Abstract
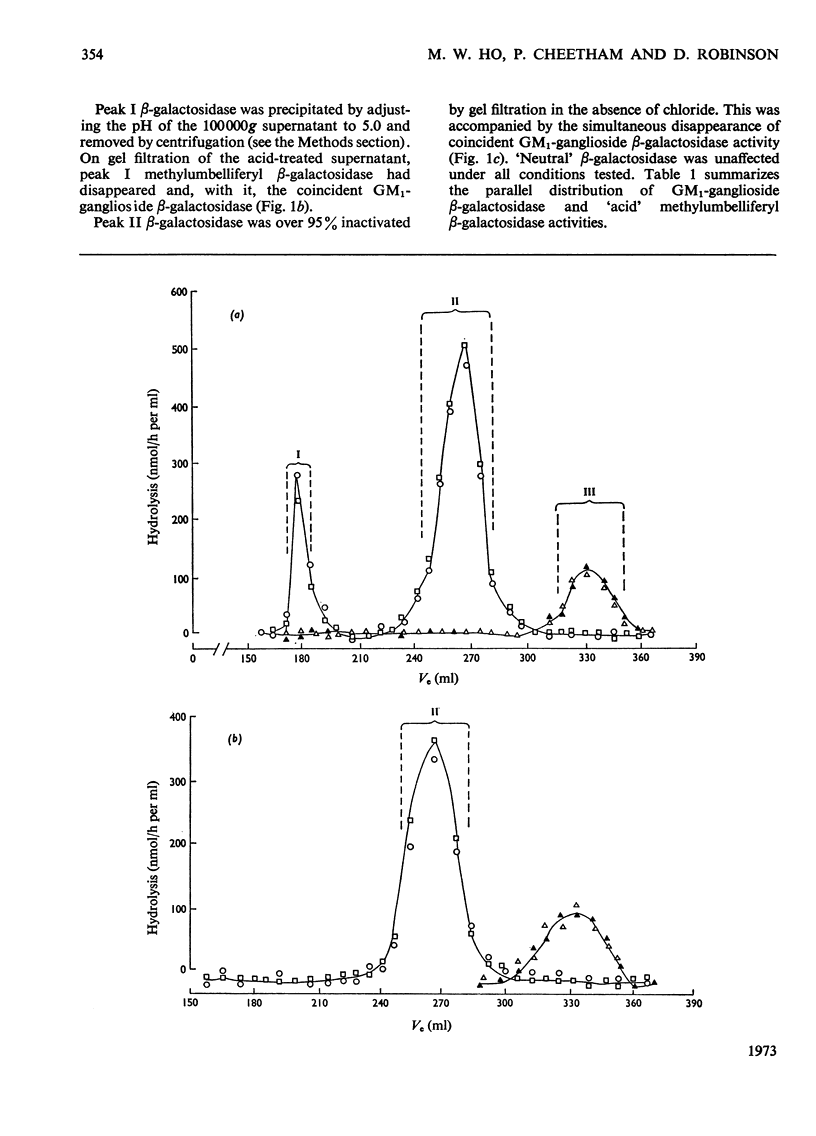
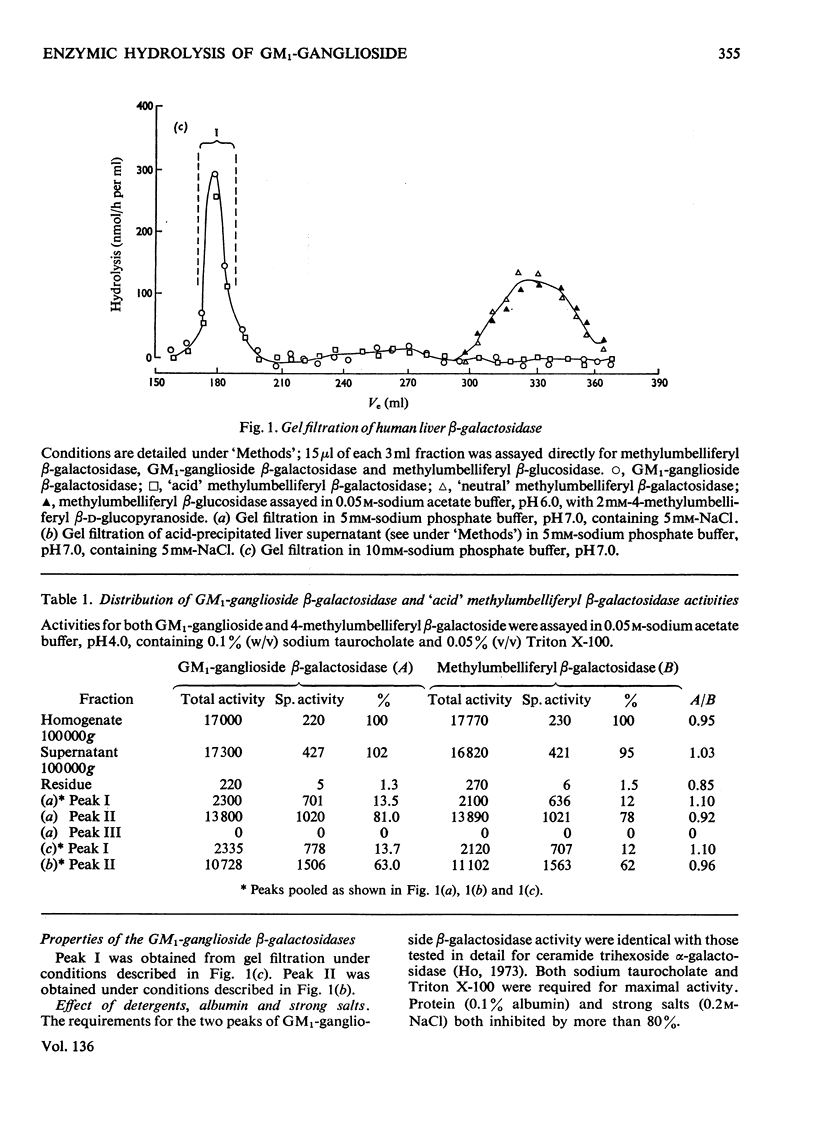
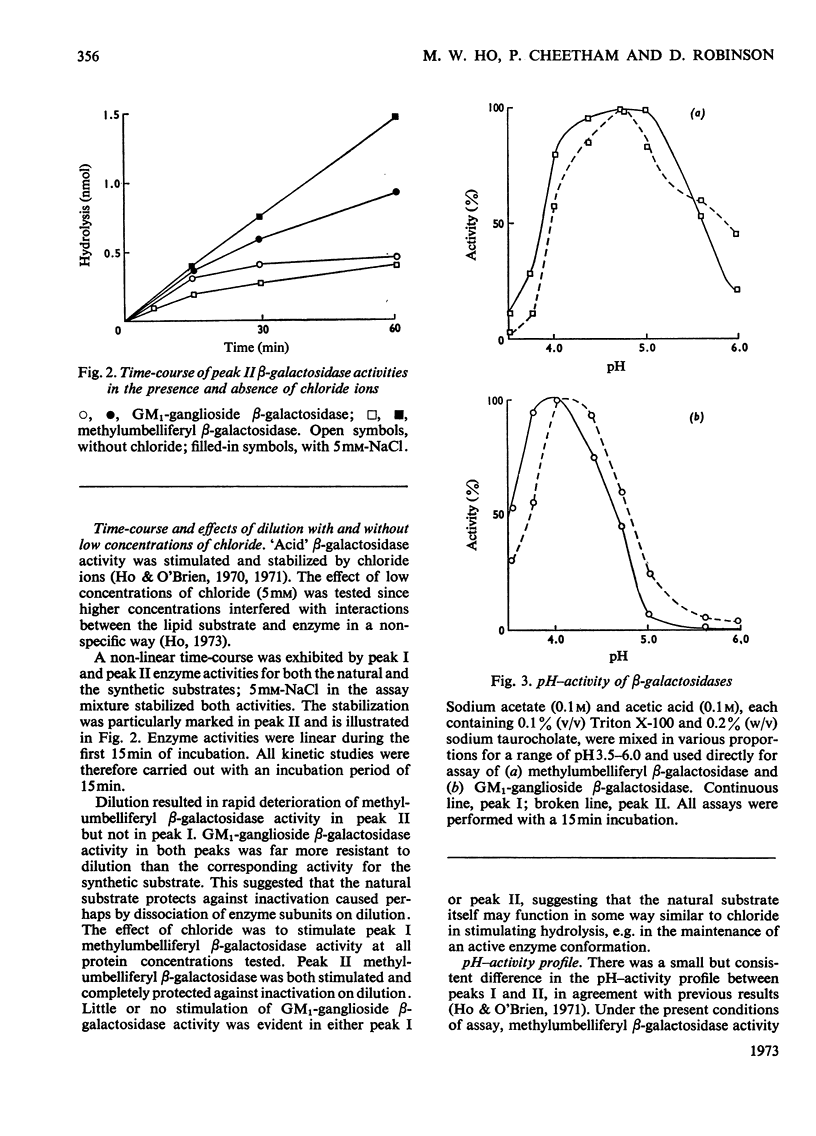
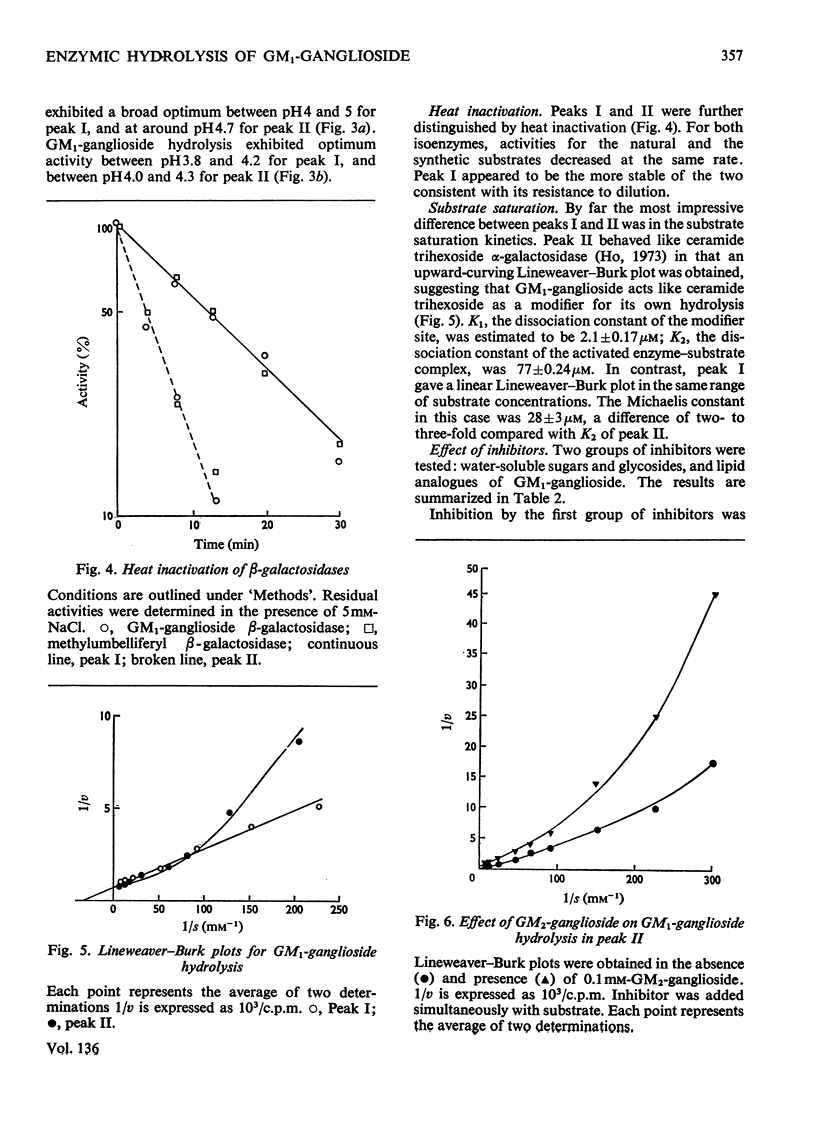
1. GM1-ganglioside, specifically tritiated in the terminal galactose, was hydrolysed by two forms of `acid' methylumbelliferyl β-galactosidase isolated on gel filtration. 2. Identification of GM1-ganglioside β-galactosidase activity with the `acid' methyl-umbelliferyl β-galactosidases was based on the following: coincident elution profiles on gel filtration; simultaneous inactivation by heat and other treatments; stabilization of both activities by chloride ions; mutual inhibition of hydrolysis by the two substrates. 3. The two isoenzymes (I) and (II) showed general requirements for a mixture of anionic and nonionic detergents in the hydrolysis of the natural substrate. 4. Isoenzyme (I) differed from (II) in molecular size, pH–activity profile, relative resistance to dilution and in sensitivity to various inhibitors. 5. The most significant difference between the isoenzymes is in substrate saturation kinetics: (I) was hyperbolic whereas (II) was sigmoid. The apparent Michaelis constants were 28μm for (I) and 77μm for (II). Isoenzyme (I) was insensitive to GM2-ganglioside whereas (II) was inhibited, consistent with the hypothesis that GM1-ganglioside (and its analogue) acts as modifier in isoenzyme (II) but not in (I). 