondrial succinate-tetrazolium reductase and adenosine triphosphatase. Biochem J. 1961 Sep;80:649–654. doi: 10.1042/bj0800649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PENNINGTON R. J., PFANDER W. H. The metabolism of short-chain fatty acids in the sheep. V. Some interrelationships in the metabolism of fatty acids and glucose by sheep-rumen epithelial tissue. Biochem J. 1957 Jan;65(1):109–111. doi: 10.1042/bj0650109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PENNINGTON R. J. The metabolism of short-chain fatty acids in the sheep. I. Fatty acid utilization and ketone body production by rumen epithelium and other tissues. Biochem J. 1952 May;51(2):251–258. doi: 10.1042/bj0510251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PRITCHARD G. I., TOVE S. B. Interrelationships between the metabolism of short-chain fatty acids by ruminant liver slices. Biochim Biophys Acta. 1960 Jun 17;41:130–137. doi: 10.1016/0006-3002(60)90378-4. [DOI] [PubMed] [Google Scholar]
- Quastel J. H., Wheatley A. H. Oxidation of fatty acids in the liver. Biochem J. 1933;27(6):1752.1–171762. doi: 10.1042/bj0271752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quraishi S., Cook R. M. Utilization of volatile fatty acids in ruminants. IV. Relative activities of acetyl CoA synthetase and acetyl CoA hydrolase in mitochondria and intracellular localization of acetyl CoA synthetase. J Agric Food Chem. 1972 Jan-Feb;20(1):91–95. doi: 10.1021/jf60179a003. [DOI] [PubMed] [Google Scholar]
- Scholte H. R., Wit-Peeters E. M., Bakker J. C. The intracellular and intramitochondrial distribution of short-chain acyl-CoA synthetases in guinea-pig heart. Biochim Biophys Acta. 1971 May 4;231(3):479–486. doi: 10.1016/0005-2760(71)90115-9. [DOI] [PubMed] [Google Scholar]
- Smith R. M. Interactions of acetate, propionate and butyrate in sheep liver mitochondria. Biochem J. 1971 Oct;124(5):877–881. doi: 10.1042/bj1240877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens C. E., Stettler B. K. Factors affecting the transport of volatile fatty acids across rumen epithelium. Am J Physiol. 1966 Feb;210(2):365–372. doi: 10.1152/ajplegacy.1966.210.2.365. [DOI] [PubMed] [Google Scholar]
- Stevens C. E., Stettler B. K. Transport of fatty acid mixtures across rumen epithelium. Am J Physiol. 1966 Jul;211(1):264–271. doi: 10.1152/ajplegacy.1966.211.1.264. [DOI] [PubMed] [Google Scholar]
- Weigand E., Young J. W., McGilliard A. D. Extent of propionate metabolism during absorption from the bovine ruminoreticulum. Biochem J. 1972 Jan;126(1):201–209. doi: 10.1042/bj1260201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson D. H., Bates M. W., Krebs H. A. Activity and intracellular distribution of enzymes of ketone-body metabolism in rat liver. Biochem J. 1968 Jul;108(3):353–361. doi: 10.1042/bj1080353. [DOI] [PMC free article] [PubMed] [Google Scholar]


