Abstract
The development and origin of animal body forms have long been intensely explored, from the analysis of morphological traits during antiquity to Newtonian mechanical conceptions of morphogenesis. Advent of molecular biology then focused most interests on the biochemical patterning and genetic regulation of embryonic development. Today, a view is arising of development of multicellular living forms as a phenomenon emerging from non-hierarchical, reciprocal mechanical and mechanotransductive interactions between biochemical patterning and biomechanical morphogenesis. Here we discuss the nature of these processes and put forward findings on how early biochemical and biomechanical patterning of metazoans may have emerged from a primitive behavioural mechanotransducive feeding response to marine environment which might have initiated the development of first animal multicellular organisms.
Subject terms: Evolutionary developmental biology, Embryonic induction, Morphogenesis
Technical advances have enabled simultaneous study of the molecular and biomechanical inputs involved in regulating development. In this Perspective, the authors give a historical view of these advances and propose that biomechanical cues in the marine environment may have helped foster metazoan evolution.
Introduction
A 2 millennium journey of animal morphogenesis conceptualization in developmental biology. Before the advent of molecular biology, the observable properties of the development of living forms and of their evolution were largely morphological in nature. The shapes and the physiologically active structures of living forms were thought to have developed either via pre-formation or self-organization, with these two views alternating for a long period of time. These views eventually converged towards a self-organised model of morphogenesis.
Indeed, the first concepts on the principles that underlie the formation of animal shapes were reported in Antiquity around 2400 years ago. In Plato’s view, all material forms, including the living, were the realization of pre-existing patterns present in a non-visible world, considered at the time to be immaterial1. Shortly after, his disciple Aristotle opposed this hypothesis with a theory of morphogenesis based on an auto-inductive cascade of causes and effects with no need of pre-existing patterns of the developed shape. Specifically on the development of embryonic living shapes, Aristotle developed his model from the observation of chicken embryo morphology at distinct stages of their development. He reported that the distinct parts of the embryonic body were generated in a sequential process rather than simultaneously. This seminal observation gave rise to a self-organized view of embryonic development called “epigenesis”, through which the embryo’s shape at a given stage is a causal condition for the generation of the next stage’s shape2.
During the Middle Ages, many scientific theories, including embryogenetic models of development, were based on Aristotle’s views3. In the 17th century, however, the theory of the “homunculus” was proposed, in which preformed miniature animal bodies entirely formed within the gametes were believed to solely grow in size until birth. The pre-formed structure of the body’s shape was here considered to be material, albeit too small to be seen (Fig. 1a). It would then be passed on completely unchanged from one generation to the next, exclusively in male or female gametes. Supported by Leeuwenhoek, Hartsoeker, Bonnet and Spallanzani, this theory of development was the subject of lengthy controversy until the early 20th century, with opposing scientists, including Wolff, Maupertuis, Buffon and Geoffroy de Saint-Hilaire4. Indeed, the latter criticised that this pre-formative model of development would fix the forms and characters of living bodies generation after generation in a way that was incompatible with observations, such as the mixing of female and male characters in offspring, and with the evolution of species4. The 19th and early 20th centuries then saw the resurgence in self-organizing models of development. Following the Newtonian revolution in physics, embryologists such as His, Leduc and D’arcy Thompson saw mechanics and mathematics as the disciplines of choice for describing the morphogenesis of animal forms as an essentially passive mechanical or hydrodynamic process in response to active tissues growth (Fig. 1b). Based on the causal laws of Newtonian physics, the form of a given stage here was thought to emerge sequentially and naturally as a mechanical causal consequence of the form of the preceding stage, in a simple self-organizing process5,6.
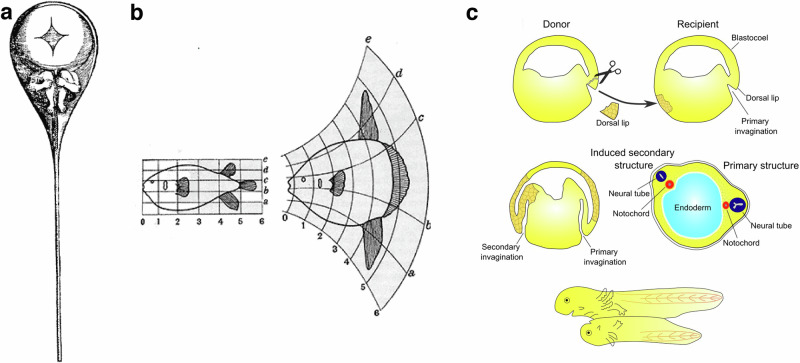
Fig. 1. Historical views of embryonic development.
a The preformed homunculus (Nicolaas Hartsoeker, 1695). b Modulation of animal shape through tissue growth modulation (d’Arcy Thompson, 1917). c- Induction in development (Speeman and Mangold, 1923).
Shortly after, the physiologists Speeman and Mangold demonstrated that transplanting the structure of the dorsal blastoporal domain of an early Triton embryo into the ventral part of another Triton embryo initiated the formation of a second embryo on the ventral part. The second embryo developed in parallel and linked to the normal embryo (Fig. 1c). This seminal experiment was followed by others that confirmed the existence of self-organized principles in development, with a given stage of the embryo being the condition that induces the next stage of developmental structures. This time the active induction processes involved were a priori independent of any mechanical considerations7. Therefore, after a long period in which pre-formation and auto-organisation concepts were debated, mechanical Newtonian approaches of morphogenesis and physiological inductive approaches of tissue specification both orientated to a self-organized based understanding of development.
Here we will begin by describing the historical progression of these developmental concepts and associated observations up to the present day. We will then depict subsequent findings that have recently revealed the existence of a crosstalk between biochemical patterning and biomechanical morphogenesis based on mechanical and mechanotransductive cues, and how these may have played a central role in the evolutionary emergence and development of first multicellular metazoan organisms.
The advent of molecular biology: the genetics of biochemical patterning in development
In the second half of the 20th century, the discovery of the genome8 and the rise of molecular biology revealed the biochemical nature of the factors involved in the active induction processes. Developmental biology then focused much of its efforts on studying the genetic control of the establishment of a patterning feature distinct from biomechanical morphogenesis: the biochemical differentiation of cells and tissues that determines the physiologically functional body plan of the future organism. In parallel, the physico-chemical principles of molecular reaction-diffusion, leading to self-induced chemical patterning were proposed and included in the framework of biochemical patterning of living systems9. Here we describe the coexistence and intricate relations between self-induced and prepatterned biochemical signals occurring in embryonic development.
The biochemical patterning of the antero-posterior axis
For a long time, the biochemical inductive cascades, which convert patterns into other more complex patterns were not as easily accessible in vertebrate embryos as in Drosophila embryos. Therefore, many examples in this review have been taken from Drosophila embryogenesis.
Anteroposterior patterning of all animals depends on the expression of specific genes from a developmental toolbox. In the Drosophila embryo, for example, 15 genes involved in the anteroposterior segmentation of the embryo have been identified10,11. The proteins produced by these genes biochemically pattern the embryo along its anteroposterior axis. This patterning is determined by an inductive biochemical process, which is initiated by the establishment of an antero-posterior gradient of the transcription factor Bicoid, and a posterior-anterior gradient of the transcription factor Nanos, as well as by the Torso-like protein expressed at both poles of the embryo. These antero-posterior patterns are transmitted to the egg by anterior and posterior signals of the already polarized mother12,13.
The early Drosophila embryo in which anteroposterior patterning occurs is a syncytium with nearly 6000 nuclei on the surface of the membrane surrounding the yolk. The nuclei of the syncytium are therefore directly subjected to the protein gradients. Depending on the sensed concentrations of Bicoid, Nanos and Torso-like and therefore on their position along the antero-posterior axis, the nuclei consequently express specific genes that begin to segment the embryo—the gap genes and paired ruled genes14. These genes control the expression of two specific classes of developmental genes: the segmentation genes and the Hox genes15. Hox genes are expressed segmentally anterior to posterior of the embryo and biochemically induce the specification of the tissues that will form the body parts of the adult fly. For example, Dfd is involved in the specification of the head of the animal, AbdB the bottom, AbdA the abdomen and Antp the wing-bearing posterior thorax (Fig. 2a-up)16. The establishment of the animal’s anteroposterior body plan is therefore the consequence of a cascade of biochemical induction processes regulated in space and time. Indeed, the biochemical pattern of proteins involved in a given developmental stage (Bicoid, Nanos, Torso-like) is here the cause of the development of the next stage (Hox genes) in a self-organizing process, here downstream of the antero-posterior patterning by the mother.
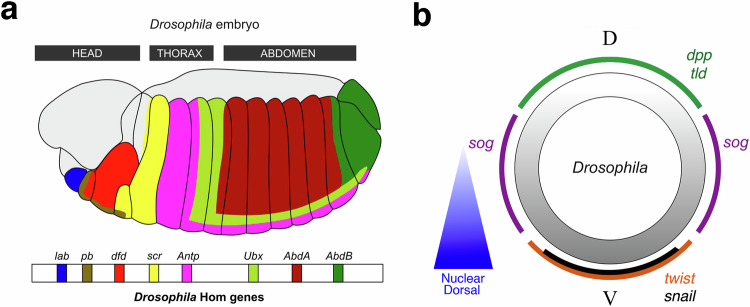
Fig. 2. The biochemical patterning of an early bilaterian embryo.
Up-a The antero-posterior biochemical patterning of Drosophila embryos by Hox genes. Down-a Hox genes location on the 3 R chromosome arm of Drosophila. Adapted from16. b Dorso-ventral biochemical patterning in early Drosophila embryos. Adapted from81.
Strikingly, the fly’s Hox genes are positioned along the DNA of one chromosome (on chromosomal arm 3 R) in the same order as they are expressed along the anterior-posterior axis of the animal’s body (Fig. 2a-down)16. In other words, the structure of the body plan seems to be predetermined by the structure of the chromosome containing the Hox genes.
Indeed, in vertebrates, the structure of the Hox gene cluster on the chromosome is translated into an identical body plan structure via the successive reading of genes along the chromosome as the body grows from what will be the animal’s head17. Specifically, the expression of the first anterior Hox genes is triggered by Wnt3 signalling in the gastrulating primitive streak of the embryo, through a Wnt-responsive element 3’ to the Hox genes cluster18. It is followed by activation of other Hox genes via 3’ to 5’ paced successive opening and reading of the more posterior Hox loci and genes during tissue growth from the anterior to the posterior, concluding in the posterior trunk19,20. Interestingly, Wnt3 also induces primitive streak gastrulation, here operating through an epithelial-mesenchymal transition (EMT)-driven ingression21. Hence, Wnt3 coordinates and synchronizes the initiation of gastrulation and antero-posterior Hox gene patterning of the primitive streak of the embryo. Unlike in the fly, this process does thus not represent a biochemical induction cascade initiated by morphogenetic gradients pre-patterned by the mother. It is rather a self-organized process, in which the structure of the body plan is established by the combination of the growth dynamics of a multi-cellular structure and the chromosome reading dynamics of a molecular structure.
Overall, in both processes a self-organized process coexists and is coupled with pre-patterned biochemical maternal signals or chromosomal structures, resulting in the anteroposterior axis of the body plan.
The biochemical patterning of the dorso-ventral axis
In Drosophila embryos, the polarized nuclear translocation of the transcription factor Dorsal determines dorso-ventral polarity. Dorsal specifies the ventral domain. It acts via repression of dpp, which encodes for a secreted protein homologous to BMP, and activation of the endomesoderm genes twist and snail, which encode for transcription factors (Fig. 2b)13. In this species, the Dpp protein is secreted apically outside the embryo and is confined between the membrane and the protective vitelline membrane of the embryo. Interestingly, the establishment of robust dorso-ventral polarity is ensured by the fact that Dpp complexed to Sog (expressed ventro-laterally) does not signal and extensively diffuses around the embryo. Only after cleavage of Sog by Tolloid (Tld), expressed dorsally, Dpp signals on the dorsal pole of the embryo and does not diffuse22. Similar genetic networks underlie the robust establishment of dorso-ventral polarity in early vertebrate embryos (see third section). Dorso-ventral polarity of the embryo is therefore the product of the polarized expression of genes like dpp, sog, tld or twist pre-established by the nuclear translocation of factor Dorsal (downstream of the activation of Toll12) at the ventral pole of the embryo, combined with a self-organizing process involving diffusion and biochemical reactions.
Hence, self-induced biochemical morphogenetic processes are again combined with pre-patterned biochemical patterning processes, such as in the establishment of the antero-posterior polarity of the Drosophila embryo. Here, however, the primary dorso-ventral polarity that leads to polarized expression of Sog, does not appear to be genetically pre-patterned into the chromosome structure like for the Hox genes. It is rather the product of an early spontaneous symmetry-breaking process associated with the random position of the fertilized nucleus in relation to the egg membrane12.
Reaction-diffusion-based spontaneous self-organized biochemical patterning
Biochemical patterning in development can also be initiated by fluctuations in morphogen concentrations that are subsequently enhanced and structured by reaction-diffusion processes9. These processes act upstream of cell differentiation and involve cellular local auto-activating and lateral inhibitory regulations (LALI)23,24. Indeed, based on short-range autoactivation and long-range lateral repression of elements of a given genetic network, the higher expressing domains of the fluctuations spontaneously increase and the lower domains decrease. This generates an amplification in fluctuation small differences of expression between the two domains, eventually leading to steady-state periodic patterns. Robustness can be ensured by additional layers of regulation, including redundant activator-inhibitor modules25.
LALI was for instance suggested to underlie the formation of bar bones and digits in vertebrate limb development. In the chicken, this occurs by local autoactivation of the TGFβ pathway—required for chondrocyte and cartilage formation—in association with activation of Notch that represses the TGF-β pathway in lateral cells, within the context of a pre-patterned gradient of FGF emanating from the apical ectodermal ridge26. Digit and joint formation were more recently theoretically and experimentally proposed to be regulated similarly, by a 3 node Turing diffusion-reaction like process involving Wnt, Sox9 and Bmp in mice27,28. Non-prepatterned reaction-diffusion is at the origin of mouse hair follicle spacing and cat pigmentation marks, both involving Wnts as low diffusing activator and Dkk as a rapidly diffusing repressor29,30. Fingerprints form via similar process31. Finally, the breaking of symmetry that polarizes Hydra aggregates during regeneration relies on a reaction-diffusion-like process involving two opposing Wnt loops interacting with each other32.