6. Isoenzyme (I) was membrane-bound whereas (II) was soluble; the former probably represents isoenzyme (II) bound to membrane components, thereby becoming activated. 7. Membranes may serve a dual role in enzyme catalysis involving lipids: as a medium where both enzyme and substrate are effectively concentrated, and as actual activator of enzymes through binding of the latter to specific membrane components.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alpers D. H. Separation and isolation of rat and human intestinal beta-galactosidases. J Biol Chem. 1969 Mar 10;244(5):1238–1246. [PubMed] [Google Scholar]
- Asp N. G., Dahlqvist A. Rat small-intestinal beta-galactosidases. Kinetic studies with three separated fractions. Biochem J. 1968 Nov;110(1):143–150. doi: 10.1042/bj1100143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen D. M., Radin N. S. Properties of cerebroside galactosidase. Biochim Biophys Acta. 1968 May 1;152(3):599–610. doi: 10.1016/0005-2760(68)90100-8. [DOI] [PubMed] [Google Scholar]
- Brady R. O., O'Brien J. S., Bradley R. M., Gal A. E. Sphingolipid hydrolases in brain tissue of patients with generalized gangliodosis. Biochim Biophys Acta. 1970 Jun 9;210(1):193–195. doi: 10.1016/0005-2760(70)90079-2. [DOI] [PubMed] [Google Scholar]
- CLELAND W. W. Computer programmes for processing enzyme kinetic data. Nature. 1963 May 4;198:463–465. doi: 10.1038/198463a0. [DOI] [PubMed] [Google Scholar]
