Abstract
Chronic pain is often accompanied by anxiety, and gradually increasing anxiety makes the pain itself more protracted. Berberine has been found to be able to cross the blood–brain barrier to treat psychiatric disorders, but its neurocirculatory mechanisms remain unclear. Here, we found that neurons in cingulate area 2 (Cg2) of the caudal anterior cingulate cortex (cACC), but not in Cg1 of the cACC, projected to the ventral lateral thalamus (VLT). Next, we induced chronic inflammatory pain by plantar injection of complete Freund’s adjuvant (CFA) and observed stable anxiety-like behaviors until two weeks postinjection. We specifically activated VLT-projecting cACC (Cg2) neurons in one-week-old CFA-induced mice without anxiety-like behaviors and in normal control mice to induce anxiety-like behaviors. We inhibited the activation of VLT-projecting cACC (Cg2) neurons in two-week-old CFA-treated mice with anxiety-like behaviors and observed that their anxiety-like behaviors were alleviated. On this basis, we further screened the effective dose of berberine for anxiolysis in two-week-old CFA-treated mice. We observed that the effective dose of berberine obtained above decreased the activity of VLT-projecting cACC (Cg2) neurons. The activation of VLT-projecting cACC (Cg2) neurons abrogated the anxiolytic effect of berberine in two-week-old CFA-treated mice.
Subject terms: Neural circuits, Emotion
Berberine alleviates comorbid anxiety symptoms in chronic pain by inhibiting the activation of VLT-projecting cACC (Cg2) neurons in male mice
Introduction
Comorbid anxiety symptoms (CAS) associated with chronic pain frequently occur1. Increasing evidence shows that CAS could be one of the important reasons for the chronification of pain2,3. CAS often appears first, and persistent anxiety further causes a series of more serious diseases, such as depression, sleep disturbance, and cognitive impairment4–6. To prevent CAS and chronic pain from interacting with each other and further resulting in more severe symptoms, greater clinical burden, and greater treatment difficulty, in-depth research on the pathological mechanisms of CAS is urgently needed.
The anterior cingulate cortex (ACC) is a key cortical area closely associated with the CAS in chronic pain7,8. Multiple afferent projections of the ACC have previously been shown to regulate CAS in chronic pain9,10. However, whether the efferent projections of the ACC are associated with CAS in chronic pain has rarely been reported.
The ACC is generally divided into rostral and caudal parts. In our previous studies, whole-brain efferent connections of the rostral ACC (rACC) revealed that the ventral lateral thalamus (VLT) is one of the main descending projections of the rACC11. Magnetic resonance studies of the human brain have shown a strong correlation between anxiety symptoms caused by painful experiences and VLT12. Deep brain stimulation via the VLT can alleviate anxiety and improve patient quality of life13. Our previous study also revealed that VLT-projecting rACC neurons are involved in the regulation of CAS in chronic pain. However, it is unclear whether the caudal ACC (cACC) projects to the VLT or whether VLT-projecting cACC neurons are involved in CAS in chronic pain.
Berberine (BBR) is a compound isolated from the Chinese medicinal plant Coptis chinensis14. BBR has received increasing attention because of its ability to cross the blood–brain barrier easily and its therapeutic effects on psychiatric disorders15. Previous studies on the anxiolytic properties of BBR have focused predominantly on its molecular mechanism16. However, it is unclear whether BBR has a therapeutic effect on CAS in chronic pain and whether BBR acts by interfering with related neural circuits.
In this study, we first microinjected anterograde and retrograde tracer adeno-associated virus (AAV) and clarified the VLT-projecting cACC subregion (cingulate area 1 (Cg1) and/or cingulate area 2 (Cg2)). Next, we imitated a persistent pain model by unilateral plantar injection of complete Freund’s adjuvant (CFA) and investigated stable anxiety-like behaviors until the pain persisted for two weeks. We then specifically activated VLT-projecting cACC neurons in mice one week after CFA injection (without anxiety-like behaviors) and normal control mice to observe whether anxiety-like behaviors appeared and inhibited the activation of this circuit in mice with anxiety-like behaviors two weeks after CFA injection to determine whether anxiety-like behaviors could be alleviated. Finally, we investigated the effect of the intervention and effective dose of BBR on CAS in chronic pain and this circuit.
Results
cACC (Cg2) neurons project to the VLT
To determine whether cACC neurons project to the VLT, we first delivered an anterograde tracer AAV (AAV2/9-hSyn-EGFP) into the cACC (Cg1) or cACC (Cg2) (Supplementary Fig. 1a, b, d, e). We found that neurons in the cACC (Cg2), but not in the cACC (Cg1), projected to the VLT (Supplementary Fig. 1c, f). To further confirm these results, we delivered a retrograde tracer, AAV (AAV2/Retro-hSyn-tdTomato), into the VLT (Supplementary Fig. 1g, h). The somata in the cACC (Cg2), but not in the cACC (Cg1), were infected via retrograde tracing from the VLT (Supplementary Fig. 1i). These results suggest that cACC (Cg2) neurons project to the VLT.
CFA injection evoked anxiety-like behaviors two weeks after injection
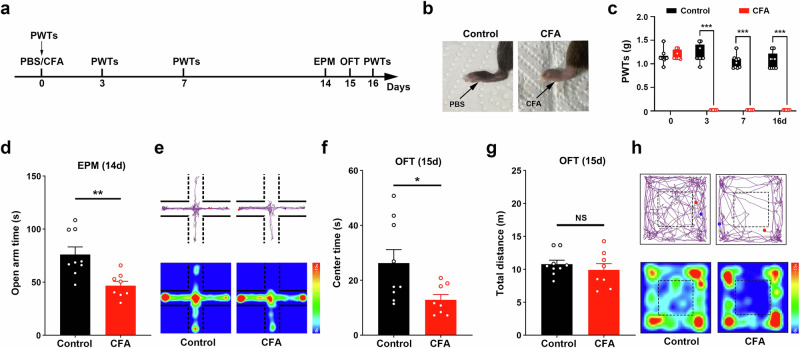
To explore the role of VLT-projecting cACC (Cg2) neurons in CAS associated with chronic pain, we established a persistent pain model via unilateral plantar injection of CFA (Fig. 1a, b). The results of the mechanical pain tests revealed that the mechanical hyperalgesia induced by CFA was maintained until 16 days after CFA injection (Fig. 1c). The results of the elevated plus maze (EPM) test revealed that the mice spent significantly less time moving in the open arms on Day 14 after the injection of CFA (Fig. 1d). The open field test (OFT) results revealed that the mice spent significantly less time moving in the center area on Day 15 after the injection of CFA (Fig. 1e). These results suggest that CFA caused significant anxiety-like behaviors two weeks after injection.