Thus, reaction-diffusion-dependent biochemical patterning can spontaneously emerge from random fluctuations of patterning in tissues that are not pre-patterned.
Back to the future: the interaction between biomechanical morphogenesis and biochemical patterning
The last decades have been characterized by a resurgence of interest in biomechanical morphogenesis in embryonic development, first through its control by biochemical patterning, second by the finding of the reverse—control of biochemical patterning by biomechanical morphogenesis. Here we describe examples of such reciprocal interplay between these types of morphogenetic processes.
Biochemical patterning as a producer of physical forces
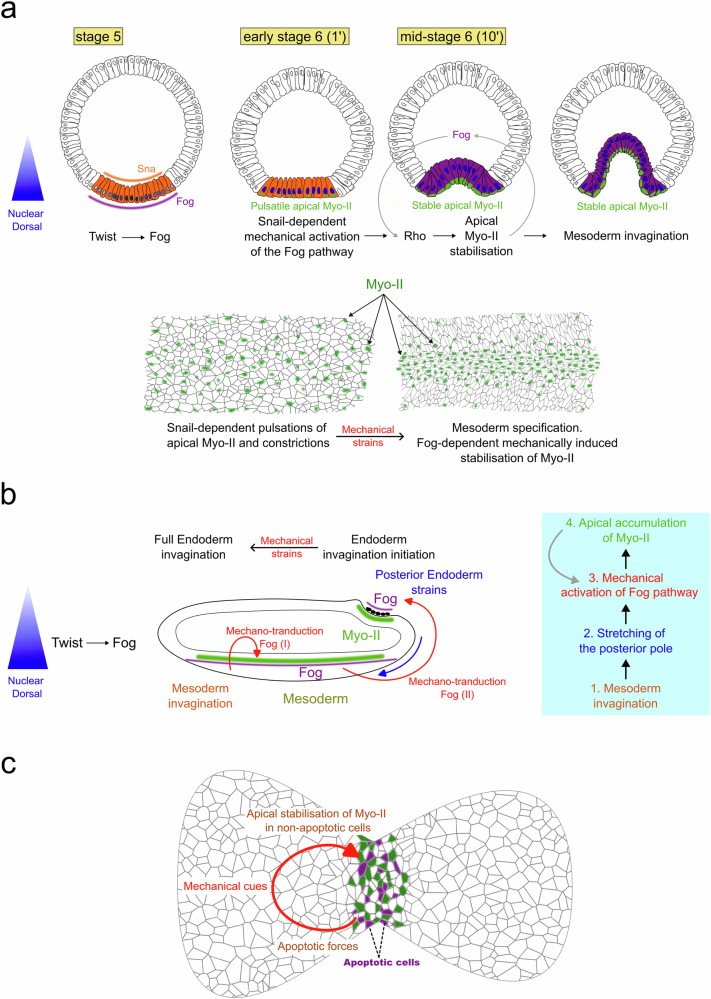
In the late 20th century, several studies investigated the role of genetically controlled biochemical patterns in the generation of biomechanical forces resulting in morphogenesis. One of the first discoveries was the genetically regulated, anisotropic intracellular distribution of the molecular motor Myosin-II (Myo-II), as a driving force of embryonic morphogenetic movements. Indeed, apical accumulation of Myo-II specifically in the posterior cells of the early Drosophila embryo leads to acto-myosin contraction of the posterior pole’s apical surface33. The difference of surface area between the apical and basal sheets of the multi-cellular tissue consequently generates its inward curvature and initiates morphogenesis of the embryo’s endodermal hindgut33. The same process has been found to drive mesoderm invagination in Drosophila embryos34. In both cases, the genetically regulated and spatially patterned expression of the apically secreted factor Fog is required to activate the Rho pathway, leading to apical Myo-II accumulation within the first 10 min of gastrulation34 (Fig. 3a, b, see Box 1).
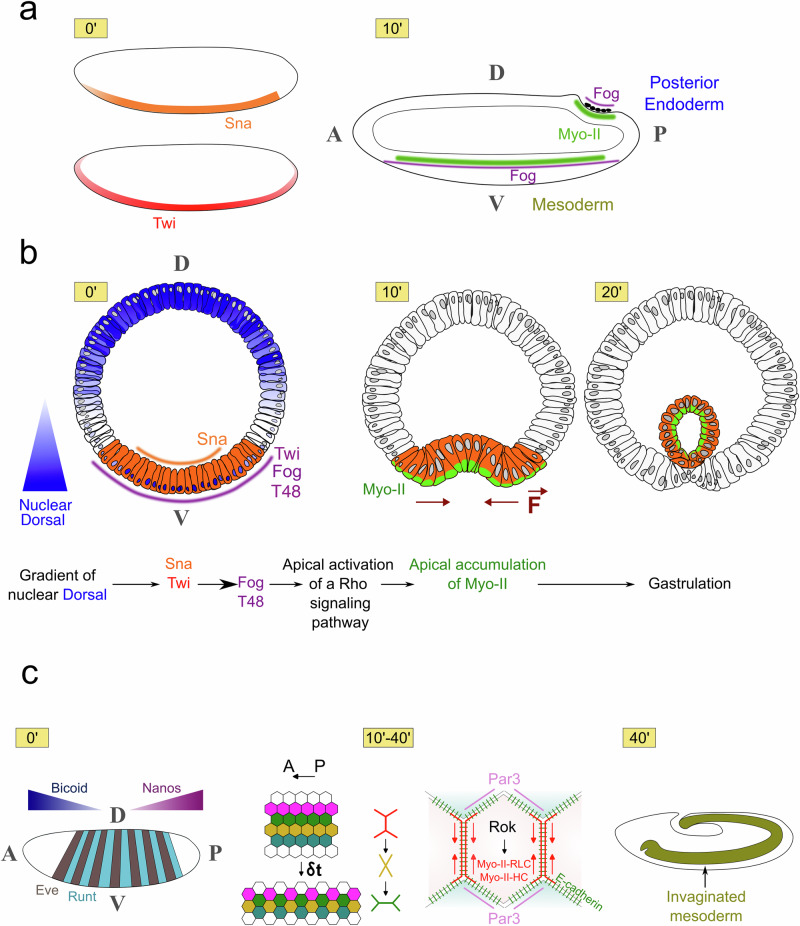
Fig. 3. The biomechanical patterning of an early bilaterian embryo.
a Apical accumulation of Myo-II (in green) triggers mesoderm and posterior endoderm invaginations, and is biochemically patterned by the expression of Fog in early Drosophila embryos. Antero-posterior cross-cut view of the embryo. b Pre-patterning of Fog and T48 expression by Dorsal and Twist in the mesoderm, and Rho-dependent activation of Myo-II medio-apical accumulation downstream of Fog and T48. T = 0’ is the beginning of mesoderm invagination. Dorso-ventral cross-cut view of the embryo. c Biochemically patterned Myo-II anisotropy in morphogenetic movements of convergent-extension in Drosophila embryos, prepatterned by the antero-posterior polarity genes Bicoid and Nanos, and pair-rule genes Eve and Runt, adapted from35. Antero-posterior surface view of the embryo.
In Drosophila, the embryonic morphogenetic movements of convergence-extension were also found to be generated by the anisotropic intracellular distribution of Myo-II, which is genetically controlled. In this case, the anisotropy is planar, with Myo-II accumulating on the apical adherent surfaces between two cells that are perpendicular to the anteroposterior axis of the early embryo. This provokes the acto-myosin contraction of these surfaces, which in turn generates the intercalation of superior and inferior cells between the contracting cells (Fig. 3c 10’-40’)35.
This ultimately leads to a movement of cell convergence along the dorso-ventral axis and elongation along the antero-posterior axis, the so called germ-band extension36. Here, Myo-II anisotropy is regulated by the embryo’s anterior-posterior segmentation patterning genes: a striped pattern of Runt and Eve, mediated by a combination of striped expression of Toll-family proteins that locally mobilizes Src and PI3K activity via a process remaining to be fully understood37. The latter regulates the enrichment of Par-3 on adherent surfaces parallel to the anterior-posterior axis, where it prevents the accumulation of Myo-II at junctions38,39.
In the vertebrate Xenopus mesoderm, cell intercalation similarly directs the embryonic morphogenetic movements of convergence and extension40. Here, planar polarity genes polarize acto-myosin activities via an anisotropic distribution of Septin41. Wnt/Planar polarity genes relatedly regulate convergent extension during zebrafish gastrulation42–44. Biochemically regulated cell migration movements are also involved in the physical forces that lead to morphogenetic movements, such as the formation of primitive streaks regulated by gradients of growth factors such as EGF, VEGF and Wnt in amniotes45. A graded contractile supracellular actomyosin ring at the margin between the embryonic and extraembryonic territories, coupled with cell divisions, drives the forces leading to the fluid-like vortexes involved in avian gastrulation46.
In addition to generating forces, biochemical patterning was also proposed to regulate tissue stiffness. Such a phenomenon was recently uncovered in Hydra, where the Wnt gradient established at the onset of development is stabilized in the adult. This gradient was found to control the adult mechanical properties by translating into a stiffness gradient via Wnt-dependent regulation of ECM production47. The initiation of complex biomechanical morphogenesis of vertebrate tissues such as nose, ear or mouth might also be the result of tissue growth within the context of biochemically pre-patterned stiffness variations in the tissue48.
Therefore, molecular biochemical patterning has extensively been shown to regulate multi-cellular biomechanical patterning via the establishment of intracellular anisotropies in Myo-II and multi-cellular actomyosin contractile patterns.
Box 1 The role of Fog in the apical accumulation of Myo-II and in mesoderm and posterior endoderm invaginations in the Drosophila embryo.
Fog is sufficient for apical stabilisation of Myo-II, and necessary for normal rates of apical stabilisation of Myo-II in the mesoderm and posterior endoderm34. The apical Fog signalling pathway involves its G-protein coupled receptors Mist and Smog74,130. Mist expression is induced by Snail in the mesoderm, whereas Smog is homogeneously expressed. The Fog pathway acts upstream of Cta, a G-protein alpha subunit of the Gα12/13 class. Cta activates apical RhoGEF2, leading to the apical stimulation of the Rho pathway that stimulates the medio-apical activation of Myo-II (detailed in Fig. 3b for the mesoderm)34. In zygotic mutants of fog, apical accumulation of Myo-II is severely reduced, leading to delayed and anomalous mesoderm invagination, and lack of posterior endoderm invagination34,131. Consistently, mutants of cta show a delay in Myo-II apical accumulation in the mesoderm and a strong reduction of Myo-II apical accumulation132.
The establishment of an invagination, albeit imperfect, in the absence of zygotic Fog133 indicates a role of a maternal component of Fog134, or a secondary pathway34. In the mesoderm, the transmembrane protein T48, known to be involved in apical accumulation of RhoGEF2, was suggested to be required for apical activation of Myo-II by Fog135, and was thus proposed to be such a secondary synergic pathway (Fig. 3b)132. Mesodermal patterned expression of Fog and T48 are regulated by the expression of the first ventral patterning gene twist34,135. Hence, maternal Fog in zygotic mutants of fog could indeed be at the origin of the medio-apical residual activation of Myo-II, requiring T48-dependent apical localisation of RhoGEF2.
In the posterior endoderm, T48 is not expressed135. Maternal Fog, and/or another secondary pathway, might be required for robust Myo-II medio-apical accumulation.
Physical forces as inducers of biochemical patterning
Biochemical patterning and physical forces interact not only via biochemical control of force production, but also the inverse—the mechanical control of biochemical patterning, via mechanotransduction.
The ability to control cell differentiation by mechanical constraints was discovered in early Drosophila embryos. A gentle and short uni-axial lateral deformation of the embryo was sufficient to induce the anomalous expression of the ventral mesoderm patterning genes twist and snail in the dorsal part of the embryo just before gastrulation (Fig. 4ai)49. The use of high throughput deformation meso-fluidic devices allowed further investigation of this property50.
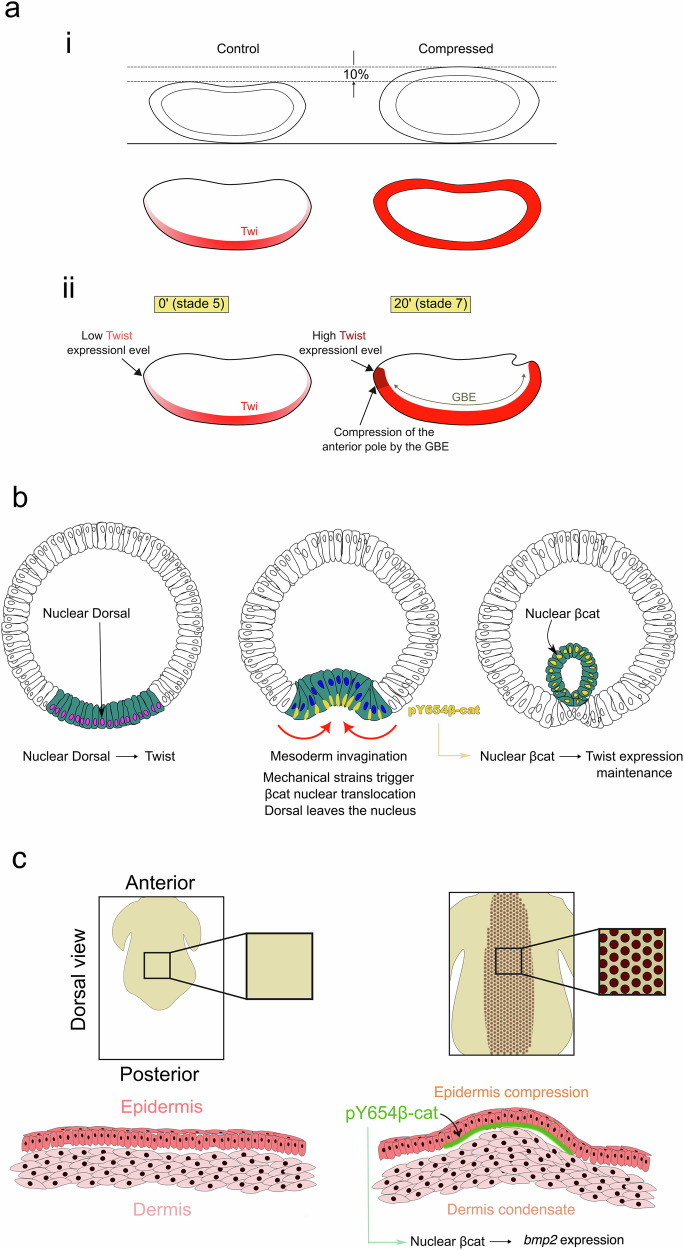
Fig. 4. Mechanical induction of specification in bilaterian early embryogenesis and development.
a i Ectopic mechanical induction of the expression of the endomesoderm twist gene and Twist protein in response to soft (on the order of 10%) global lateral deformation of Drosophila embryos. Similar results were obtained for snail gene expression (not shown). ii Mechanical stimulation of Twist expression in the presumptive anterior midgut of the Drosophila embryo, in response to its compression by the morphogenetic movement of germ-band extension at gastrulation. Adapted from49,51. b Mesoderm invagination maintains Twist expression via the mechanical stimulation of the β-catenin pathway, by Y654-βcat mechanotransductive phosphorylation leading to translocation of β-cat from the junctions to the cytoplasm. c Periodic compression and buckling of the epidermis epithelial cells after condensation of the underlying dermis cells leads to mechanical activation of Y654β-cat phosphorylation, upstream of Fgf20 expression that specifies feather follicle primordia formation during chicken development. Adapted from55.