- Dawson G., Stein A. O. Lactosyl ceramidosis: catabolic enzyme defect of glycosphingolipid metabolism. Science. 1970 Oct 30;170(3957):556–558. doi: 10.1126/science.170.3957.556. [DOI] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- FRIEDEN C. TREATMENT OF ENZYME KINETIC DATA. I. THE EFFECT OF MODIFIERS ON THE KINETIC PARAMETERS OF SINGLE SUBSTRATE ENZYMERS. J Biol Chem. 1964 Oct;239:3522–3531. [PubMed] [Google Scholar]
- Furth A. J., Robinson D. Specificity and multiple forms of beta-galactosidase in the rat. Biochem J. 1965 Oct;97(1):59–66. doi: 10.1042/bj0970059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatt S. Enzymatic hydrolysis of sphingolipids. V. Hydrolysis of monosialoganglioside and hexosylceramides by rat brain beta-galactosidase. Biochim Biophys Acta. 1967 Feb 14;137(1):192–195. doi: 10.1016/0005-2760(67)90027-6. [DOI] [PubMed] [Google Scholar]
- Gatt S., Rapport M. M. Enzymic hydrolysis of sphingolipids: Hydrolysis of ceramide lactoside by an enzyme from rat brain. Biochem J. 1966 Dec;101(3):680–686. [PMC free article] [PubMed] [Google Scholar]
- Gray G. M., Santiago N. A. Intestinal beta-galactosidases. I. Separation and characterization of three enzymes in normal human intestine. J Clin Invest. 