Fig. 1. Anxiety-like behaviors were evoked two weeks after CFA injection.
a Schematic of the experimental design. b Gross macroscopic view of left hindpaw after CFA or PBS injection into the left hindpaw of mice. c Statistical results of 50% paw withdrawal thresholds (PWTs). d Statistical results of time spent in the open arms in the EPM test. e Representative images of movement trajectory (top) and activity time (bottom) in the EPM test. f, g Statistical results of time spent in the central area and total distance traveled in the OFT. h Representative images of movement trajectory (top) and activity time (bottom) in the OFT. Data are the mean ± SEM; *P < 0.05, **P < 0.01, ***P < 0.01. CFA complete Freund’s adjuvant, EPM elevated plus maze, OFT open field test.
One week after CFA injection, anxiety-like behaviors could be evoked by the activation of VLT-projecting cACC (Cg2) neurons
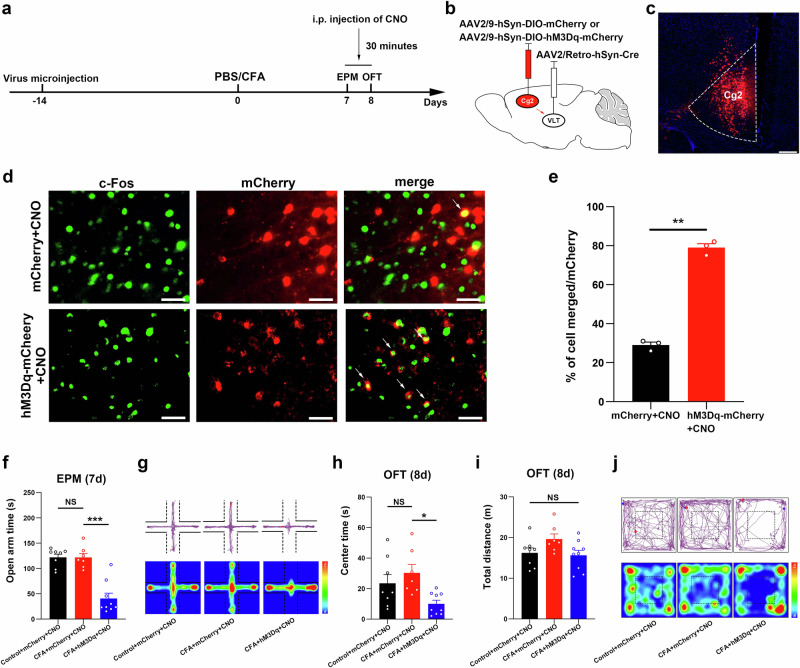
To observe the effects of VLT-projecting cACC (Cg2) neurons on chronic pain without CAS, we first examined anxiety-like behavioral performance in mice one week after CFA injection. The results of both the EPM test and OFT revealed that CFA did not induce significant anxiety-like behaviors one week after injection (Fig. 2f–j). To specifically activate VLT-projecting cACC (Cg2) neurons one week after CFA injection, we delivered the retrograde transport virus AAV2/Retro-hSyn-Cre into the VLT, and a Cre-dependent virus encoding the neuronal activator DREADD hM3Dq was injected into the cACC (Cg2) (Fig. 2a–c). An examination of the coexistence of c-Fos with AAVs (mCherry-labeled), revealed a significant increase in neuronal activity in VLT-projecting cACC (Cg2) neurons labeled with hM3Dq (Fig. 2d, e). The results of the EPM test revealed that mice, with specifically activated VLT-projecting cACC (Cg2) neurons, spent significantly less time moving in the open arms on Day 7 after injection of CFA (Fig. 2f, g). The results of the OFT revealed that mice, with specifically activated VLT-projecting cACC (Cg2) neurons, spent significantly less time moving in the center area on Day 8 after injection of CFA (Fig. 2h–j). These results suggest that the activation of VLT-projecting cACC (Cg2) neurons induces anxiety-like behaviors in chronic pain model mice without CAS.
Fig. 2. After one week of CFA injection, anxiety-like behaviors could be evoked by activation of VLT-projecting cACC (Cg2) neurons.
a Schematic of the experimental design. b, c Schematic for specific infection of VLT-projecting cACC (Cg2) neurons with mCherry or hM3Dq-mCherry. Scale bar, 200 μm. d Representative images showed that intraperitoneal injection of CNO induced c-Fos expression in VLT-projecting cACC (Cg2) neurons with mCherry or hM3Dq-mCherry. Scale bar, 20 μm. e Percentage of labeled mCherry+ neurons expressing c-Fos in mCherry+CNO group and hM3Dq-mCherry+CNO group. f Statistical results of time spent in the open arms in the EPM test. g Representative images of movement trajectory (top) and activity time (bottom) in the EPM test. h, i Statistical results of time spent in the central area and total distance traveled in the OFT. j Representative images of movement trajectory (top) and activity time (bottom) in the OFT. Data are the mean ± SEM; *P < 0.05, **P < 0.01, ***P < 0.001, NS not significant. CFA complete Freund’s adjuvant, VLT ventral lateral thalamus, cACC caudal anterior cingulate cortex, Cg2 cingulate area 2, CNO Clozapine N-oxide, EPM elevated plus maze, OFT open field test.
Activation of VLT-projecting cACC (Cg2) neurons induced anxiety-like behaviors in normal mice
To observe whether the activation of VLT-projecting cACC (Cg2) neurons induces anxiety-like behaviors in normal mice, we delivered the retrograde transport virus AAV2/Retro-hSyn-Cre into the VLT, and a Cre-dependent virus encoding the neuronal activator DREADD hM3Dq was injected into the cACC (Cg2) (Supplementary Fig. 2a–c). The results of the EPM and OFT revealed that mice, with specifically activated VLT-projecting cACC (Cg2) neurons, spent significantly less time in the open arms and center area (Supplementary Fig. 2d–h). These results suggest that the activation of VLT-projecting cACC (Cg2) neurons induces anxiety-like behaviors in normal mice.