Indeed, this feature was found to be involved in Drosophila embryonic development. During germ-band extension, the morphogenetic movement of the ventral part of the embryo (see subsection above) compresses the anterior endoderm—the presumptive anterior mid-gut of the embryo—at its anterior pole (Fig. 4aii). This compression, in turn, leads to the induction of Twist expression to a level required for the functional and vital specification of the anterior midgut at later stages of development49,51. A combination of low-heat 2-photon methodologies and the use of magnetic nanoparticles uncovered these regulatory relationships without affecting the genetic background of the wild-type embryo. Low-heat 2-photons methodologies generate tissue photo-destruction and were used to prevent the mechanical compression of the embryo’s anterior pole by germ-band extension. This was followed by the injection and magnetic manipulation of magnetic nanoparticles in the epithelium to rescue the compression with physiological forces from the inside of the embryo, hence mimicking the force of germ-band extension in the compression-defective embryo51. These experiments uncovered mechanical stimulation of anterior midgut specification as one of the functions of the morphogenetic movement of germ-band extension, whose function had been questioned by its subsequent retraction during development36,49. In response to germ-band extension, the mechanical state of chromatin itself has been suggested modulate the expression of genes involved in anterior-posterior segmentation, such as Engrailed, via mechanotransduction52.
In addition to the mechanical specification of the anterior endoderm, the mechanical stresses of mesoderm invagination in gastrulating Drosophila tissues induce and maintain high levels of Twist expression via mechanotransduction (Fig. 4b). Indeed, in mutants of snail that do not gastrulate, Twist expression strongly fades in the mesoderm at stages corresponding to its full invagination in the WT. Mechanically rescuing mesoderm invagination in mutants of snail (see subsection below) re-establishes the physiological levels of Twist expression. In this case, it was proposed that the underlying function of this mechanotransductive process is a robust coordination of the biomechanical with the biochemical patterning in mesoderm formation. Indeed, this property ensures that only cells that experienced the mechanical stresses of invagination maintain the high levels of Twist expression required to specify themselves as mesodermal cells. As a consequence, only the cells that have internalized after invagination will develop into mesoderm (Fig. 4b)53.
Interestingly, the underlying molecular mechanism of this mechanotransduction process involves the mechanical activation of the beta-catenin (βcat) pathway, independent of its associated Wnt ligand, which is not expressed at these early stages of embryo development49,53. Mechanical stress caused by mesoderm invagination induces a molecular conformation change to the main βcat interaction site with E-cadherins at junctions, Y654. This increases Y654’s accessibility to phosphorylation by the Src-family kinase Src42A, resulting in βcat’s release from junctions into the cytoplasm and nucleus51,53. Mesoderm specification is initiated by the nuclear localisation of Dorsal that leads to the expression of Twist before gastrulation (see Fig. 3b)13, and Dorsal protein concentration in the ventral nuclei decreases shortly before the onset of gastrulation54. However, β-cat-dependent mechanical stimulation of Twist expression occurs at gastrulation—after the initiation of mesoderm specification by Dorsal—and was indeed found to be required for the maintenance of high levels of Twist expression during gastrulation. Strikingly, mesoderm specification is also initiated in a Wnt-independent Y654-β-cat-dependent mechanotransductive process in response to epiboly initiation in presumptive mesoderm in zebrafish embryos. β-cat-dependent mechanical induction via Y654-βcat mechanotransductive phosphorylation hence represents a conserved signalling process in mesoderm specification between ecdysozoa arthropods and vertebrate chordate bilaterians53.
In vertebrates, the latter process has also been identified in the specification of chicken embryo feather follicle primordia, via βcat-dependent mechanical induction of Fgf20, due to mechanical stimulation of Y654-βcat phosphorylation specifically in the compressed buckling domains of the epidermis55. These epidermis buckling domains were proposed to be induced by an FGF/BMP LALI process leading to periodically patterned condensates in the dermis (Fig. 4c)56,57, similarly to bar bones and digits in vertebrate limb development (see first section). This is another example of the intricateness of biochemical patterning and biomechanical morphogenesis in generating and using internal forces. This additionally indicates that mechanical stimulation of Y654 phosphorylation is involved in diverse developmental processes.
It has been suggested that not only βcat, but also the mechanosensitive Yap/Taz pathway is involved in cell specification events during early mouse embryonic development. Indeed, after the first asymmetric cell division following fertilization, the cell characterized by higher surface tension is engulfed into the cell with lower cortical tension, a process blocked by surface tension inhibition in both cells when treated with Blebbistatin. In addition, the mechanosensitive phosphorylation of Yap and associated repression of the Cdx2 marker are activated in the inner cell, thereby specifying the inner cell mass in pre-implantation mouse embryos. As both tension-defective cells treated with Blebbistatin display symmetric phosphorylation of Yap, tension induced by un-phosphorylated Yap, and the decrease of surface tension associated with Cadherin-1 cell-cell surface interaction during engulfment may underlie this process58,59. This suggests the coordination of biochemical morphogenesis with biomechanical morphogenesis similar to Drosophila mesoderm internalization.
Later in mouse development, the mechanical constraints of the uterine walls applied to the embryo stimulate the expression of Dve (distal visceral endoderm) genes60 involved in the establishment of antero-posterior and primitive streak polarity at the posterior pole of the embryo before gastrulation21, through an unknown mechanotransductive molecular process.
The mechanosensitivity of the Yap/Taz pathway to substrate adhesion is also involved in zebrafish somite formation, as a permissive signal enabling the Delta-Notch-dependent spontaneous genetic oscillation clock that leads to biochemical somite patterning61. Taz mechanosensitivity is also at work in zebrafish oogenesis. Here, the oocyte cells that express more Taz, by fluctuations, grow faster than the others. One consequence of this growth asymmetry is a greater confinement of the other cells, with compression leading to the mechanical stimulation of Taz repression. As a result of this mechanotransductive loop process, a single cell eventually expresses Taz and will define the oocyte micropile through which the spermatozoids will enter the protective eggshell62. In this case, photo-ablation experiments preventing mechanical stress coupled with local rescue by pipet micro-aspiration uncovered the mechanotransductive process.
The hydrostatic pressure of the blastocoel, an embryonic cavity next to the prospective neural crest, serves as a mechanical signal regulating neural crest induction in Xenopus embryos. Here, competence for neural crest identity has been shown to decrease and eventually be lost along with the increase in hydrostatic pressure of the blastocoel. The underlying mechanism is the inhibition of Yap signaling leading to impairment of Wnt activation in the responding tissue that is required for neural crest induction63.
Biochemically regulated static forces are also involved in biochemical patterning. Acto-myosin cables at compartment boundaries, for example, robustly define the biochemical patterns of anteroposterior segmentation64,65. Cell sorting, due to differences in surface tension in differentiated cells, either because of differences in acto-myosin cortical tension or because of adhesive properties associated with cadherins, has also been suggested as a driver of biochemical patterning66,67. Indeed, cell-cell surface interactions and tensions are believed to regulate the early stages of ascidian embryo morphogenesis, in a process relatively autonomous from any biochemical pre-pattern68–70.
Therefore, mechanical forces play a major role in biochemical patterning. These can either be associated with segregating sorting forces, or directly inductive of cell differentiation through mechanotransduction.
Physical forces as a trigger of self-induced, Myosin-dependent active biomechanical patterning
The mechanical stresses developed by active morphogenetic movements not only retroact on the biochemical structuring of the embryo, but also stimulate the activation of myosin-dependent processes that trigger subsequent active biomechanical morphogenesis.
For example, by detailed examination of the genetic control of mesoderm invagination in early Drosophila embryos, it was found that expression of the patterning gene snail was required in addition to twist (see first subsection beyond) for the apical stabilization of Myo-II that leads to inward invagination71. Precisely, this apical accumulation begins with a sna-dependent transient, unstable and uncoordinated build-up of Myo-II spots at the apex. This leads to uncoordinated pulses of apex size in the mesoderm, yet insufficient to generate the invagination. twist-dependent apical stabilization and accumulation of Myo-II spots rapidly follow, which lead to coordinated and stable apex contraction and mesoderm invagination71. Strikingly, the mechanical stresses developed by snail-dependent pulsation activate the mechanosensitive Fog signalling pathway72,73, with Fog being expressed under the control of twist and upstream of the Rho-dependent process leading to apical stabilization of Myo-II (see first section34 (Fig. 5a).
Fig. 5. Mechanotransductive triggers of active morphogenetic movements in bilaterian early embryogenesis and organogenesis.
a,b- Fog-dependent mechanical induction of Rho-dependent apical stabilisation of Myo-II by snail-dependent Myo-II-dependent pulsations (red loop I in b), potentially auto-amplified (grey loop arrow in mid-sage 6 (10’)). Fog-dependent mechanical stimulation of Myo-II-dependent posterior endoderm invagination initiation by the stretching induced by mesoderm invagination (red loop II in b) auto-amplified (blue box, grey loop arrow (potentially Fog dependent)), suggested as also Fog-independent (black arrow in b leading to full endoderm invagination). Adapted from72. c Mechanical induction of apical stabilisation of Myo-II by contractile forces of apoptotic cells in folding initiating joint formation in Drosophila leg organogenesis. Adapted from78.
Rescue of sna-dependent mechanical apex pulsations in sna-defective embryos by application of an indent, or pulsatile micromagnets in combination with mesoderm-injected ultramagnetic liposomes, rescued medio-apical accumulation of Myo-II and mesoderm invagination72,73. These processes were inhibited in sna twi-defective embryos lacking zygotic expression of Fog and in sna-defective embryos injected with fog RNAi, respectively72,73. Therefore, the sna-dependent mechanical pulses of apexes are sufficient to trigger apical stabilisation of Myo-II and initiate mesoderm invagination in early Drosophila embryos in a Fog-dependent mechanotransductive process, via at least the ubiquitously expressed Fog receptor Smog74. In Fog zygotic mutants, such a mechanotransducive pathway might additionally act in response to snail-dependant mechanical pulsations in a delayed and less efficient way, through the maternal components of Fog and in a T48-permissive process. In case of failure of the mechanotransductive process, a secondary biochemical pathway such as T48 might robustly compensate (see Box 1).
Subsequently, the mechanical constraints developed at the poles of the embryo by the invagination of the mesoderm lead to mechanical stimulation of apical Myo-II accumulation at the posterior pole, in which the Fog mechanosensitive pathway is also expressed. This initiates the invagination of the posterior endoderm (Fig. 5b)72. Indeed, magnetically rescuing the stretching of the posterior pole in sna mutant embryos lacking mesoderm invagination and injected with ultra-magnetic liposomes, rescued the apical accumulation of Myo-II lacking at the onset of mesoderm invagination, in a Fog-dependent process, as well as the initiation of posterior pole invagination72. Finally, it has been proposed that the invagination of the endoderm is amplified and achieved by a third mechanosensitive process that appears to be independent of Fog (Fig. 5b)75 and reminiscent of theoretical predictions for a self-induced wave of active invagination76. A similar process was proposed to be involved in cephalic furrow formation in the Drosophila embryo, with a cluster of median contractile cells triggering bi-directional propagation of the contractile furrow both ventrally and dorsally77.
There thus exists a self-induced cascade of active morphogenetic movements mediated by mechanical stimulation of Myo-II activation, with one morphogenetic movement triggering activation of the next, from snail-dependent pulsations to mesoderm invagination and posterior endoderm gastrulation. Interestingly, the characteristic time scales of this cascade, i.e. the order of a minute between each event in the cascade, are too dynamic to imply a process of mechanical induction of biochemical patterning events dependent on gene expression. The cascade is, however, tightly predetermined by the expression of snail, which initiates pulsatile morphogenetic movements in the mesoderm only. It is predetermined as well by the expression of the mechanosensitive Fog signalling pathway in the presumptive mesoderm and posterior endoderm only72.
These examples again demonstrate how self-induced biomechanical morphogenetic processes cooperate with pre-patterned biochemical processes. Here, the primary dorso-ventral polarity of the embryo, leading to the patterned polarized expression of twist, fog and snail, is the product of spontaneous symmetry breaking associated with the random position of the fertilized nucleus relative to the egg membrane. This leads to a polarized activation of Toll, upstream of the nuclear translocation of the transcription factor Dorsal12.
Epithelial folding during organogenesis is another event dependent on mechanical stimulation of Myo-II activity. In Drosophila embryos, the morphogenesis of leg and wing formation involves the folding of the leg and wing discs that morphologically separate the different parts of the organs. Instead of pulses, folding is initiated here by cell apoptosis. As with endomesoderm invagination, the mechanical stresses developed by apoptosis-induced tissue folding on neighbouring cells have been proposed to activate apical stabilization of Myo-II, ultimately leading to complete folding of the epithelium (Fig. 5c)78. In addition to folding, actomyosin contraction-based mechanosensitivity has been proposed to enhance cell intercalation in avians, which is the driving force of convergent-extension79.