1969 Apr;48(4):716–728. doi: 10.1172/JCI106029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho M. W. Hydrolysis of ceramide trihexoside by a specific -galactosidase from human liver. Biochem J. 1973 May;133(1):1–10. doi: 10.1042/bj1330001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho M. W., O'Brien J. S. Differential effect of chloride ions on -galactosidase isoenzymes: a method for separate assay. Clin Chim Acta. 1971 May;32(3):443–450. doi: 10.1016/0009-8981(71)90446-3. [DOI] [PubMed] [Google Scholar]
- Ho M. W., O'Brien J. S., Radin N. S., Erickson J. S. Glucocerebrosidase: reconstitution of activity from macromolecular components. Biochem J. 1973 Jan;131(1):173–176. doi: 10.1042/bj1310173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho M. W., O'Brien J. S. Stimulation of acid beta-galactosidase activity by chloride ions. Clin Chim Acta. 1970 Nov;30(2):531–534. doi: 10.1016/0009-8981(70)90147-6. [DOI] [PubMed] [Google Scholar]
- Jungalwala F. B., Robins E. Glycosidases in the nervous system. 3. Separation, purification, and substrate specificities of beta-galactosidases and beta-glucuronidase from brain. J Biol Chem. 1968 Aug 25;243(16):4258–4266. [PubMed] [Google Scholar]
- MacBrinn M. C., Okada S., Ho M. W., Hu C. C., O'Brien J. S. Generalized gangliosidosis: impaired cleavage of galactose from a mucopolysaccharide and a glycoprotien. Science. 1969 Feb 28;163(3870):946–947. doi: 10.1126/science.163.3870.946. [DOI] [PubMed] [Google Scholar]