Inhibition of VLT-projecting cACC (Cg2) neurons alleviated anxiety-like behaviors induced by CFA
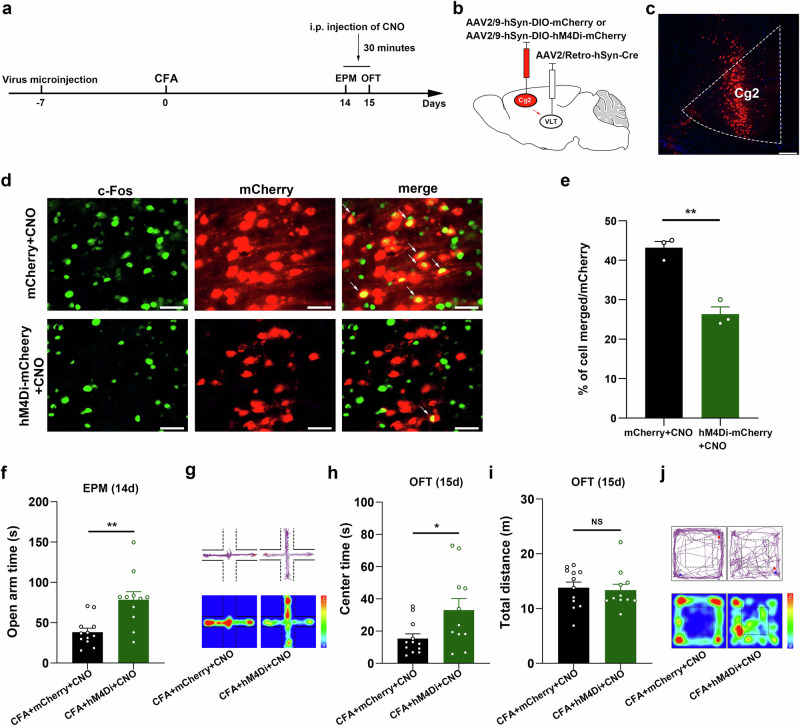
To observe the effects of VLT-projecting cACC (Cg2) neuron activation on CAS in chronic pain, we delivered the retrograde transport virus AAV2/Retro-hSyn-Cre into the VLT, and a Cre-dependent virus encoding the neuronal inhibitor DREADD hM4Di was injected into the cACC(Cg2) (Fig. 3a–c). An examination of the coexistence of c-Fos with the AAVs (mCherry-labeled) revealed a significant decrease in neuronal activity in VLT-projecting cACC(Cg2) neurons labeled with hM4Di (Fig. 3d, e). The results of the EPM test revealed that mice in which VLT-projecting cACC (Cg2) neuron activation was specifically inhibited spent significantly more time moving in the open arms on Day 14 after injection of CFA (Fig. 3f, g). The OFT results revealed that mice in which VLT-projecting cACC (Cg2) neuron activation was specifically inhibited spent significantly more time moving in the center area on Day 15 after injection of CFA (Fig. 3h–j). These results suggest that the inhibition of VLT-projecting cACC (Cg2) neuron activation alleviates anxiety-like behaviors in chronic pain model mice.
Fig. 3. Inhibition of VLT-projecting cACC (Cg2) neuron activation alleviated anxiety-like behaviors induced by CFA.
a Schematic of the experimental design. b, c Schematic for specific infection of VLT-projecting cACC (Cg2) neurons with mCherry or hM4Di-mCherry. Scale bar, 200 μm. d Representative images showed that intraperitoneal injection of CNO induced c-Fos expression in VLT-projecting cACC (Cg2) neurons with mCherry or hM4Di-mCherry. Scale bar, 20 μm. e Percentage of labeled mCherry+ neurons expressing c-Fos in mCherry+CNO group and hM4Di-mCherry+CNO group. f Statistical results of time spent in the open arms in the EPM test. g Representative images of movement trajectory (top) and activity time (bottom) in the EPM test. h, i Statistical results of time spent in the central area and total distance traveled in the OFT. j Representative images of movement trajectory (top) and activity time (bottom) in the OFT. Data are the mean ± SEM; *P < 0.05, **P < 0.01, NS not significant. CFA complete Freund’s adjuvant, VLT ventral lateral thalamus, cACC caudal anterior cingulate cortex, Cg2 cingulate area 2, CNO Clozapine N-oxide, EPM elevated plus maze, OFT open field test.
BBR alleviated anxiety-like behaviors induced by CFA
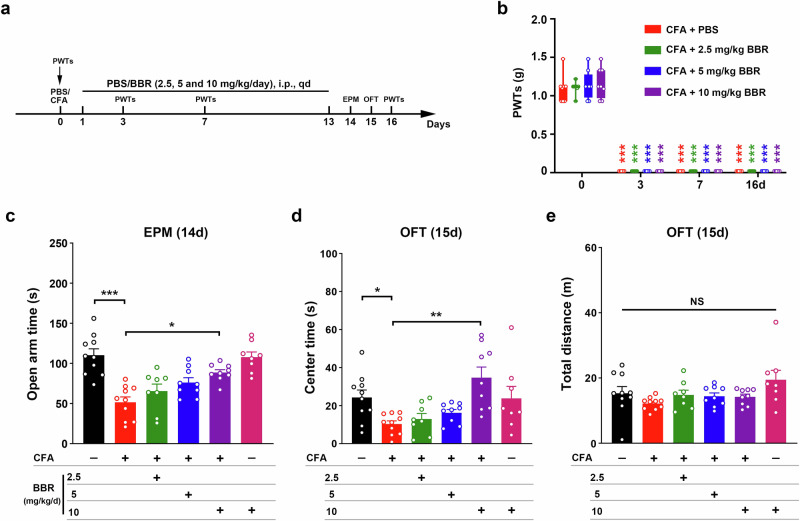
To observe the effect of BBR on CAS in chronic pain, we selected different concentrations of BBR (2.5, 5 and 10 mg/kg/day) for effective dose screening (Fig. 4a). We first observed the effect of BBR on the mechanical pain threshold and found that different doses of BBR (2.5, 5 and 10 mg/kg/day) did not alleviate mechanical hyperalgesia (Fig. 4b). The results of the EPM test revealed a significant increase in the activity time in the open arms of the mice after intraperitoneal (i.p.) injection of 10 mg/kg BBR, but no significant changes were observed for either 2.5 mg/kg or 5 mg/kg BBR (Fig. 4c). The OFT results also revealed a significant increase in the activity time in the center area in mice after i.p. injection of 10 mg/kg BBR, but no significant changes were observed for either 2.5 mg/kg or 5 mg/kg BBR (Fig. 4d, e). These results suggest that 10 mg/kg BBR alleviated anxiety-like behaviors in mice after two weeks of CFA injection.