Therefore, mechanotransductive signals are key intermediate inductive steps during biochemical patterning and active biomechanical morphogenesis. Indeed, biochemical patterns produce active biomechanical strains, whose mechanotransductive effects produce further biochemical patterns and active morphogenetic forces. The interplay between biochemical patterning and biomechanical morphogenesis is thus reciprocal. This has two important implications: First, the biochemical patterning cascade can be both cause and effect of biomechanical morphogenetic processes and reversely, without a priori systematic hierarchical causality on the one to the other. Second, biomechanical morphogenetic cascades can be self-induced like biochemical induction cascades, within a biochemical pre-patterned framework. Such intricate relationships between biochemical patterning and biomechanical morphogenesis thus makes their disentangling, required to understand their interplay, particularly challenging.
Forward to the past: emergence of biochemical and biomechanical patterns at the evolutionary origin of earliest primitive multi-cellular metazoan organisms
How the first biochemical and biomechanical patterns that defined first multicellular organisms and metazoan animals emerged is a long debated, yet open question in evolutionary biology. Here we will highlight how gastrulation regulated by mechanotransduction may represent a unifying characteristic of endomesoderm formation in metazoan species, whether it responds to internal and/or environmental marine mechanical strains. We will also emphasize how, in today’s non-metazoan multicellular choanoflagelattes, mechanotransduction of marine strains controls the process of inversion, which can be seen as a primitive form of gastrulation. From this, we will propose that a major primitive condition of today’s metazoans could have been Myo-II-dependent gut formation upon mechanotransduction of marine strains, with a newly associated mechanosensitive pathway leading to its endomesodermal specification: β-catenin. The evolutionary origin of today’s endomesoderm development would then have relied on the autonomization from the marine environment, achieved by embedding this core mechanism within internal biochemical and/or biomechanical patterns. This could have occurred in parallel with the diversification of animals with different reproductive strategies and body plans, leading to very diverse conditions for this embedding.
Genetic clues to the existence of a common ancestor for all bilaterian metazoans
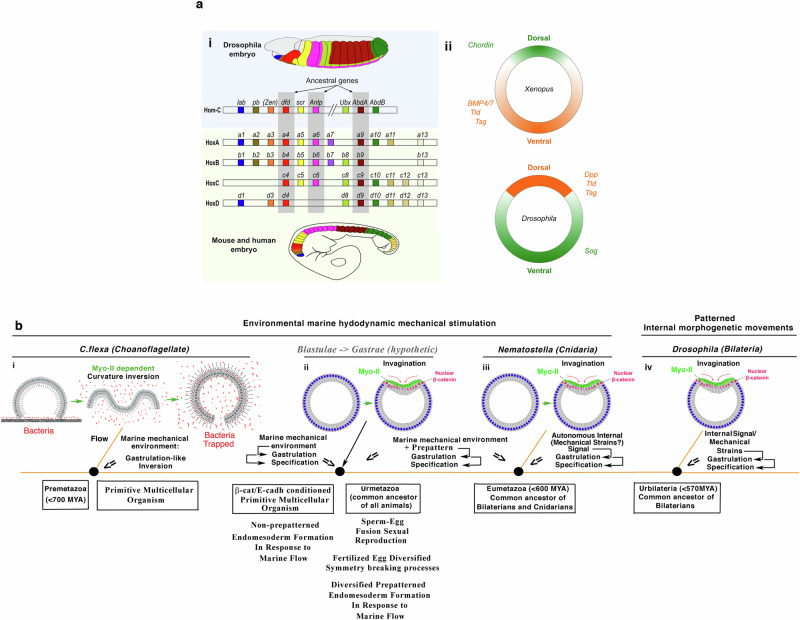
Hox genes and their specific roles in determining specific domains of the animal’s anteroposterior body plan are remarkably conserved across species, for example between flies and vertebrates, including xenopus, mice and humans (Fig. 6ai)16. This appears to confirm the hypothesis of a certain unity of plan for all antero-posteriorly segmented bilaterians, after a long and famous controversy that began over 2 centuries ago, pitting Saint-Hilaire against Cuvier80.
Fig. 6. Genetic and mechanotransductive common origin of Bilateria and of first multicellular animal organisms.
a Conservation of i Hox genes and of their expression along the anteroposterior axis of the embryo across species, here in Drosophila versus mouse and human embryos, ii and of embryonic early dorso-ventral patterning genes, inverted in vertebrates compared to insects (here in Xenopus and Drosophila early embryos). Adapted from16 and81. b Conservation of Myo-II-dependent hydrodynamic stimulation of i gastrulation-like inversion in C.flexa and of iii gastrulation in Nematostella, whose last common ancestor was a pre-metazoan dating back to at least 700 million years ago. ii Environmental hydrodynamic stimulation of endomesoderm morphogenesis and specification from β-cat/E-cadherin conditioned multicellular colony of cells, subsequently prepatterned after egg-sperm fusion sexual reproduction, having hypothetically led to the Gastrae (black arrow). iv Substitution of environmental mechanical strains by patterned internal mechanical strains stimulated gastrulation in Bilaterians such as Drosophila embryos, whose last common ancestor dates back to 570 million years ago. Adapted from86.
However, one of the most critical points of the controversy crystallized around the fact that when comparing the dorso-ventral structures of arthropods, such as crustaceans and insects, with those of vertebrates, these were inverted, with most of the dorsal structures of the one being found as ventral in the other81. This, from the outset of the controversy, seemed to disfavour the hypothesis of a unity of plan for all animal species, unless an inversion in the development of dorso-ventral structures occurred in a given super-phyla relative to the other in the course of evolution, as Saint-Hilaire suggested. And indeed, the same set of genes has been found to be involved in the initiation of dorso-ventral structure in vertebrates like Xenopus and the arthropod Drosophila, but reversed with regard to ground position: the BMP/Chordin complex82.
In Xenopus, the ventral inducer BMP4/7 (homologous to the dorsal inducer of Drosophila embryos Dpp) is repressed by Chordin (homologous to Sog) at the dorsal pole of the embryo, thereby determining the dorsal nature of the tissue in the absence of BMP proteins. In other words, the BMP/Chordin polarity reversed in the control of ventral and dorsal genes between vertebrates and arthropods but remained conserved in the establishment of dorso-ventral polarity in bilaterians (Fig. 6aii)81.
These observations along with analyses of molecular phylogenies16,83 converged on the existence of a common ancestor for bilaterian animals81, having diverged around 570 million years ago from radially symmetrical cnidarian animals.
Gastrulation upon mechanotransduction as an ancestral and unifying mechanism to understand metazoan evolutionary emergence and early development
Hydrodynamic stimulation of early endomesoderm morphogenesis and specification
The first metazoan multi-cellular organisms are thought to have arisen with the formation of a first organ from a multicellular colony of cells: the primitive gut84,85. In today’s early embryos, this process is called gastrulation and involves both the biomechanical morphogenesis of the inward invagination leading to the primitive gut, and its biochemical specification in regard to the ectoderm. However, it is striking to note that although the molecular motor Myo-II is involved as the driving force of this invagination in most of the species of different superphyla tested, the upstream molecular pathways that regulate Myo-II activation differ86. Therefore, these do not appear to be conserved from one superphyla to another. The same can be said for the conditions that led to the specification of the invaginated primitive gut86. Indeed, endomesoderm (EM) specification was so far found not to be induced by the Wnt/β-cat pathway in insects and crustacea, and more generally in protostomes, in contrast to other species87,88, as well as induced by β-cat but not initiated by Wnt in the earliest specification of zebrafish mesoderm53.
Therefore, while genetic and biochemical clues point to the existence of an ancestor common to all metazoans (see subsection above), there are no straightforward biochemical or genetic clues as to the conditions of the emergence of such a primitive organism. These conditions may have diversified during evolution, for instance, via loss of the Wnt/β-cat-dependent induction of EM specification in protostomes and a partial loss in some vertebrates, for instance, Wnt in zebrafish earliest mesoderm specification. Alternatively, these conditions might not have been purely genetic or biochemical in nature.
Indeed, just as in Drosophila bilaterian embryos, in which the Myo-II activation that triggers gastrulation is mechanically stimulated by pulsating internal morphogenetic movements (see second section above), a gentle marine hydrodynamic flow mimicking soft waves on the sea-shore was found to be able to trigger active gastrulation in a Myo-II-dependent process in the cnidarian sea anemone N. vectensis (Fig. 6biii)86. The common ancestor of cniadian like N. vectensis with bilaterians like Drosophila, dates back at least 600 million years. Such hydrodynamic stimulation, as well as vortexing89, was also shown to lead to a Myo-II-dependent gastrulation-like reversal in the open multicellular choanoflagellate C. flexa (Fig. 6bi)86,90, which shares a common pre-metazoan ancestor with cnidarians and bilaterians dating back at least 700 million years91.
In N. vectensis, invagination mechanotransductively contributes to endomesoderm (primitive gut) specification, through mechanical activation of the β-cat pathway via Y654-βcat phosphorylation (Fig. 6biii)86, like in early endomesoderm specification in the bilateria Drosophila (Fig. 6biv) and zebrafish embryos51,53. This is also the case in mesoderm specification of human embryonic stem cells, where mechanical stimulation of the β-cat pathway by Y654-βcat phosphorylation has been shown to potentiate BMP4-dependent mesoderm specification via the expression of brachyury, and its subsequent stabilization via the expression of Wnt in human gastruloids92 and in zebrafish embryos53. Yap, known as mechanosensitive, also plays a role in gastruloid patterning of human embryonic stem cells93.
Strikingly, Y654−β-cat, and its E-cadherin D655 interaction site, are strongly conserved in metazoans across all super-phyla94. There is thus a conservation of the mechanotransductive stimulation of endomesoderm formation and specification in evolutionary distant species across at least 700 million years ago, activable by environmental hydrodynamic flows. This raises the possibility that the emergence of a first organ from a multi-cellular structure—the first multi-cellular organism—was stimulated by the inextricable requirement of the natural environment of the marine cell colony and the existence of mechanosensitive molecular processes leading to activation of Myo-II and βcat86. The organism would thus have been stabilized by natural selection for its advantageous ability to capture and digest more prey by sensing flow. Indeed, this configuration makes feeding more effective because preys are more trappable in suspension stirred by flow through inversion than sedimented on the ground without flow and without inversion (Fig. 6bi, bacteria preys in red), as experimentally demonstrated in C.flexa86.
Consequently, the mechanosensitivity of Myo-II activation in response to the environment may have played a major role in the evolutionary emergence of the first multicellular organisms in the pre-metazoan common ancestor of choanoflagellates, cnidarians and bilaterians, more than 700 million years ago (Fig. 6bi).
In such a scenario, the evolutionary emergence of a motor-sensorial behavioural response to environmental mechanical strains and the evolutionary emergence of the first multicellular organisms would have been one and the same unified event. This indicates that, at least in its early stages, the development of multicellular organisms may have been shaped by the evolution of their active response to the environment, before being stabilized by the selective pressure of the environment. This makes the environment both an active stimulating and a passive selective parameter in the evolutionary emergence of multicellular organisms. Subsequently, the mechanosensitivity of β-cat pathway activation in response to invagination mechanical stimulation by the marine environment may have played a major role in the emergence of EM specification in the first multi-cellular organisms, in which multi-cellularity was specifically ensured by junctional β-cat/Ecadh complexes (Fig. 6bii) (see Box 2).
Autonomisation of endomesoderm specification from the environment
Internal morphogenetic movements, such as snail-dependent pulsations in Drosophila embryos, may then have substituted environmental mechanical signals and triggered gastrulation in a mechanotransductive manner, thereby initiating embryogenesis (Fig. 6biv)86. Such mechanical signals may have emerged spontaneously and homogenously around the embryo as active mechanical fluctuations through non-patterned expression of genes like snail. Local invaginations would then have been triggered by local instabilities in the inward curvature, formed by a particularly strong fluctuation that was auto-amplified by Myo-II mechanical stimulation. After the emergence of β-cat/E-cadherin-dependent multi-cellularity that is specific of metazoans, such a spontaneous symmetry-breaking process caused by a local instability could then have been reinforced by β-cat pathway-mediated mechanical stimulation initiated by Y654-β-cat phosphorylation. This would have led to the expression of patterning genes such as the endomesoderm gene twist upstream of fog, thereby enhancing the efficiency of the Myo-II/inward curvature self-induced positive feedback loop, and the robustness of the formation of invaginations. The emergence of the β-cat/E-cadherin junctions characteristic of metazoan multi-cellularity might additionally have been the condition for more robust adherent multi-cellular structures ensuring their full and solid closing (Fig. 6bii-Left).
More generally, such β-cat-dependent mechanotransductive processes might also have been involved in the emergence of other patterns. For example, blastopore specification, which specifies the ectoderm of the pharynx and has been proposed to separate the ectoderm from other tissues95,96, was suggested to be mechanically stimulated by morphogenetic movement during gastrulation97. Within this framework, it might also be interesting to consider whether the mechanical activation of the β-cat pathway could have not only been at the evolutionary origin of endomesoderm formation, but also participated in addition to the Wnt3-dependent canonical Wnt/β-cat pathway to trigger the genetic program that impulses Hox genes antero-posterior patterning (see first section).
Therefore, the primitive metazoan multi-cellular organism, i.e the first metazoan multi-cellular living structure characterized by β-cat/E-cadherin junctions and a functional organ specified as an endomesoderm, could have been a closed multi-cellular structure. It would have been characterized by the ability to display and adapted behavior in reaction to hydrodynamic flow, allowed by the formation and specification of a primitive gut through Myo-II-dependent mechanotransduction (Fig. 6bii). Biochemically regulated internal mechanical fluctuations would have then replaced environment cues as mechanotransductive triggers.