- Mahadevan S., Dillard C. J., Tappel A. L. Degradation of polysaccharides, mucopolysaccharides, and glycoproteins by lysosomal glycosidases. Arch Biochem Biophys. 1969 Feb;129(2):525–533. doi: 10.1016/0003-9861(69)90210-0. [DOI] [PubMed] [Google Scholar]
- Okada S., O'Brien J. S. Generalized gangliosidosis: beta-galactosidase deficiency. Science. 1968 May 31;160(3831):1002–1004. doi: 10.1126/science.160.3831.1002. [DOI] [PubMed] [Google Scholar]
- Radin N. S., Hof L., Bradley R. M., Brady R. O. Lactosylceramide galactosidase: comparison with other sphingolipid hydrolases in developing rat brain. Brain Res. 1969 Jul;14(2):497–505. doi: 10.1016/0006-8993(69)90124-3. [DOI] [PubMed] [Google Scholar]
- SVENNERHOLM L. CHROMATOGRAPHIC SEPARATION OF HUMAN BRAIN GANGLIOSIDES. J Neurochem. 1963 Sep;10:613–623. doi: 10.1111/j.1471-4159.1963.tb08933.x. [DOI] [PubMed] [Google Scholar]
- SVENNERHOLM L. Quantitative estimation of sialic acids. II. A colorimetric resorcinol-hydrochloric acid method. Biochim Biophys Acta. 1957 Jun;24(3):604–611. doi: 10.1016/0006-3002(57)90254-8. [DOI] [PubMed] [Google Scholar]
- Suzuki K., Suzuki Y. Globoid cell leucodystrophy (Krabbe's disease): deficiency of galactocerebroside beta-galactosidase. Proc Natl Acad Sci U S A. 1970 Jun;66(2):302–309. doi: 10.1073/pnas.66.2.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenger D. A., Wardell S. Action of neuraminidase from Clostridium perfringens of Tay-Sachs ganglioside. Physiol Chem Phys. 1972;4(3):224–230. [PubMed] [Google Scholar]