Fig. 4. BBR alleviated anxiety-like behaviors induced by CFA.
a Schematic of the experimental design. b Statistical results of 50% paw withdrawal thresholds (PWTs). ***P < 0.001 vs. baseline in the same group. c–e Statistical results of time spent in the open arms in the EPM test and the central area in the OFT after different gradient concentrations of BBR (2.5, 5 and 10 mg/kg/day). Data are the mean ± SEM; *P < 0.05, **P < 0.01, ***P < 0.001, NS not significant. BBR Berberine, CFA complete Freund’s adjuvant, EPM elevated plus maze, OFT open field test.
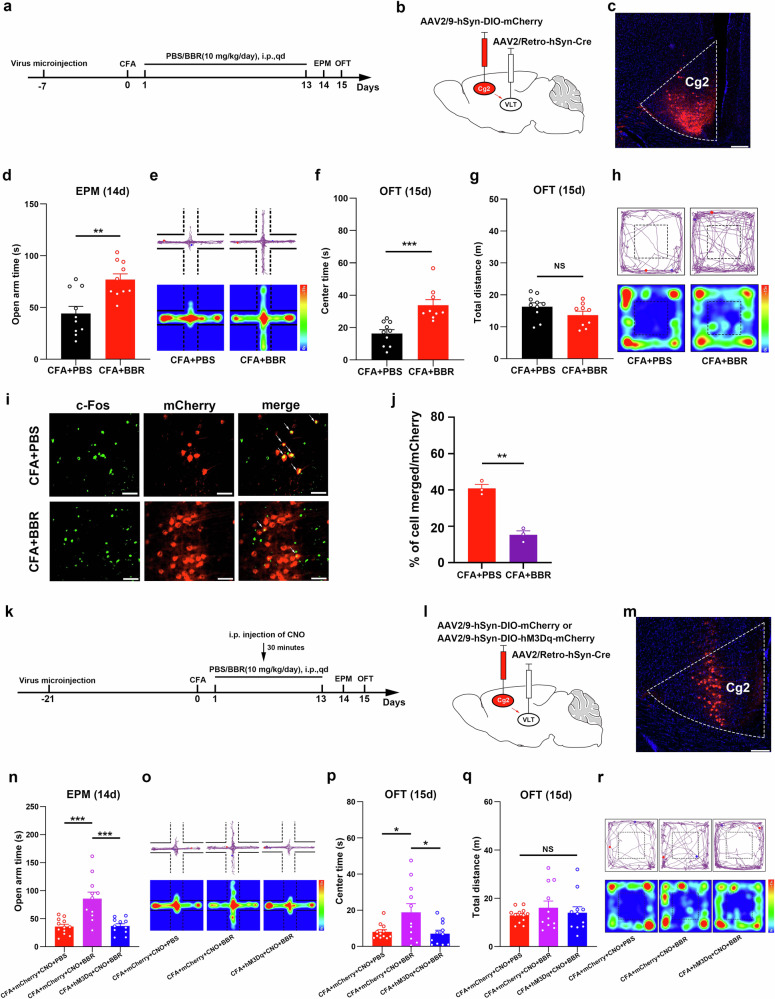
Inhibition of VLT-projecting cACC (Cg2) neurons is required for the ability of BBR to relieve anxiety-like behaviors induced by CFA
To further demonstrate whether 10 mg/kg BBR exerts anxiolytic effects through VLT-projecting cACC (Cg2) neurons, we first observed changes in the activity of VLT-projecting cACC (Cg2) neurons after 10 mg/kg BBR intervention (Fig. 5a). We labeled VLT-projecting cACC (Cg2) neurons by injecting the retrograde transport virus AAV2/Retro-hSyn-Cre into the VLT and a Cre-dependent virus (mCherry-labeled) into the cACC (Cg2) (Fig. 5b, c). On the basis of the results of the EPM and OF tests, which demonstrated that BBR indeed alleviated anxiety-like behaviors (Fig. 5d–h), we examined the effect of BBR on the activity of VLT-projecting cACC (Cg2) neurons. When c-Fos was coexpressed with the AAVs (mCherry-labeled), a significant decrease in neuronal activity was observed in VLT-projecting cACC (Cg2) neurons after 10 mg/kg BBR intervention (Fig. 5i, j). These results suggest that the activity of VLT-projecting cACC (Cg2) neurons decreases, when BBR alleviates anxiety-like behaviors in CFA-treated mice.
Fig. 5. Inhibition of VLT-projecting cACC (Cg2) neuron activation was required for the effect of BBR on relieving anxiety-like behaviors induced by CFA.
a Schematic of the experimental design. b, c Schematic for specific infection of VLT-projecting cACC (Cg2) neurons with mCherry. Scale bar, 200 μm. d–h Statistical results of time spent in the open arms in the EPM test and the central area in the OFT after intraperitoneal injection of BBR (10 mg/kg/day). i Representative images showed that intraperitoneal injection of PBS or BBR induced c-Fos expression in VLT-projecting cACC (Cg2) neurons with mCherry. Scale bar, 20 μm. j Percentage of labeled mCherry+ neurons expressing c-Fos in CFA + PBS group and CFA + BBR group. k Schematic of the experimental design. l, m Schematic for specific infection of VLT-projecting cACC (Cg2) neurons with mCherry or hM3Dq-mCherry. n Statistical results of time spent in the open arms in the EPM test. o Representative images of movement trajectory (top) and activity time (bottom) in the EPM test. p, q Statistical results of time spent in the central area and total distance traveled in the OFT. r Representative images of movement trajectory (top) and activity time (bottom) in the OFT. Data are the mean ± SEM; *P < 0.05, **P < 0.01, ***P < 0.001, NS not significant. VLT ventral lateral thalamus, cACC caudal anterior cingulate cortex, Cg2 cingulate area 2, BBR Berberine, CFA complete Freund’s adjuvant, EPM elevated plus maze, OFT open field test, CNO Clozapine N-oxide.