The evolutionary steps towards endomesoderm pre-patterning
Today’s embryos are pre-patterned in the expression of endo-mesodermal genes prior to the initiation of gastrulation morphogenetic movements, as mentioned above. In addition, gastrulation can mechanically stimulates the maintenance of endomesoderm specification, possibly in reminiscence of its ancestral mechanical stimulation by the environment49,53. Such a pre-pattern ensures local expression of genes such as twist and fog, but also snail, that generate mechanical stimulation and a mechanosensitive invagination response in endomesoderm-specified domains in Drosophila embryos. However, how was such a pre-patterned domain of endomesoderm gene expression established in the course of evolution? As already mentioned, the random localisation of the nucleus in the fertilised egg membrane determines the initial Dorso-Ventral polarities leading to the expression of mesoderm genes in a spontaneously symmetry-breaking manner in Drosophila embryos12. Antero-posterior axis polarity, involved in endoderm specification, is transmitted by the mother to the egg in these embryos12. In Xenopus, it is thought that the entry point of the spermatozoid triggers such symmetry breaking and determines the location of the active β-cat signal leading to the early specification of the endomesoderm98. We propose here that simple geometric symmetry breaking, for instance, due to random localisation of the nucleus in the fertilised egg, or to the point of sperm entry after egg-sperm fusion, substituted for the Myo-II dependent environmentally stimulated morphogenetic movements of gastrulation to biochemically pattern the endomesoderm. Other processes potentially at play include reaction-diffusion-based spontaneous symmetry breaking or transmission of the polarity by the mother9,12,23.
These processes would have been upstream of the patterned activation of biochemical pathways leading to the early expression of genes and proteins, whose functions substituted for the environment in the Myo-II dependent and β-cat dependent mechanical endomesoderm induction, such as Snail expression leading to mechanical strains and Fog expression leading to Myo-II dependent mechanical stimulation of gastrulation (Fig. 6biv). Some of these genes might have been endomesoderm genes like twist, that would have already been selected for to be both downstream and upstream of the Myo-II/β-cat-dependent mechanosensitive inductive processes for endomesoderm formation before the age of sperm-egg fusion-based reproduction (see previous subsection).
Indeed, in the first primitive pre-metazoan and metazoan organisms, sperm-egg fusion is assumed not yet to exist. Therefore, the emergence of sexual reproduction by sperm-egg fusion might have introduced a wide variety of symmetry-breaking processes in the early specification of the domain of endomesodermal genes. This would have led to pre-patterning the self-induced processes of endomesoderm biochemical specification and biomechanical morphogenesis, which had previously been stimulated by non-patterned environmental or active internal mechanical stimulation, through diverse non-conserved biochemical signaling pathways.
Overall, initiation of endomesoderm specification has been described to be Toll-dependent (pre-gastrulation stage, Drosophila), β-cat dependent but Wnt-independent (epiboly earliest stage, zebrafish mesoderm), and Wnt/β-cat dependent (gastrulation stage N. Vectensis). Further it has been shown to be mechanically stimulated at early gastrulation stage in Drosophila and N.Vectensis, and at early epiboly stage in zebrafish via the mechanical activation of β-cat Y654 phosphorylation, which is strongly conserved in all metazoans. This indicates a conserved role of mechanically induced endomesoderm specification by first morphogenetic movements of embryogenesis in contrast to a less conserved role of biochemical signalling induction. Hence, mechanical induction is probably older than biochemical induction in endomesoderm specification. Importantly, this is in line with the fact that an endomesoderm Wnt/β-cat specification signalling pathway has not been conserved in metazoans, which one would expect if it had prepatterned the endomesoderm Myo-II dependent gastrulation process in the metazoan common ancestor. Indeed, in the pre-metazoan ancestor, gastrulation-like events could be Myo-II dependent and mechanically stimulated, as seen for C.flexa inversion, but in a Wnt/β-cat independent process, as these molecules are metazoan-specific (see Box 2).
Interestingly, in addition to physico-mechanical cues, physico-chemical cues play a key role in embryonic development. This is the case of nitrite oxide (NO) regulated by estrogens in the establishment of murine embryo pre-implantation99, or in heart looping during embryonic development that is regulated by yolk NO100. NO was also found to trigger C.flexa inversion, but not found so far to be involved in metazoan EM formation89.
The mechanosensitivity of the β-cat pathway could have been inherent to its role in metazoan multicellularity through its interaction with E-cadherin in the formation of adherens junctions via its Y654 residue, phosphorylable by evolutionarily preexisting Src family kinases94,101. It may also have helped curvature formation by softening the invaginating tissue by the mechanotransductively induced loss of 20% of junction β-cat after phosphorylation94, in addition to supplying mechanosensitive sensors once the β-cat pathway had connected upstream to Myo-II (see above in this section and following section), in this new metazoan context where cells are more strongly linked together in a stiffer tissue.
Once this core mechanosensitive mechanism was in place during early metazoan evolution, different possibilities could have opened for its diversification throughout evolution. As discussed, these include the possible subsequent use of: internal fluctuations to replace hydrodynamic strains; sperm entry, nucleus location in the fertilized egg, or Wnt ligand to pre-pattern internal or environmental mechanotransducive stimulation of Myo-II activation in a β-cat or other signalling pathway dependent process like Dorsal. These mechanisms could also have replaced each other throughout evolution, with apparent hierarchies being reversed, with no simple rule for all eumetazoans.
In summary, we propose that Myo-II, β-cat and their mechanosensitivity might have played central roles in early metazoan emergence, through the following sequence of events: i/ the β-cat-dependent emergence of multi-cellularity specific to metazoans, via β-cat/E-cadh formation of epithelial junctions102, leading to ii/ endomesoderm morphogenesis and specification via β-cat mechanosensitivity in response first to environmentally, then to internally stimulated gastrulation by an older pre-metazoan property of Myo-II mechanosensitivity, iii/ with subsequent evolvement of diversified symmetry breaking processes in the fertilised egg, such random localisation of the nucleus, transmission of polarities from the mother to the egg or by the entry point of the spermatozoid. These would have prepatterned the expression of proteins like Fog or Snail downstream of Dorsal, involved in the mechanosensitivity of Myo-II activation and in internal active mechanical stimulation.
Following this view, biomechanical, biochemical and geometrical cues would have been at constant interplay in self-inducing the emergence of the first multi-cellular metazoan organism and initiation of animal embryogenesis, via a non-hierarchical, reciprocal biochemical patterning and biomechanical morphogenetic canalisation process through evolution.
Box 2 Ancestrality of Myo-II dependent hydrodynamic stimulation of multi-cellular tissue inversion to endomesoderm specification.
In this scenario, Myo-II-dependent hydrodynamic mechanotransductive stimulation of multi-cellular tissue inversion would have been ancestral to the mechanotransductive stimulation of tissue specification by inversion. Such an order of events is supported by the notion that the multi-cellular choanoflagelattes C flexa, in which the metazoan β-cat/E-cadh complex is absent101, can invert in a Myo-II dependent process136, and in response to hydrodynamic mechanical strains86,89,90 as N. vectensis does86. Hence, the mechanotransductive stimulation of Myo-II dependent inversion could have been present in the pre-metazoan common ancestor of choanoflagelattes and cnidarians in the absence of β-cat-dependent specification of the EM by tissue inversion mechanical strains (Fig. 6bi).
Indeed, the first pre-metazoan multi-cellular living structure characterized by a functional organ, the primitive gut, was suggested to be a C.flexa-like unclosed multi-cellular structure. It was characterized by the formation of a full gastrulation-like inversion, which trapped preys through mechanotransduction in reaction to hydrodynamic flow that puts bacterial preys in suspension (Fig. 6bi, right)86. Therefore, the emergence of the first multi-cellular organism might have been due to the emergence of a favourable mechano-sensorial behaviour of producing a primitive gut. Once selected by its evolutionary advantage, such a property would have naturally become a feeding-reflex behavioural property of gastrulation by inversion.
What would thus have been the mechanosensitive pathway involved? The mechanosensitive Fog pathway expressed during initiation of mesoderm and endoderm invagination in Drosophila embryos (see Fig. 3a, b) is specific for insects137. Therefore, mechanical activation of Myo-II via ligands of small protein G receptors (RCPG) secreted apically with the same effect on Myo-II activation as Fog could have been responsible. Indeed, mechanical inhibition of Fog endocytosis by membrane tension was found to underlie the mechanical stimulation of Myo-II apical accumulation. This was proposed to prevent the endocytosis of Fog with its receptors, consequently maintaining and stimulating their activation73. The ligands upstream of such RCPGs may thus subsequently have diversified, with a conservation of the underlying mechanotransducive mechanism stimulating medio-apical accumulation of Myo-II. Alternatively, such RCPGs may have been directly mechanosensitive123 and insensitive to ligand dispersion by flow in the pre-metazoan common ancestor of choanoflagellates and cnidarians. Sensitivity would then have enhanced during the course of evolution by substitution with RCPG ligand-specific mechanical modulation of endocytosis in embryos not subjected to flow, or protected from flow by a vitelline membrane.
Legacy from the past: Filtering of ubiquitous mechano-biochemical coupling and evolution of selected functional mechanosensitive processes
Here we will first use the adult mouse colon to first illustrate how β-cat mechanosensitivity is made visible today in tumorigenic conditions, where tumors produce forces, compressing neighbouring cells and inducing their proliferation via β-cat. This model uncovered how β-cat mechanosensitivity is filtrated in normal conditions by the expression of APC that, together with GSK3β, degrades cytoplasmic β-cat before it can enter the nucleus to regulate transcription. We will describe how this filtering is counteracted in stem/regenerative cells through Wnt-dependent inhibition of APC, combined with stimulation by peristaltic-like myogenic mechanical pulses. We will more generally detail the different types of filters identified in early Drosophila embryos and in the adult mice colon, from already activated kinases that permit the mechanical activation of the signal (Filter type 1), to the expression of the kinases that are directly mechanically activated (Filter types 2), in addition to downstream filtering by the APC/ GSK3 complex (Filter type 3). In addition to the existence of these specific filter types, we will speculate on general structures of regulatory networks that could act as discriminators of biomechanical and biochemical signals or as their respective buffering. We will then propose an evolutionary scenario for the emergence of first specific filter types in the formation of the endomesoderm from the non-prepatterned hydrodynamically induced gastrulation-like of first multicellular organisms to modern early embryos.
Exploiting and filtering the evolutionary inherited mechanosensitive β-cat pathway in metazoan epithelia
The endomesoderm is often considered to have been the common ancestor of most metazoan organs95. Thus, if the ancestral endomesoderm was induced by mechanotransduction, one would expect that the mechanosensitivity of the biochemical pathways involved would remain functional in the epithelia of all metazoan organs. This is indeed the case in epithelia of the adult mouse colon, where pressures of 1 kPa generated by pathological tumour growth activate the β-cat pathway via Y654 phosphorylation-mediated mechanical stimulation in neighbouring nontumour cells compressed by the tumour103. Undeniably, once in place during the establishment of metazoan multicellularity, the β-cat/Ecadh complex could not escape the laws of physics. Indeed, here the latter led to an increase in the probability of opening the major βcat Y654 interaction site with E-cadherins to phosphorylation by Src family kinases94, in all metazoan epithelia, including the primitive, embryonic51,53,55,92, or adult in kidney canine cells104 and mice gut103. In the abovementioned colonic epithelium of adult mice, it activates a proliferative pathway in neighbouring nontumour cells, playing a mechanotransductive role in tumour progression (Fig. 7a-iii)103.
Fig. 7. Sorting and filtering mechanical from biochemical signalling.
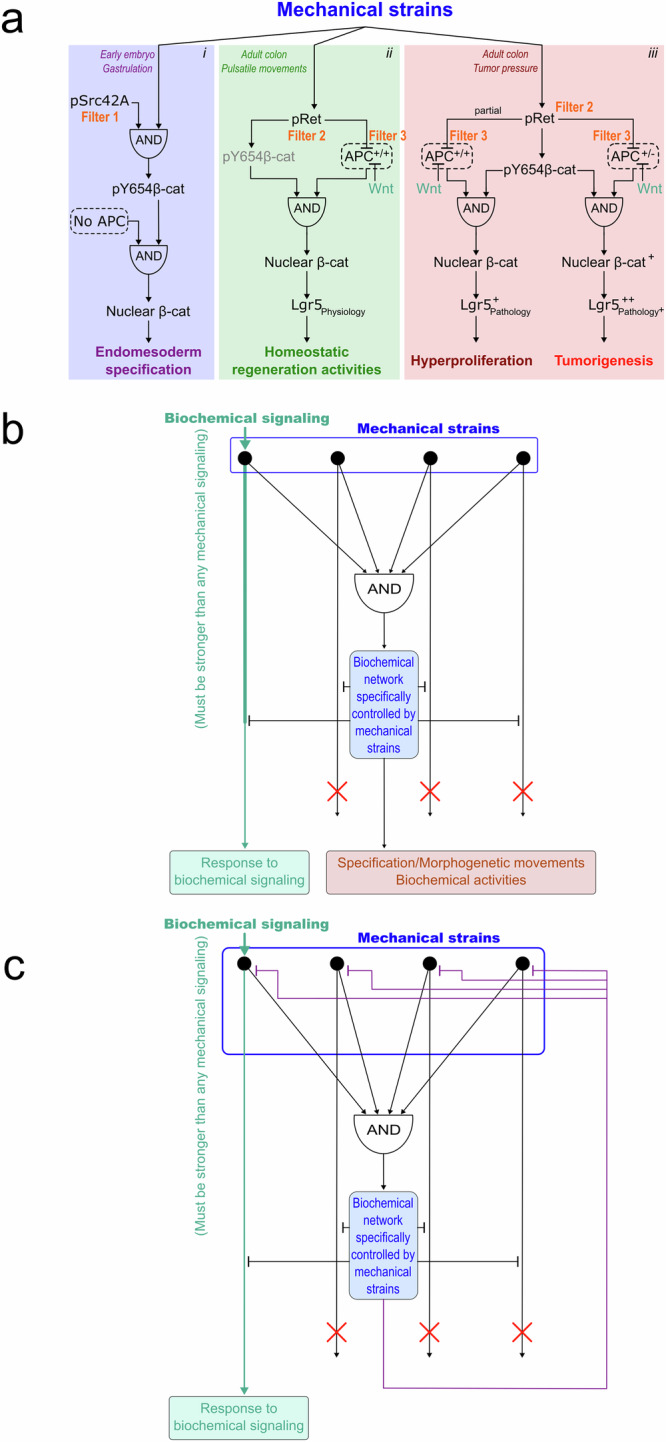
a β-cat dependent mechanosensitive regulatory network in i- early Drosophila embryo mesoderm and endoderm specification, ii- maintenance of homeostatic levels of Lgr5-expressing stem cells in WT mice colon and iii- production of tumorigenic levels in Lgr5+ hyperproliferative and Lgr5 + + tumorigenic stem cells number. Each link between the nodes of the network consists in a non-binary percentage of activation or repression. b Hypothetic generic structure to filter and separate biochemical from mechanical regulatory responses of the cell. c- Hypothetic generic structure to prevent mechanical perturbation of biochemical activities of the cell. Biochemical signalling is assumed to be stronger than mechanical signalling (see text).
However, 1 kPa is also the amplitude of the peristaltic and pulsatile mechanical stresses present in the mouse colon, associated with the physiological function of intestinal transit105. Thus, how are these physiological mechanical stresses filtered so as not to over-activate the βcat tumorigenic pathway, triggering tumourigenesis? One possibility is that, because they are pulsed, these physiological stresses are cumulatively less intense over time than the permanent pathological stress of tumour growth. A second possibility, experimentally demonstrated, is that the genetic network downstream of βcat activation filters the signal to prevent activation of the tumorigenic target genes of βcat. This is indeed the case, due to the expression of the APC/GSK3β complex in WT mice, which sends most cytoplasmic βcat in degradation, after its junctional release due to mechanical stimulation of Y654 phosphorylation of βcat (Fig. 7a-ii, Filter 3). Interestingly, APC is not expressed in early Drosophila embryos at the time of gastrulation but is induced afterwards (Fig. 7a-i)106. This suggests that the transcriptional power of the mechanotransductive pathway involved in the specification of the endomesoderm of early embryos needs to be blocked by APC/GSK3β expression once specification is completed, as for instance in the adult tissues of the mouse colon107. Indeed, the βcat-dependent signal involved in stimulating tumour progression is not fully operational to amplify tumorigenesis in WT adult mice colon, and only leads to hyperproliferation. In the tissues of heterozygous APC-mutated mice—where mutant homozygosity is known to initiate colon tumorigenesis—this is, however, not the case. Rather, with the APC filter being 50% less effective than in WT, mechanical induction of tumorigenesis occurs (Fig. 7a-iii)103.
Strikingly, the physiological high-frequency mechanical pulses of the colon activate the βcat signal, leading to the production of new Lgr5 positive regenerative and proliferative stem cells in the WT105, cells in which the Wnt signal is known to have an inactivation role via the APC/GSK3β filter (Fig. 7a-ii)108. Consequently, in the adult colon, APC is used as a filter to block the transcriptional power of the mechanosensitive pathway that possibly led to the emergence of the ancestral primitive gut organ86, except in a subpopulation of cells in which patterned expression of Wnt represses the APC filter. This seemingly restores to these cells to an embryonic-like developmental function in the regeneration of the organ by producing physiological levels of stem cells in response to physiological pulsatile mechanical stress, like in the common ancestor stimulated by the wavelets (Fig. 7a-i,ii).
Back to embryogenesis, it has been suggested that the overall structure of medaka fish embryos is maintained against gravity-induced deformations by a Yap/Taz-dependent mechanosensitive process that activates the Rho pathway in a counter-action process opposing tension to gravity forces109. During medaka gastrulation, this process is believed to be at work in maintaining embryonic structures in resistance to the internal forces of convergent cell migration at the origin of midline assembly110. A similar mechanism seems to be involved in the formation of the notochord and neural tube in the mouse embryo, where Yap-dependent rigidity is required111. Notably, these processes are reminiscent of Myo-II accumulation at junctions increasing the mechanical resistance of epithelial tissue to morphogenetic movements of convergence and extension in early Drosophila embryos112.
Therefore, biochemical patterning indeed serves as a filtering regulator of mechanotransductive physiological processes, by combining the patterned expression of permissive (Filters 1,2) and buffering (Filter 3) proteins in mechanical signalling. In parallel, in addition to be actively involved in biomechanical patterning (see second section), mechanotransduction can also serve to actively counterbalance and buffer undesired mechanical perturbations to robustly maintain the biomechanical structure of the embryo.
Exploiting and filtering the numerous mechanosensitive pathways in living systems
Many of the main signalling pathways have been shown to be mechanosensitive113–118, even though the underlying molecular mechanisms of the transduction of a mechanical signal into a biochemical signal have, in many cases, not yet been found. Indeed, there exists an a priori large number of proteins that are physically associated with mesoscopic or macroscopic mechanical structures, and that can therefore change conformation to become activable or active in response to mechanical stress. It is therefore expected that most known pathways would have key elements of pathways that are mechanically activable in the absence of ligands. For instance, in cultured cells, mechanical elongation of the adherens complex protein p130Cas, involved among other processes in cell migration, was found to open its phosphorylation sites to Src kinase activity119. In mouse colon tumors, phosphorylation of Y654-βcat could be mechanically induced similar to Drosophila embryos103 (see second section). In this case, the Src family kinase involved was found to be Ret, and its activation through the phosphorylation of its Y1062 site was shown to be mechanosensitive as well103. In the case of the cytoplasmic adapter protein Talin, mechanically induced opening of the α-helix R1-R3 rod domains allows its interaction with Vinculin that stimulates focal adhesion formation120–122. This could also be the case for transmembrane ligand receptors, which can change conformation in response to a change in membrane tension123.
However, without the knowledge of detailed mechanisms, it remains difficult to anticipate the nature of accompanying filtering mechanisms, like for the abovementioned filtering of βcat mechanical stimulation. Nevertheless, one can speculate on the general mechanisms that might be at work in such filtering processes.
As mentioned above, in addition to filtering systems downstream of activated mechanotransductive pathways similar to the APC/GSK3β complex, the filters could also act upstream of such activation. Mechanically induced phosphorylation requires the expression of kinases like Src42A or Ret capable of phosphorylating tyrosines made accessible by mechanically induced conformational changes94,119. Pre-established expression of these tyrosines in space and time would be natural direct candidates for such filtering (Fig. 7a-i,ii,iii, Filters 1,2).
The structure of the mechanosensitive pathway network could potentially play a major role in filtering mechanosensitive pathways in space and time. For example, the non-specific activation of all mechanosensitive pathways could synergise through an AND gate124 to activate proteomic or transcriptional networks. Here the AND gate would take the form of the requirement of the activation of the downstream elements of all the mechanosensitive pathways to trigger the activation of a biochemical network specifically controlled by mechanical strain (Fig. 7b, black arrows). This could be the generalisation to multi-pathways of two pathways representing AND gates similar to Fig. 7aii,iii in which both the repression of GSK3β/APC and the phosphorylation of Y654−β-cat are required for nuclear translocation of β-cat. Such networks would therefore become specific to the mechanical stress experienced and the macroscopic shape of the embryo. To do so, the activated network would have to negatively feedback to all other pathways downstream of each mechanosensitive pathway that do not converge at the AND node (Fig. 7b, black inhibiting arrows). Such negative feedback signalling could take place both at the proteomic and transcriptomic level, via the expression of proteins masking interactions or phosphorylation sites, such as Sog that couples to Dpp (see first section)22, or by blocking transcription like Snail, which represses E-cadherin expression in humans125. To not prevent individual pathways from being efficiently used by individual biochemical ligands during mechanical activation, the efficiency of mechanically activated pathways would need to remain lower than that of the biochemically stimulated pathways (Fig. 7b, bold green arrows). Would such a sophisticated network exist in nature, it would naturally allow the proteome or genome to separate biochemically induced from mechanically induced signals, namely, to distinguish the detection of the biochemical pattern of the embryo from the detection of the biomechanical morphology of the embryo.
Interestingly, AND gates are also known to protect regulatory networks from high-frequency noise124. This is the case insofar as one of the upstream pathways is characterised by a longer temporal response than the others, due for example to its transcriptional nature (10 to 40 min depending on the species, compared with the tiny time scale of direct protein activation). An alternative setup exists if one of the pathways is multi-step in nature, compared with other pathways involved that would be single-step or with fewer intermediate steps in their cascade of biochemical intercations124. Such an AND structure of the filtering network could therefore also protect downstream mechanosensitive events from mechanical noise, such as transient accidental shocks or natural mechanical fluctuations active at high frequency in Myo-II activities.
Inversely, negative feedback from the network downstream of the first AND node, applied downstream of every individual mechanically activated pathway would ensure that the system is insensitive to mechanical stress, and only sensitive to the individual biochemical stimulations activating the individual pathways (Fig. 7c). These could, for instance, act through the repression of Filters type 1 and Filter types 2, or the activation of Filter type 3.
Therefore, in addition to combining the patterned expression of specific permissive and buffering proteins of mechanically activated biochemical signals, sophisticated general motifs in the regulatory networks for mechanotransduction could have evolved to participate in the discrimination of biomechanical from biochemical signals or in their buffering.
Initiation and evolution of mechanotransductive filtering in primitive multi-cellular metazoan organisms
However, be it those demonstrated (Fig. 7a) or speculative (Fig. 7b, c), the latter sophisticated pathways likely did not exist in the first primitive metazoan organisms. This raises the questions of what might have been the primitive processes of specific filtering of physiological mechanical signals and how they may have evolved into today’s filtering networks.
As mentioned, in the first primitive metazoan organisms as defined in previous section, symmetry breaking in the fertilized embryo following sperm-egg fusion that prepatterns the mechanosensitive stimulation of EM formation is assumed not to have existed. This would have de facto prevented any simple biochemical pre-pattern filter to be present in a specific domain of the embryo, for example by repressing APC/GSK3 activity by Wnt expression, or by allowing Src expression, in specific domains of the embryonic tissue, as in previous subsections. As described in previous section, such filtering has possibly emerged spontaneously and progressively, before sperm-egg fusion dependent symmetry breaking arose.
Indeed, in the C.flexa-like opened sphere structure of the pre-metazoan primitive multi-cellular organism (Fig. 6bi-Left), global deformation due to hydrodynamic flow leads to a gastrulation-like complete inversion that per se defines the primitive gut (Fig. 6bi-Right). In this case, there is no need for the establishment of a patterned filter to form the mechanically induced primitive gut. Here the critical amplitude of the passive fluctuations at which Myo-II is activated would be the only constitutive filter of the process.
In the closed metazoan primitive multicellular organism (Fig. 6bii), however, the establishment of a self-induced patterning process is required for local tissue curvature inversion to lead to gastrulation, due to the fact that the closed sphere cannot fully invert itself. Indeed, in response to hydrodynamic stimulation, one can imagine that one of the curvature deformations that is by chance greater than the others activate the Myo-II pathway. As a result, the curvature of this deformation will actively increase, which results in further increasing Myo-II activation and strain. This in turn would further increase the curvature in a non-linear positive feedback loop with instability ultimately leading to a local primitive gastrulation. This should also be true in response to snail-like internal active mechanical fluctuations. However, the lateral mechanical tensions due to invagination in the lateral domains should also activate Myo-II outside the invagination, thus preventing the formation of the invagination into a complete furrow126. A first step in increasing the efficiency of local invagination formation might have been to increase feedback non-linearity by adding another feedback loop. This, for instance, may have occurred by mechanotransductively increasing the expression of the proteins of the mechanosensitive pathway in response to the invagination strains. In turn, this would have increased the efficiency of mechanotransductive feedback within the invagination compared to external tissues. This could possibly have occurred by mechanosensitive transcriptional processes leading to the expression of the proteins involved in Myo-II mechanical activation, for instance in a β-cat dependent manner (Fig. 6bii-Right, see asssociated section). The coupling of the biochemical patterning of the endomesoderm downstream of its biomechanical patterning would then have begun. In this case, the critical amplitudes of the mechanical strain, which activated Myo-II and β-cat in response to non patterned and initiation of invagination induced deformations would have been the only constitutive filter.
Interestingly, mechano-biochemical interplay emerging directly from geometrical shape fluctuations was proposed to be involved in the establishment of the antero-posterior axis of Hydra during regeneration, leading to local stimulation of Wnt3 in a Yap-dependent mechanotransductive process127,128. This added to reaction-diffusion biochemical models independent of mechanics32. On the other hand, spheroids produced at the onset of regeneration have been found to be pre-patterned through a structural inheritance of the actomyosin organization that directs body-axis formation during Hydra regeneration from the adult129, which might have an impact in pre-patterning the location of Wnt3 expression. However, regeneration is a mode of reproduction that is not widely shared, hence not conserved, in different species.
Once self-induced mechanical stimulation ensuring gastrulation in a closed surface (Fig. 6bii, Primitive multicellular organism) was possibly replaced by its pre-patterning after symmetry breaking in the fertilized embryo in the common ancestor of metazoan animals (Fig. 6bii, Urmetazoa) (see associated section). The consequent local activation of downstream transcription factors would have led to the prepatterned expression of the proteins initially involved in the mechanostransductive generation of gastrulation (like the mechanical stimulator Snail and the mechanosensors Twit/Fog in Drosophila embryos) of its Fig. 6bi-Right ancestor. In other words, this would have led to a first pre-patterned filter locally favouring the mechanotransductive response to environmental or internal mechanical strains, that conditions the formation of local gastrulation in a closed multi-cellular system, based on the conservation of the mechanotransductive principles that had led to the gastrulation-like inversion of their opened first multi-cellular Fig. 6bi ancestor.
Therefore, the replacement of self-induced mechano-biochemical morphogenesis and patterning of endomesoderm formation in earliest multi-cellular organisms in response to marine environment hydrodynamic stimulation by the symmetry breaking of egg and sperm fusion induced by sexual reproduction may have led to the pre-pattern of such ancestral mechanosensitive pathways, and/or of internal mechanical deformation producers, in the initiation of first metazoans embryogenesis. Downstream of such primary embryonic patterning, the biochemical patterning of other stages of development, such as the patterned expression of Wnt or Src family kinases, could also serve as a pre-patterned filter for subsequent mechanotransductive processes stimulated by internal active biomechanical morphogenetic processes, like Wnt in adult mice colon.