To confirm whether 10 mg/kg BBR exerts an anxiolytic effect by inhibiting VLT-projecting cACC (Cg2) neurons, we specifically activated VLT-projecting cACC (Cg2) neurons at the same time as BBR intervention to observe whether the anxiolytic effect of BBR was reversed (Fig. 5k–m). The results of the EPM test revealed that BBR increased the activity of CFA-treated mice in the open arms, but the activity of CFA-treated mice in the open arms was reduced again after VLT-projecting cACC (Cg2) neurons were activated (Fig. 5n, o). In the OFT, BBR increased the activity time of CFA-treated mice in the center area of the open field, but after VLT-projecting cACC(Cg2) neurons were activated, the activity time of CFA mice in the center area of the open field was significantly reduced (Fig. 5p–r). The above results suggest that the anxiolytic effect of BBR in CFA-treated mice is eliminated by the activation of VLT-projecting cACC (Cg2) neurons. Therefore, BBR may exert its anxiolytic effect in CFA-treated mice by inhibiting VLT-projecting cACC (Cg2) neurons.
Discussion
In this study, we first microinjected anterograde and retrograde tracer AAVs and clarified that neurons in the cACC (Cg2), but not in the cACC (Cg1), project to the VLT. Next, we established a persistent pain model by unilateral plantar injection of CFA and investigated stable anxiety-like behaviors until pain persisted for two weeks. We then specifically activated VLT-projecting cACC (Cg2) neurons in CFA-treated mice without anxiety-like behaviors and in normal mice to induce anxiety-like behaviors. We inhibited the activation of VLT-projecting cACC (Cg2) neurons in CFA-treated mice with anxiety-like behaviors and observed that these behaviors were alleviated. On this basis, we further screened the effective dose of BBR for anxiolysis in CFA-treated mice with anxiety-like behaviors. We observed that the effective dose of BBR obtained above decreased the activity of VLT-projecting cACC (Cg2) neurons. The activation of VLT-projecting cACC (Cg2) neurons reversed the anxiolytic effect of BBR in CFA-treated mice. Together, the data of this study suggest that BBR alleviates CAS in chronic pain by inhibiting the activation of VLT-projecting cACC (Cg2) neurons.
The ACC is a central hub for the development of comorbid mental disorders associated with chronic pain17. The ACC is divided into the rACC and cACC. The rACC is dominated by the Cg1 region. The cACC consists of two regions, Cg1 and Cg2, together. Our preliminary studies and others have shown that the rACC (Cg1) is strongly associated with pain-induced negative emotions, independent of pain itself18–20. Other studies have shown that in addition to the rACC (Cg1), the cACC (Cg1) is also involved in modulating pain affect21. Although the literature suggests that Cg2 is the subregion in the ACC with the strongest connections to other brain regions and is recommended as a potential target for treating neuropsychiatric disorders22, relatively few in-depth studies on the involvement of the cACC (Cg2) in the development of CAS exist.
The ACC internal signaling and descending projections are the key parts of top-down processing23. Understanding these internal processing dynamics can help us better understand the expression processing of inner emotions. The VLT used to be considered an internal thalamic region that encodes motor information24. In addition to cerebellar and basal ganglia afferents (related to motor function), the VLT receives pronounced input from frontal areas and the prefrontal cortex25. The VLT could be a key hub that integrates multiple types of information to make motor decisions or choices26. Recent studies have shown that the VLT is necessary for preparing and planning movements27,28. CAS tends to cause avoidance behaviors in rodents, which could be caused by negative motor planning (anticipatory) induced by internal negative emotion29–31. Therefore, we hypothesize that there could be a corticothalamic connection between the cACC (Cg2) and VLT that manipulates the explicit expression of inner emotions.
BBR is a promising agent for anxiety symptoms in various neuropsychiatric diseases32,33. Xinlu Li et al.34 indicated that BBR mitigated the degree of anxiety and anxiety-like behaviors in transgenic mice with Alzheimer’s disease. Yuan Fang et al.35 observed that ovariectomy significantly aggravated anxiety-like behaviors and that BBR ameliorated ovariectomy-induced anxiety-like behaviors. Additionally, BBR could be used as a potential agent for the treatment of methamphetamine addiction-induced anxiety symptoms36 and anxiety-like behaviors caused by post-traumatic stress disorder37. In the present study, we focused on the interventional effects of BBR on chronic pain-induced anxiety-like behaviors and reported that BBR significantly alleviated chronic inflammatory pain-induced anxiety-like behaviors. This study expands the spectrum of diseases that can be treated with BBR.
Since the discovery of the ability of BBR to penetrate the blood‒brain barrier, BBR has been increasingly researched for the treatment of disorders of brain function. Zongshi Qin et al.16 reported that the anxiolytic and antidepressant effects of BBR were accompanied by the inhibition of the NLRP3 inflammasome in the prefrontal cortex and hippocampus. Bombi Lee et al.38 indicated that BBR attenuated anxiety-like behaviors by blocking the increase in hypothalamic corticotrophin-releasing factor expression and tyrosine hydroxylase expression in the locus coeruleus. Pingyuan Ge et al.39 reported a therapeutic role of BBR in anxiety and depression through the downregulation of glutamate levels in the cortex and striatum. Currently, studies on neurocircuitry related to the anxiolytic effects of BBR are lacking. The present study expands our understanding of the conventional anxiolytic targets of BBR. The Findings of this work prompted us to further explore the antipsychotic effects of BBR from the perspective of brain neural connections in the future.