Discussion
In conclusion, the coexistence and interplay of self-induced and pre-patterned biochemical and biomechanical morphogenetic processes found in today’s embryonic early development (first and second section) might have evolutionarily self-emerged through the mechanotransductive coupling of its biochemical and biomechanical patterning, stimulated and initiated by the mechanical perturbations induced by the mechanical environment (third section). It would have been selected as a multi-cellular primitive behavioural feature by Darwinian pressure having favoured such a gastrulating structure initiated by marine flow, that feed more efficiently than an inert structure86. The formation of such a primitive multi-cellular organism would then have possibly evolved toward formation of first primitive animals and embryogenesis through its pre-patterning after sperm-egg fusion by symmetry breaking, with mechanotransduction processes being still present as conserved processes required for proper gastrulation today (third and fourth section). Such prepatterns could have thus played a role as natural filters, allowing mechanosensing locally only, as seen today in the prepatterned process of endomesoderm specification and gastrulation. While permissive pre-patterning is involved in the mechanotransductive processes involved in adult endoderm structures, other filtering processes, still hypothetical or to be conceptualised, remain to be experimentally tested and discovered (fourth section).
Acknowledgements
The research of the lab related to the article was and is supported by the OCAV grants n° ANR-10-IDEX-0001-02 PSL, the FRM (grant Équipe labellisée FRM 2015 DEQ20150331702, grant Équipe labellisée FRM 2019 EQU201903007805), the Inca (PLBIO-13-172, PLBIO-03-ICR-1, PLBIO 2024-125), the ANR (16-CE14-002801), the Labex Cell(n)Scale (grants ANR-11-LABX-0038, ANR-10-IDEX-0001-02), the ARC (ARCPGA2021120004327_4871) and the INSERM (PCSI-24CP054-00).
Author contributions
Conception: E.F. and M.N. Writing—original draft: E.F. Writing— review and editing: E.F. and M.N.
Peer review
Peer review information
Nature Communications thanks James Glover, Sophie Pantalacci and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Plato. Timée. (358 before JC)
- 2.Aristote. Generation of Animals. II, 22, 523 a 515
- 3.van der Lugt, M. Generation in the Middle Ages. Past, Present, Future. Micrologus’ Libraryhttps://hal.science/hal-04005451 (2023).
- 4.Preformationism, https://en.wikipedia.org/wiki/Preformationism.
- 5.Leduc, S. La Biologie Synythétique. Etude de Biophysique. (1912).
- 6.Thomson, D. A. W. On growth and Form. (1917).
- 7.Spemann, H. & Mangold, H. Induction of embryonic primordia by implantation of organizers from a different species. 1923. Int J. Dev. Biol.45, 13–38 (2001). [PubMed] [Google Scholar]
- 8.Watson, J. D. & Crick, F. H. Molecular structure of nucleic acids; a structure for deoxyribose nucleic acid. Nature171, 737–738 (1953). [DOI] [PubMed] [Google Scholar]
- 9.Turing, A. M. The chemical basis of morphogenesis. 1953. Bull. Math. Biol.52, 153–197 (1990). [DOI] [PubMed] [Google Scholar]
- 10.Lewis, E. B. A gene complex controlling segmentation in Drosophila. Nature276, 565–570 (1978). [DOI] [PubMed] [Google Scholar]
- 11.Nusslein-Volhard, C. & Wieschaus, E. Mutations affecting segment number and polarity in Drosophila. Nature287, 795–801 (1980). [DOI] [PubMed] [Google Scholar]
- 12.Roth, S. The origin of dorsoventral polarity in Drosophila. Philos. Trans. R. Soc. Lond. B Biol. Sci.358, 1317–1329 (2003). discussion 1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.St Johnston, D. & Nusslein-Volhard, C. The origin of pattern and polarity in the Drosophila embryo. Cell68, 201–219 (1992). [DOI] [PubMed] [Google Scholar]
- 14.Lawrence, P. A. The making of a fly. The genetics of animal design. (1992).
- 15.Sanson, B. Generating patterns from fields of cells: Examples from Drosophila segmentation. EMBO Rep.2, 1083–1088 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gilbert, S. F. & Barresi, M. J. F. Developmental Biology. 11th edn, 372 (Sinauer Associates, 2016).
- 17.Kmita, M. & Duboule, D. Organizing axes in time and space; 25 years of colinear tinkering. Science301, 331–333 (2003). [DOI] [PubMed] [Google Scholar]
- 18.Neijts, R. et al. Polarized regulatory landscape and Wnt responsiveness underlie Hox activation in embryos. Genes Dev30, 1937-1942 (2016). [DOI] [PMC free article] [PubMed]
- 19.Rekaik, H. et al. Sequential and directional insulation by conserved CTCF sites underlies the Hox timer in stembryos. Nat. Genet55, 1164–1175 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rekaik, H. & Duboule, D. A CTCF-dependent mechanism underlies the Hox timer: relation to a segmented body plan. Curr. Opin. Genet Dev.85, 102160 (2024). [DOI] [PubMed] [Google Scholar]
- 21.Bardot, E. S. & Hadjantonakis, A. K. Mouse gastrulation: Coordination of tissue patterning, specification and diversification of cell fate. Mech. Dev.163, 103617 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eldar, A. et al. Robustness of the BMP morphogen gradient in Drosophila embryonic patterning. Nature419, 304–308 (2002). [DOI] [PubMed]
- 23.Gierer, A. & Meinhardt, H. A theory of biological pattern formation. Kybernetik12, 30–39 (1972). [DOI] [PubMed] [Google Scholar]
- 24.Green, J. B. & Sharpe, J. Positional information and reaction-diffusion: two big ideas in developmental biology combine. Development142, 1203–1211 (2015). [DOI] [PubMed] [Google Scholar]
- 25.Landge, A. N., Jordan, B. M., Diego, X. & Muller, P. Pattern formation mechanisms of self-organizing reaction-diffusion systems. Dev. Biol.460, 2–11 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu, J., Zhang, Y. T., Alber, M. S. & Newman, S. A. Bare bones pattern formation: a core regulatory network in varying geometries reproduces major features of vertebrate limb development and evolution. PloS one5, e10892 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Raspopovic, J., Marcon, L., Russo, L. & Sharpe, J. Modeling digits. Digit patterning is controlled by a Bmp-Sox9-Wnt Turing network modulated by morphogen gradients. Science345, 566–570 (2014). [DOI] [PubMed] [Google Scholar]
- 28.Hiscock, T. W., Tschopp, P. & Tabin, C. J. On the Formation of Digits and Joints during Limb Development. Dev. Cell41, 459–465 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaelin, C. B., McGowan, K. A. & Barsh, G. S. Developmental genetics of color pattern establishment in cats. Nat. Commun.12, 5127 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sick, S., Reinker, S., Timmer, J. & Schlake, T. WNT and DKK determine hair follicle spacing through a reaction-diffusion mechanism. Science314, 1447–1450 (2006). [DOI] [PubMed] [Google Scholar]
- 31.Glover, J. D. et al. The developmental basis of fingerprint pattern formation and variation. Cell186, 940–956 e920 (2023). [DOI] [PubMed] [Google Scholar]
- 32.Meinhardt, H. Modeling pattern formation in hydra: a route to understanding essential steps in development. Int J. Dev. Biol.56, 447–462 (2012). [DOI] [PubMed] [Google Scholar]
- 33.Young, P. E., Pesacreta, T. C. & Kiehart, D. P. Dynamic changes in the distribution of cytoplasmic myosin during Drosophila embryogenesis. Development111, 1–14 (1991). [DOI] [PubMed] [Google Scholar]
- 34.Dawes-Hoang, R. E. et al. folded gastrulation, cell shape change and the control of myosin localization. Development132, 4165–4178 (2005). [DOI] [PubMed] [Google Scholar]
- 35.Bertet, C., Sulak, L. & Lecuit, T. Myosin-dependent junction remodelling controls planar cell intercalation and axis elongation. Nature429, 667–671 (2004). [DOI] [PubMed] [Google Scholar]
- 36.Costa, M., Sweeton, D. & Wieschaus, E. in The development ofDrosophila Melanogaster”. (eds M. Bate & A. Martinez-Arias) 425-464 (Cold Spring Harbor Laboratory Press, 1993).
- 37.Tamada, M. et al. Toll receptors remodel epithelia by directing planar-polarized Src and PI3K activity. Dev. Cell56, 1589–1602 e1589 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bertet, C. & Lecuit, T. Planar polarity and short-range polarization in Drosophila embryos. Semin Cell Dev. Biol.20, 1006–1013 (2009). [DOI] [PubMed] [Google Scholar]
- 39.Pare, A. C. et al. A positional Toll receptor code directs convergent extension in Drosophila. Nature515, 523–527 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Keller, R. et al. Mechanisms of convergence and extension by cell intercalation. Philos. Trans. R. Soc. Lond. B Biol. Sci.355, 897–922 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shindo, A. & Wallingford, J. B. PCP and septins compartmentalize cortical actomyosin to direct collective cell movement. Science343, 649–652 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sepich, D. S., Usmani, M., Pawlicki, S. & Solnica-Krezel, L. Wnt/PCP signaling controls intracellular position of MTOCs during gastrulation convergence and extension movements. Development138, 543–552 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Heisenberg, C. P. et al. Silberblick/Wnt11 mediates convergent extension movements during zebrafish gastrulation. Nature405, 76–81 (2000). [DOI] [PubMed] [Google Scholar]
- 44.Ulrich, F. et al. Slb/Wnt11 controls hypoblast cell migration and morphogenesis at the onset of zebrafish gastrulation. Development130, 5375–5384 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chuai, M. & Weijer, C. J. Regulation of cell migration during chick gastrulation. Curr. Opin. Genet Dev.19, 343–349 (2009). [DOI] [PubMed] [Google Scholar]
- 46.Saadaoui, M., Rocancourt, D., Roussel, J., Corson, F. & Gros, J. A tensile ring drives tissue flows to shape the gastrulating amniote embryo. Science367, 453–458 (2020). [DOI] [PubMed] [Google Scholar]
- 47.Veschgini, M. et al. Wnt/beta-catenin signaling induces axial elasticity patterns of Hydra extracellular matrix. iScience26, 106416 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fleury, V. & Abourachid, A. A biaxial tensional model for early vertebrate morphogenesis. Eur. Phys. J. E, Soft matter45, 31 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Farge, E. Mechanical induction of Twist in the Drosophila foregut/stomodeal primordium. Curr. Biol.: CB13, 1365–1377 (2003). [DOI] [PubMed] [Google Scholar]
- 50.Shorr, A. Z., Sonmez, U. M., Minden, J. S. & LeDuc, P. R. High-throughput mechanotransduction in Drosophila embryos with mesofluidics. Lab on a chip10.1039/c8lc01055b (2019). [DOI] [PubMed]
- 51.Desprat, N., Supatto, W., Pouille, P. A., Beaurepaire, E. & Farge, E. Tissue deformation modulates twist expression to determine anterior midgut differentiation in Drosophila embryos. Dev. Cell15, 470–477 (2008). [DOI] [PubMed] [Google Scholar]
- 52.Kumar, A. & Shivashankar, G. V. Mechanical force alters morphogenetic movements and segmental gene expression patterns during Drosophila embryogenesis. PloS one7, e33089 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brunet, T. et al. Evolutionary conservation of early mesoderm specification by mechanotransduction in Bilateria. Nat. Commun.4, 2821 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Roth, S., Stein, D. & Nusslein-Volhard, C. A gradient of nuclear localization of the dorsal protein determines dorsoventral pattern in the Drosophila embryo. Cell59, 1189–1202 (1989). [DOI] [PubMed] [Google Scholar]
- 55.Shyer, A. E. et al. Emergent cellular self-organization and mechanosensation initiate follicle pattern in the avian skin. Science357, 811–815 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Painter, K. J., Ho, W. & Headon, D. J. A chemotaxis model of feather primordia pattern formation during avian development. J. Theor. Biol.437, 225–238 (2018). [DOI] [PubMed] [Google Scholar]
- 57.Ho, W. K. W. et al. Feather arrays are patterned by interacting signalling and cell density waves. PLoS Biol.17, e3000132 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Maitre, J. L. et al. Asymmetric division of contractile domains couples cell positioning and fate specification. Nature536, 344–348 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Maitre, J. L., Niwayama, R., Turlier, H., Nedelec, F. & Hiiragi, T. Pulsatile cell-autonomous contractility drives compaction in the mouse embryo. Nat. Cell Biol.17, 849–855 (2015). [DOI] [PubMed] [Google Scholar]
- 60.Hiramatsu, R. et al. External mechanical cues trigger the establishment of the anterior-posterior axis in early mouse embryos. Dev. Cell27, 131–144 (2013). [DOI] [PubMed] [Google Scholar]
- 61.Hubaud, A., Regev, I., Mahadevan, L. & Pourquie, O. Excitable Dynamics and Yap-Dependent Mechanical Cues Drive the Segmentation Clock. Cell171, 668–682 e611 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xia, P., Gutl, D., Zheden, V. & Heisenberg, C. P. Lateral Inhibition in Cell Specification Mediated by Mechanical Signals Modulating TAZ Activity. Cell176, 1379–1392 e1314 (2019). [DOI] [PubMed] [Google Scholar]
- 63.Alasaadi, D. N. et al. Competence for neural crest induction is controlled by hydrostatic pressure through Yap. Nat. Cell Biol.26, 530–541 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Monier, B., Pelissier-Monier, A., Brand, A. H. & Sanson, B. An actomyosin-based barrier inhibits cell mixing at compartmental boundaries in Drosophila embryos. Nat. Cell Biol.12, 60–69 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Landsberg, K. P. et al. Increased cell bond tension governs cell sorting at the Drosophila anteroposterior compartment boundary. Curr. Biol.19, 1950–1955 (2009). [DOI] [PubMed] [Google Scholar]
- 66.Rasoulinejad, S., Mueller, M., Nzigou Mombo, B. & Wegner, S. V. Orthogonal Blue and Red Light Controlled Cell-Cell Adhesions Enable Sorting-out in Multicellular Structures. ACS Synth. Biol.9, 2076–2086 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Steinberg, M. S. Does differential adhesion govern self-assembly processes in histogenesis? Equilibrium configurations and the emergence of a hierarchy among populations of embryonic cells. J. Exp. Zool.173, 395–433 (1970). [DOI] [PubMed] [Google Scholar]
- 68.Godard, B. G. et al. Apical Relaxation during Mitotic Rounding Promotes Tension-Oriented Cell Division. Dev. Cell55, 695–706 e694 (2020). [DOI] [PubMed] [Google Scholar]
- 69.Guignard, L. et al. Contact area-dependent cell communication and the morphological invariance of ascidian embryogenesis. Science369,10.1126/science.aar5663 (2020). [DOI] [PubMed]
- 70.Robert, A. Sur le développement des Troques PhD thesis, Faculté des Sciences de l’Université de PAris, (1903).
- 71.Martin, A. C., Kaschube, M. & Wieschaus, E. F. Pulsed contractions of an actin-myosin network drive apical constriction. Nature457, 495–499 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mitrossilis, D. et al. Mechanotransductive cascade of Myo-II-dependent mesoderm and endoderm invaginations in embryo gastrulation. Nat. Commun.8, 13883 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pouille, P. A., Ahmadi, P., Brunet, A. C. & Farge, E. Mechanical Signals Trigger Myosin II Redistribution and Mesoderm Invagination in Drosophila Embryos. Sci. Signal.2, ra16 (2009). [DOI] [PubMed] [Google Scholar]
- 74.Kerridge, S. et al. Modular activation of Rho1 by GPCR signalling imparts polarized myosin II activation during morphogenesis. Nat. Cell Biol.18, 261–270 (2016). [DOI] [PubMed] [Google Scholar]
- 75.Bailles, A. et al. Genetic induction and mechanochemical propagation of a morphogenetic wave. Nature572, 467–473 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Odell, G. M., Oster, G., Alberch, P. & Burnside, B. The mechanical basis of morphogenesis. I. Epithelial folding and invagination. Dev. Biol.85, 446–462 (1981). [DOI] [PubMed] [Google Scholar]
- 77.Popkova, A. et al. A mechanical wave travels along a genetic guide to drive the formation of an epithelial furrow during Drosophila gastrulation. Dev. Cell59, 400–414 e405 (2024). [DOI] [PubMed] [Google Scholar]
- 78.Monier, B. et al. Apico-basal forces exerted by apoptotic cells drive epithelium folding. Nature518, 245–248 (2015). [DOI] [PubMed] [Google Scholar]
- 79.Sknepnek, R., Djafer-Cherif, I., Chuai, M., Weijer, C. & Henkes, S. Generating active T1 transitions through mechanochemical feedback. eLife12,10.7554/eLife.79862 (2023). [DOI] [PMC free article] [PubMed]
- 80.Panchen, A. L. Etienne Geoffroy St.-Hilaire: father of “evo-devo”? Evol. Dev.3, 41–46 (2001). [DOI] [PubMed] [Google Scholar]
- 81.De Robertis, E. M. Evo-devo: variations on ancestral themes. Cell132, 185–195 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.De Robertis, E. & Sasai, Y. A common plan for dorsoventral patterning in bilateralia. Nature380, 37–40 (1996). [DOI] [PubMed] [Google Scholar]
- 83.Heger, P., Zheng, W., Rottmann, A., Panfilio, K. A. & Wiehe, T. The genetic factors of bilaterian evolution. eLife9, 10.7554/eLife.45530 (2020). [DOI] [PMC free article] [PubMed]
- 84.Haeckel, E. Die Gastraea-Theorie, die phylogenetische Classification des Thierreichs und die Homologie der Keimblätter. Jena. Z. Nat.8, 1–55 (1874). [Google Scholar]
- 85.Arendt, D. in Gastrulation (eds C. D. Stern) Ch. 51, 679-693 (University College London, 2004).
- 86.Nguyen, N. M. et al. Mechano-biochemical marine stimulation of inversion, gastrulation, and endomesoderm specification in multicellular Eukaryota. Front Cell Dev. Biol.10, 992371 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Schneider, S. Q. & Bowerman, B. Animal development: an ancient beta-catenin switch? Curr. Biol.23, R313–R315 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Martindale, M. Q. The evolution of metazoan axial properties. Nat. Rev. Genet6, 917–927 (2005). [DOI] [PubMed] [Google Scholar]
- 89.Reyes-Rivera, J. et al. Nitric oxide signaling controls collective contractions in a colonial choanoflagellate. Curr. Biol.32, 2539–2547 e2535 (2022). [DOI] [PubMed] [Google Scholar]
- 90.Nguyen, N. M. et al. Evolutionary Emergence of First Animal Organisms Triggered by Environmental Mechano-Biochemical Marine Stimulation. bioRxiv12, 407668 (2020). [Google Scholar]
- 91.dos Reis, M. et al. Uncertainty in the Timing of Origin of Animals and the Limits of Precision in Molecular Timescales. Curr. Biol.25, 2939–2950 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Muncie, J. M. et al. Mechanical Tension Promotes Formation of Gastrulation-like Nodes and Patterns Mesoderm Specification in Human Embryonic Stem Cells. Dev. Cell55, 679–694 e611 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Stronati, E. et al. YAP1 regulates the self-organized fate patterning of hESC-derived gastruloids. Stem Cell Rep.17, 211–220 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Roper, J. C. et al. The major beta-catenin/E-cadherin junctional binding site is a primary molecular mechano-transductor of differentiation in vivo. eLife7,10.7554/eLife.33381 (2018). [DOI] [PMC free article] [PubMed]
- 95.Servetnick, M. D. et al. Cas9-mediated excision of Nematostella brachyury disrupts endoderm development, pharynx formation and oral-aboral patterning. Development144, 2951–2960 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Steinmetz, P. R. H., Aman, A., Kraus, J. E. M. & Technau, U. Gut-like ectodermal tissue in a sea anemone challenges germ layer homology. Nat. Ecol. Evol.1, 1535–1542 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pukhlyakova, E., Aman, A. J., Elsayad, K. & Technau, U. beta-Catenin-dependent mechanotransduction dates back to the common ancestor of Cnidaria and Bilateria. Proc. Natl. Acad. Sci. USA10.1073/pnas.1713682115 (2018). [DOI] [PMC free article] [PubMed]
- 98.Chang, P., Perez-Mongiovi, D. & Houliston, E. Organisation of Xenopus oocyte and egg cortices. Microsc Res Tech.44, 415–429 (1999). [DOI] [PubMed] [Google Scholar]
- 99.Gouge, R. C., Marshburn, P., Gordon, B. E., Nunley, W. & Huet-Hudson, Y. M. Nitric oxide as a regulator of embryonic development. Biol. Reprod.58, 875–879 (1998). [DOI] [PubMed] [Google Scholar]
- 100.Siamwala, J. H. et al. Nitric Oxide Reverses the Position of the Heart during Embryonic Development. Int. J. Mol. Sci.2010.3390/ijms20051157 (2019). [DOI] [PMC free article] [PubMed]
- 101.Fernandez-Sanchez, M. E., Brunet, T., Roper, J. C. & Farge, E. Mechanotransduction’s impact on animal development, evolution, and tumorigenesis. Annu Rev. Cell Dev. Biol.31, 373–397 (2015). [DOI] [PubMed] [Google Scholar]
- 102.Miller, P. W., Clarke, D. N., Weis, W. I., Lowe, C. J. & Nelson, W. J. The evolutionary origin of epithelial cell-cell adhesion mechanisms. Curr. Top. Membr.72, 267–311 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Fernandez-Sanchez, M. E. et al. Mechanical induction of the tumorigenic beta-catenin pathway by tumour growth pressure. Nature523, 92–95 (2015). [DOI] [PubMed] [Google Scholar]
- 104.Benham-Pyle, B. W., Pruitt, B. L. & Nelson, W. J. Cell adhesion. Mechanical strain induces E-cadherin-dependent Yap1 and beta-catenin activation to drive cell cycle entry. Science348, 1024–1027 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Nguyen Ho-Bouldoires, T. H. et al. Ret kinase-mediated mechanical induction of colon stem cells by tumor growth pressure stimulates cancer progression in vivo. Commun. Biol.5, 137 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hayashi, S. et al. A Drosophila homolog of the tumor suppressor gene adenomatous polyposis coli down-regulates beta-catenin but its zygotic expression is not essential for the regulation of Armadillo. Proc. Natl Acad. Sci. USA94, 242–247 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Adamcikova, Z. et al. Study of the APC gene function in the mouse APC+/APC1638N model. Neuro Endocrinol. Lett.33, 26–33 (2012). [PubMed] [Google Scholar]
- 108.Reya, T. & Clevers, H. Wnt signalling in stem cells and cancer. Nature434, 843–850 (2005). [DOI] [PubMed] [Google Scholar]
- 109.Porazinski, S. et al. YAP is essential for tissue tension to ensure vertebrate 3D body shape. Nature521, 217–221 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sousa-Ortega, A. et al. A Yap-dependent mechanoregulatory program sustains cell migration for embryo axis assembly. Nat. Commun.14, 2804 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Cheng, C. et al. Yap controls notochord formation and neural tube patterning by integrating mechanotransduction with FoxA2 and Shh expression. Sci. Adv.9, eadf6927 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Fernandez-Gonzalez, R., Simoes Sde, M., Roper, J. C., Eaton, S. & Zallen, J. A. Myosin II dynamics are regulated by tension in intercalating cells. Dev. Cell17, 736–743 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Paolini, A., Fontana, F., Pham, V. C., Rodel, C. J. & Abdelilah-Seyfried, S. Mechanosensitive Notch-Dll4 and Klf2-Wnt9 signaling pathways intersect in guiding valvulogenesis in zebrafish. Cell Rep.37, 109782 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Poduri, A. et al. Endothelial cells respond to the direction of mechanical stimuli through SMAD signaling to regulate coronary artery size. Development144, 3241–3252 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Maeda, T. et al. Conversion of mechanical force into TGF-beta-mediated biochemical signals. Curr. Biol.21, 933–941 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Vincent, T. L., McLean, C. J., Full, L. E., Peston, D. & Saklatvala, J. FGF-2 is bound to perlecan in the pericellular matrix of articular cartilage, where it acts as a chondrocyte mechanotransducer. Osteoarthr. Cartil. / OARS, Osteoarthr. Res. Soc.15, 752–763 (2007). [DOI] [PubMed] [Google Scholar]
- 117.Kojic, N. et al. An EGFR autocrine loop encodes a slow-reacting but dominant mode of mechanotransduction in a polarized epithelium. FASEB J.24, 1604–1615 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ghuloum, F. I., Johnson, C. A., Riobo-Del Galdo, N. A. & Amer, M. H. From mesenchymal niches to engineered in vitro model systems: Exploring and exploiting biomechanical regulation of vertebrate hedgehog signalling. Mater. Today Bio17, 100502 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sawada, Y. et al. Force sensing by mechanical extension of the Src family kinase substrate p130Cas. Cell127, 1015–1026 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.del Rio, A. et al. Stretching single talin rod molecules activates vinculin binding. Science323, 638–641 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kumar, A. et al. Talin tension sensor reveals novel features of focal adhesion force transmission and mechanosensitivity. J. Cell Biol.213, 371–383 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Elosegui-Artola, A. et al. Mechanical regulation of a molecular clutch defines force transmission and transduction in response to matrix rigidity. Nat. Cell Biol.18, 540–548 (2016). [DOI] [PubMed] [Google Scholar]
- 123.Poudel, B., Rajeshwar, T. R. & Vanegas, J. M. Membrane mediated mechanical stimuli produces distinct active-like states in the AT1 receptor. Nat. Commun.14, 4690 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Alon, U. An introduction to systems biology. (Chapman & Hall / CRC, 2007).
- 125.Batlle, E. et al. The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nat. Cell Biol.2, 84–89 (2000). [DOI] [PubMed] [Google Scholar]
- 126.Morize, P., Christiansen, A. E., Costa, M., Parks, S. & Wieschaus, E. Hyperactivation of the folded gastrulation pathway induces specific cell shape changes. Development125, 589–597 (1998). [DOI] [PubMed] [Google Scholar]
- 127.Ferenc, J. et al. Mechanical oscillations orchestrate axial patterning through Wnt activation in Hydra. Sci. Adv.7, eabj6897 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Suzuki, R. & al., e. Spatio-temporal Coordination of Active Deformation Forces and Wnt / Hippo-Yap Signaling in Hydra Regeneration. BioRXiv10.1101/2023.09.18.558226 (2023).
- 129.Braun, E. & Keren, K. Hydra Regeneration: Closing the Loop with Mechanical Processes in Morphogenesis. BioEssays: N. Rev. Mol., Cell. Dev. Biol.40, e1700204 (2018). [DOI] [PubMed] [Google Scholar]
- 130.Manning, A. J., Peters, K. A., Peifer, M. & Rogers, S. L. Regulation of epithelial morphogenesis by the G protein-coupled receptor mist and its ligand fog. Sci. Signal.6, ra98 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zusman, S. B. & Wieschaus, E. F. Requirements for zygotic gene activity during gastrulation in Drosophila melanogaster. Dev. Biol.111, 359–371 (1985). [DOI] [PubMed] [Google Scholar]
- 132.Horo, U., Clarke, D. N. & Martin, A. C. Drosophila Fog/Cta and T48 pathways have overlapping and distinct contributions to mesoderm invagination. Mol. Biol. Cell, mbcE24020050 (2024). [DOI] [PMC free article] [PubMed]
- 133.Sweeton, D., Parks, S., Costa, M. & Wieschaus, E. Gastrulation in Drosophila: the formation of the ventral furrow and posterior midgut invaginations. Development112, 775–789 (1991). [DOI] [PubMed] [Google Scholar]
- 134.Costa, M., Wilson, E. T. & Wieschaus, E. A putative cell signal encoded by the folded gastrulation gene coordinates cell shape changes during Drosophila gastrulation. Cell76, 1075–1089 (1994). [DOI] [PubMed] [Google Scholar]
- 135.Kolsch, V., Seher, T., Fernandez-Ballester, G. J., Serrano, L. & Leptin, M. Control of Drosophila gastrulation by apical localization of adherens junctions and RhoGEF2. Science315, 384–386 (2007). [DOI] [PubMed] [Google Scholar]
- 136.Brunet, T. et al. Light-regulated collective contractility in a multicellular choanoflagellate. Science366, 326–334 (2019). [DOI] [PubMed] [Google Scholar]
- 137.Benton, M. A. et al. Fog signaling has diverse roles in epithelial morphogenesis in insects. eLife8,10.7554/eLife.47346 (2019). [DOI] [PMC free article] [PubMed]