Methods
Experimental animals
In the present study, we used male C57BL/6J mice (aged 8–10 weeks) to carry out the experiments. All the mice were purchased from Vital River Laboratories (Jiaxing, China). The mice were housed under standard environmental conditions at 23–25 °C, 40–60% humidity and a 12-h light–dark cycle (lights on at 8:00 am) and were given free access to food and water. All animal care and experimental studies followed the Guide for the Care and Use of Laboratory Animals. All animal experiments were approved by the Laboratory Animal Management and Welfare Ethical Review Committee of Zhejiang Chinese Medical University. We have complied with all relevant ethical regulations for animal use.
Animal models of CAS induced by chronic inflammatory pain
CFA was used to establish a chronic inflammatory pain model and induce anxiety-like behaviors40,41. CFA (4 mg/mL heat-killed Mycobacterium tuberculosis H37 RA, Chondrex, Catalog #7001, USA) and phosphate-buffered saline (PBS, Solarbio, Catalog #P1020, China) were mixed at a ratio of 1:1.5 to form water-in-oil emulsions of immunogens. Under brief anesthesia with isoflurane, a 1 mL BD insulin syringe was used to inject the immunogens (20 μL) into the plantar surface of the left hind paw of each mouse to establish a chronic inflammatory pain model and induce anxiety-like behaviors at two weeks after CFA injection (Figs. 1d–h and 2f–j). The control mice were injected in the same manner but with only 20 μL of PBS.
Anxiety-like behavior tests
Anxiety-like behaviors in the mice were evaluated by the EPM test and the OFT. The EPM test, which was performed 35 cm above the floor, consisted of two open arms (30 × 6 cm), two opposing closed arms (30 × 6 × 15 cm) and a central platform (6 × 6 cm). The open and closed arms were placed vertically in a cross. An ANY-maze video tracking system (ANY-maze, Stoelting, USA) was used to monitor and record the behaviors of the mice in the EPM. The activity of the mice in the EPM test was recorded for 5 min. To prevent olfactory cue bias, we used 75% alcohol to wash the apparatus after each test. Each behavioral test (including the EPM test, OFT, and mechanical pain test) was performed by a dedicated researcher.
The OFT was performed in a square acrylic box (40 × 40 × 40 cm). We divided the bottom evenly into 16 squares of equal area by the ANY-maze video tracking system. The outer 12 squares were defined as the peripheral region and the middle 4 squares were defined as the central region. The activity of the animals in the OFT were monitored and recorded in real time via an ANY-maze video tracking system. The activity of the mice was recorded for 5 min in the OFT. To prevent olfactory cue bias, we used 75% alcohol to wash the apparatus after each test.
Mechanical pain tests
We used von Frey filaments (0.02–1.4 g, Touch-Test Sensory Evaluator, North Coast Medical, Inc., Gilroy, CA, USA) to test the 50% paw withdrawal thresholds (PWTs) in the left hind paws of the mice via the up-down method. For adaptation, the mice were individually placed in a transparent box on a raised wire mesh screen for 20 min. Filaments were applied to the central plantar surface of the hindpaw for 3 sec until buckling occurred. Positive responses were defined as paw withdrawal, flinching, and licking.
AAV microinjection
The mice were fixed on a stereotaxic apparatus (RWD Life Science, 68513, China) under anesthesia with 0.3% pentobarbital sodium. AAVs were injected at a rate of 40 nL/min via a borosilicate glass capillary (Sutter Instrument, B100-58-10, USA) connected to a 10 μL syringe (World Precision Instruments, USA) controlled by a motorized microinjector (KD Scientific, Legato 130, USA).
To determine the VLT-projecting cACC subregion (Cg1 and/or Cg2), we infused an antegrade tracer AAV (AAV2/9-hSyn-EGFP, 5.04 × 1012 vg/mL, 60 nL/injection, BrainVTA, China) into Cg1 (AP, +1.00 mm; ML, ±0.31 mm; DV, −0.85 mm) or Cg2 (AP, +1.00 mm; ML, ±0.31 mm; DV, −1.40 mm) and a nontranssynaptic retrograde AAV (AAV2/Retro-hSyn-tdTomato, 1.31 × 1013 vg/mL, 60 nL/injection, Shanghai Taitool, China) into the VLT (AP, −1.06 mm; ML, ±1.13 mm; DV, −3.56 mm).
To specifically infect VLT-projecting cACC neurons with mCherry, hM3Dq-mCherry or hM4Di-mCherry, we delivered the retrograde transport virus AAV2/Retro-hSyn-Cre (5.40 × 1012 vg/mL, 80 nL/injection, BrainVTA, China) into the VLT, and a Cre-dependent virus encoding the neuronal activator/inhibitor DREADD hM3Dq/hM4Di was injected into the cACC subregion (AAV2/9-hSyn-DIO-mCherry, 5.40 × 1012 vg/mL; AAV2/9-hSyn-DIO-hM3Dq-mCherry, 5.27 × 1012 vg/mL; AAV2/9-hSyn-DIO-hM4Di-mCherry, 5.45 × 1012 vg/mL; 60 nL/injection, BrainVTA, China).
To label VLT-projecting cACC neurons (colabeled with c-Fos to determine the effect of BBR on VLT-projecting cACC neurons), we delivered AAV2/Retro-hSyn-Cre into the VLT and AAV2/9-hSyn-DIO-mCherry into the cACC subregion.
Chemogenetic manipulations of mouse behaviors
The chemogenetic manipulations were performed by i.p. injection of clozapine N-oxide (CNO, 2 mg/kg; BrainVTA, China) 30 min prior to each behavior session. To allow enough time for virus infection and expression on target circuits, the chemogenetic manipulations were executed at least 3 weeks after virus injection.
Verification of viral infection and virus labeling
All the mice were anesthetized and perfused with 0.9% saline followed by 4% (w/v) paraformaldehyde to acquire the brains. All the brains were postfixed with 4% paraformaldehyde overnight at 4 °C, and dehydrated in 15% and 30% sucrose until sectioning with a cryostat (CryoStar NX50, Thermo Fisher Scientific, USA). Coronal brain slices (30 μm) were mounted with fluorescent medium containing DAPI (ab104139; Abcam, United States) and imaged via a virtual slide microscope (VS120-S6-W; Olympus, Japan). Since Cre-dependent viruses (i.e., AAV2/9-hSyn-DIO-mCherry) can only express fluorescent proteins by binding to Cre (i.e., AAV2/Retro-hSyn-Cre), it is necessary to check for viral infection at the injection site first. Mice with no fluorescence or very low fluorescence protein expression in the target brain region were excluded from this study.
The validity of the virus was verified via an immunofluorescence method. The effectiveness of the viral labeling was determined by detecting the colabeling rate of the virus-expressed fluorescent protein and the c-Fos-labeled fluorescent protein. The brain slices were incubated with rabbit anti-c-fos antibody (1:500, ab190289, Abcam, USA) overnight at 4 °C and an Alexa Fluor 488-conjugated secondary antibody (1:800, ab15001, Abcam, USA) for 1 h at 37 °C.
BBR administration
BBR was administered by i.p. injection to the mice. In accordance with the experimental requirements, the doses of berberine used were 2.5, 5 and 10 mg/kg/day. The mice in the control and CFA groups received PBS without BBR. The mice were observed for 13 days (Days 1 to 13 after CFA or PBS injection).
Statistics and reproducibility
All the data are expressed as the means ± standard errors of the means (SEMs). PWTs data were statistically analyzed by two-way repeated-measures analysis of variance followed by the Tukey post hoc test. We used independent-sample t-tests to statistically analyze the data between the two groups. One-way analysis of variance followed by Tukey’s post hoc test was used to compare 3 or more groups. P < 0.05 was considered a statistically significant difference.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Supplementary information
Description of Additional Supplementary File
Acknowledgements
This study was supported by the National Natural Science Foundation of China (82205278), Zhejiang Provincial Natural Science Foundation of China (LY24H270006), Zhejiang Provincial Medical and Health Science and Technology Plan of China (2024KY1232), National Natural Science Foundation of China (U23A20509), and Research Project of Zhejiang Chinese Medical University (2022JKZKTS46).
Author contributions
Z.S. designed the experiments and wrote the manuscript. J.G.L., J.Q.F. and X.M.S. provided guidance on the feasibility of the experiments. M.H. and Y.X.C. performed the viral injections. P.P.F. and C.X. conducted the behavioral experiments. J.C. and S.T.Z. carried out the animal modeling and berberine administration. B.Y.L. and X.F.H. were responsible for histology and microscopy. Z.S. and J.G.L. analyzed the data.
Peer review
Peer review information
Communications Biology thanks Mehran Shayganfard for the contribution to the peer review of this work. Primary Handling Editors: Joao Valente. A peer review file is available.
Data availability
The datasets have been uploaded in accordance with the journal’s requirements (see Supplementary Data 1 file). All other relevant raw data that support the findings of the current study are available from the corresponding author upon reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Min He, Ye-Xiang Chen, Pei-Pei Feng, Jie Chen.
Contributor Information
Xiao-Mei Shao, Email: 13185097375@163.com.
Jian-Qiao Fang, Email: fangjianqiao7532@163.com.
Zui Shen, Email: shenzui1228@163.com.
Supplementary information
The online version contains supplementary material available at 10.1038/s42003-024-07372-2.
References
- 1.Cohen, S. P., Vase, L. & Hooten, W. M. Chronic pain: an update on burden, best practices, and new advances. Lancet397, 2082–2097 (2021). [DOI] [PubMed] [Google Scholar]
- 2.Gureje, O. Comorbidity of pain and anxiety disorders. Curr. Psychiatry Rep.10, 318–322 (2008). [DOI] [PubMed] [Google Scholar]
- 3.Carleton, R. N. et al. Anxiety-related psychopathology and chronic pain comorbidity among public safety personnel. J. Anxiety Disord.55, 48–55 (2018). [DOI] [PubMed] [Google Scholar]
- 4.Penninx, B. W., Pine, D. S., Holmes, E. A. & Reif, A. Anxiety disorders. Lancet397, 914–927 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coakeley, S., Martens, K. E. & Almeida, Q. J. Management of anxiety and motor symptoms in Parkinson’s disease. Expert Rev. Neurother.14, 937–946 (2014). [DOI] [PubMed] [Google Scholar]
- 6.Savulich, G., O’Brien, J. T. & Sahakian, B. J. Are neuropsychiatric symptoms modifiable risk factors for cognitive decline in Alzheimer’s disease and vascular dementia? Br. J. Psychiatry216, 1–3 (2020). [DOI] [PubMed] [Google Scholar]
- 7.Bliss, T. V., Collingridge, G. L., Kaang, B. K. & Zhuo, M. Synaptic plasticity in the anterior cingulate cortex in acute and chronic pain. Nat. Rev. Neurosci.17, 485–496 (2016). [DOI] [PubMed] [Google Scholar]
- 8.Zhuo, M. Neural Mechanisms Underlying Anxiety-Chronic Pain Interactions. Trends Neurosci.39, 136–145 (2016). [DOI] [PubMed] [Google Scholar]
- 9.Koga, K. et al. Coexistence of two forms of LTP in ACC provides a synaptic mechanism for the interactions between anxiety and chronic pain. Neuron85, 377–389 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li, X. H. et al. Oxytocin in the anterior cingulate cortex attenuates neuropathic pain and emotional anxiety by inhibiting presynaptic long-term potentiation. Cell Rep.36, 109411 (2021). [DOI] [PubMed] [Google Scholar]
- 11.Ma, X. et al. Afferent and efferent projections of the rostral anterior cingulate cortex in young and middle-aged mice. Front Aging Neurosci.14, 960868 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Casteen, E. J., Nielsen, S. R., Olson, E. A., Frederiks, K. & Rosso, I. M. Reexperiencing and anxious arousal symptoms in relation to volumes of thalamus nuclei in posttraumatic stress spectrum adults. Brain Behav.12, e2639 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huys, D. et al. Motor Improvement and Emotional Stabilization in Patients With Tourette Syndrome After Deep Brain Stimulation of the Ventral Anterior and Ventrolateral Motor Part of the Thalamus. Biol. Psychiatry79, 392–401 (2016). [DOI] [PubMed] [Google Scholar]
- 14.Zhang, Z. et al. Berberine activates thermogenesis in white and brown adipose tissue. Nat. Commun.5, 5493 (2014). [DOI] [PubMed] [Google Scholar]
- 15.Fan, J. et al. Pharmacological effects of berberine on mood disorders. J. Cell Mol. Med.23, 21–28 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qin, Z. et al. Berberine ameliorates depression-like behaviors in mice via inhibiting NLRP3 inflammasome-mediated neuroinflammation and preventing neuroplasticity disruption. J. Neuroinflammation20, 54 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kummer, K. K., Mitric, M., Kalpachidou, T. & Kress, M. The Medial Prefrontal Cortex as a Central Hub for Mental Comorbidities Associated with Chronic Pain. Int. J. Mol. Sci.21, 3440 (2020). [DOI] [PMC free article] [PubMed]
- 18.Shen, Z. et al. Electroacupuncture Alleviates Chronic Pain-Induced Anxiety Disorders by Regulating the rACC-Thalamus Circuitry. Front. Neurosci.14, 615395 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cao, H. et al. Activation of extracellular signal-regulated kinase in the anterior cingulate cortex contributes to the induction and expression of affective pain. J. Neurosci.29, 3307–3321 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johansen, J. P., Fields, H. L. & Manning, B. H. The affective component of pain in rodents: direct evidence for a contribution of the anterior cingulate cortex. Proc. Natl Acad. Sci. USA98, 8077–8082 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mussio, C. A., Harte, S. E. & Borszcz, G. S. Regional Differences Within the Anterior Cingulate Cortex in the Generation Versus Suppression of Pain Affect in Rats. J. Pain.21, 121–134 (2020). [DOI] [PubMed] [Google Scholar]
- 22.Gusain, P., Taketoshi, M., Tominaga, Y. & Tominaga, T. Functional Dissection of Ipsilateral and Contralateral Neural Activity Propagation Using Voltage-Sensitive Dye Imaging in Mouse Prefrontal Cortex. Eneuro10, 1–12 (2023). [DOI] [PMC free article] [PubMed]
- 23.LeDuke, D. O., Borio, M., Miranda, R. & Tye, K. M. Anxiety and depression: A top-down, bottom-up model of circuit function. Ann. N. Y Acad. Sci.1525, 70–87 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tucker, H. R. et al. Deep brain stimulation of the ventroanterior and ventrolateral thalamus improves motor function in a rat model of Parkinson’s disease. Exp. Neurol.317, 155–167 (2019). [DOI] [PubMed] [Google Scholar]
- 25.Vertes, R. P., Linley, S. B., Groenewegen, H. J. & Witter, M. P. in The Rat Nervous System,4th ed. (ed George Paxinos) 335–390 (Academic Press, 2015).
- 26.Kaas, J. H. & Stepniewska, I. in Encyclopedia of the Human Brain (ed V. S. Ramachandran) 159–169 (Academic Press, 2002).
- 27.Gao, Z. et al. A cortico-cerebellar loop for motor planning. Nature563, 113–116 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gaidica, M., Hurst, A., Cyr, C. & Leventhal, D. K. Distinct Populations of Motor Thalamic Neurons Encode Action Initiation, Action Selection, and Movement Vigor. J. Neurosci.38, 6563–6573 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Donner, N. C. & Lowry, C. A. Sex differences in anxiety and emotional behavior. Pflug. Arch.465, 601–626 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Straube, T., Schmidt, S., Weiss, T., Mentzel, H. J. & Miltner, W. H. Dynamic activation of the anterior cingulate cortex during anticipatory anxiety. Neuroimage44, 975–981 (2009). [DOI] [PubMed] [Google Scholar]
- 31.Koganemaru, S., Domen, K., Fukuyama, H. & Mima, T. Negative emotion can enhance human motor cortical plasticity. Eur. J. Neurosci.35, 1637–1645 (2012). [DOI] [PubMed] [Google Scholar]
- 32.Gao, Y., Nie, K., Wang, H., Dong, H. & Tang, Y. Research progress on antidepressant effects and mechanisms of berberine. Front. Pharm.15, 1331440 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shayganfard, M. Berberine: Is it a Promising Agent for Mental Disorders Treatment? Curr. Mol. Pharm.16, 307–320 (2023). [DOI] [PubMed] [Google Scholar]
- 34.Li, X. et al. Berberine ameliorates iron levels and ferroptosis in the brain of 3 × Tg-AD mice. Phytomedicine118, 154962 (2023). [DOI] [PubMed] [Google Scholar]
- 35.Fang, Y. et al. Berberine ameliorates ovariectomy-induced anxiety-like behaviors by enrichment in equol generating gut microbiota. Pharm. Res.165, 105439 (2021). [DOI] [PubMed] [Google Scholar]
- 36.Alavijeh, M. M., Vaezi, G., Khaksari, M. & Hojati, V. Berberine hydrochloride attenuates voluntary methamphetamine consumption and anxiety-like behaviors via modulation of oxytocin receptors in methamphetamine addicted rats. Physiol. Behav.206, 157–165 (2019). [DOI] [PubMed] [Google Scholar]
- 37.Lee, B., Shim, I., Lee, H. & Hahm, D. H. Berberine alleviates symptoms of anxiety by enhancing dopamine expression in rats with post-traumatic stress disorder. Korean J. Physiol. Pharm.22, 183–192 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee, B. et al. Effect of berberine on depression- and anxiety-like behaviors and activation of the noradrenergic system induced by development of morphine dependence in rats. Korean J. Physiol. Pharm.16, 379–386 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ge, P. Y. et al. Berberine ameliorates depression-like behavior in CUMS mice by activating TPH1 and inhibiting IDO1-associated with tryptophan metabolism. Phytother. Res.37, 342–357 (2023). [DOI] [PubMed] [Google Scholar]
- 40.Chen, Z. J. et al. Enhanced AMPAR-dependent synaptic transmission by S-nitrosylation in the vmPFC contributes to chronic inflammatory pain-induced persistent anxiety in mice. Acta Pharmacol. Sin.44, 954–968 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jin, Y. et al. A somatosensory cortex input to the caudal dorsolateral striatum controls comorbid anxiety in persistent pain. Pain161, 416–428 (2020). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary File
Data Availability Statement
The datasets have been uploaded in accordance with the journal’s requirements (see Supplementary Data 1 file). All other relevant raw data that support the findings of the current study are available from the corresponding author upon reasonable request.