Abstract
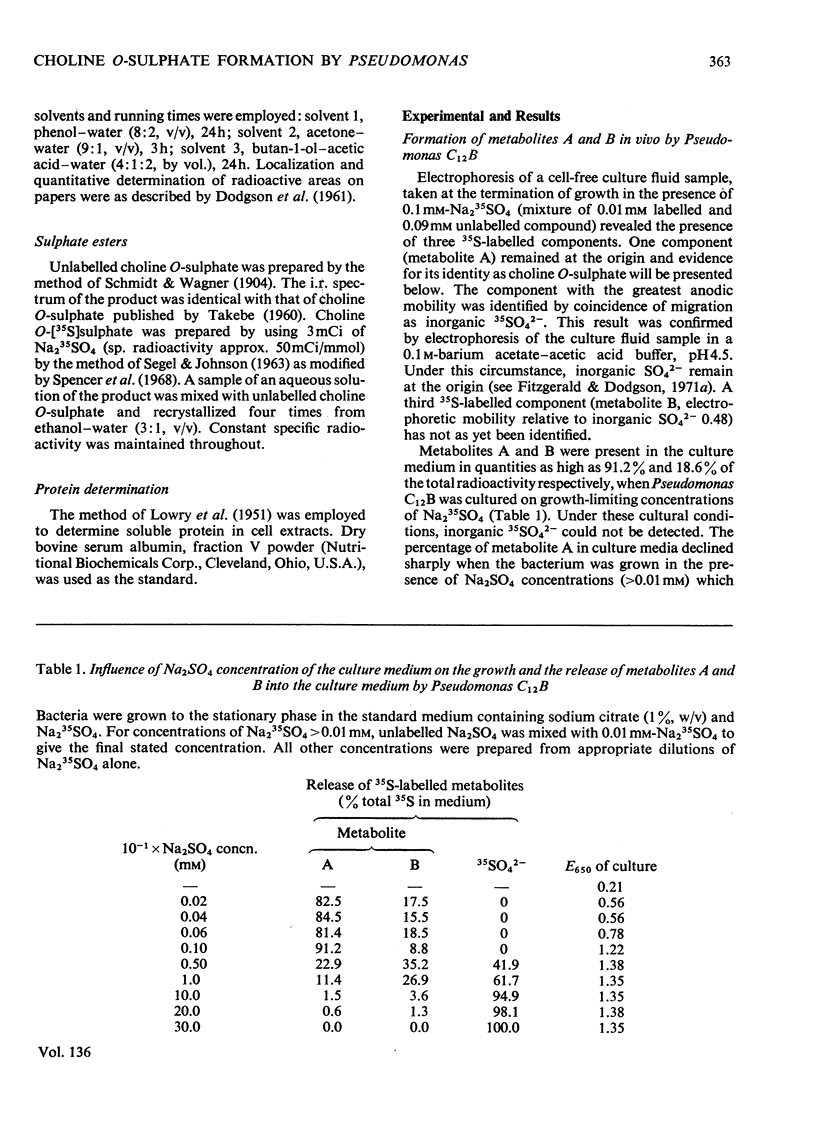
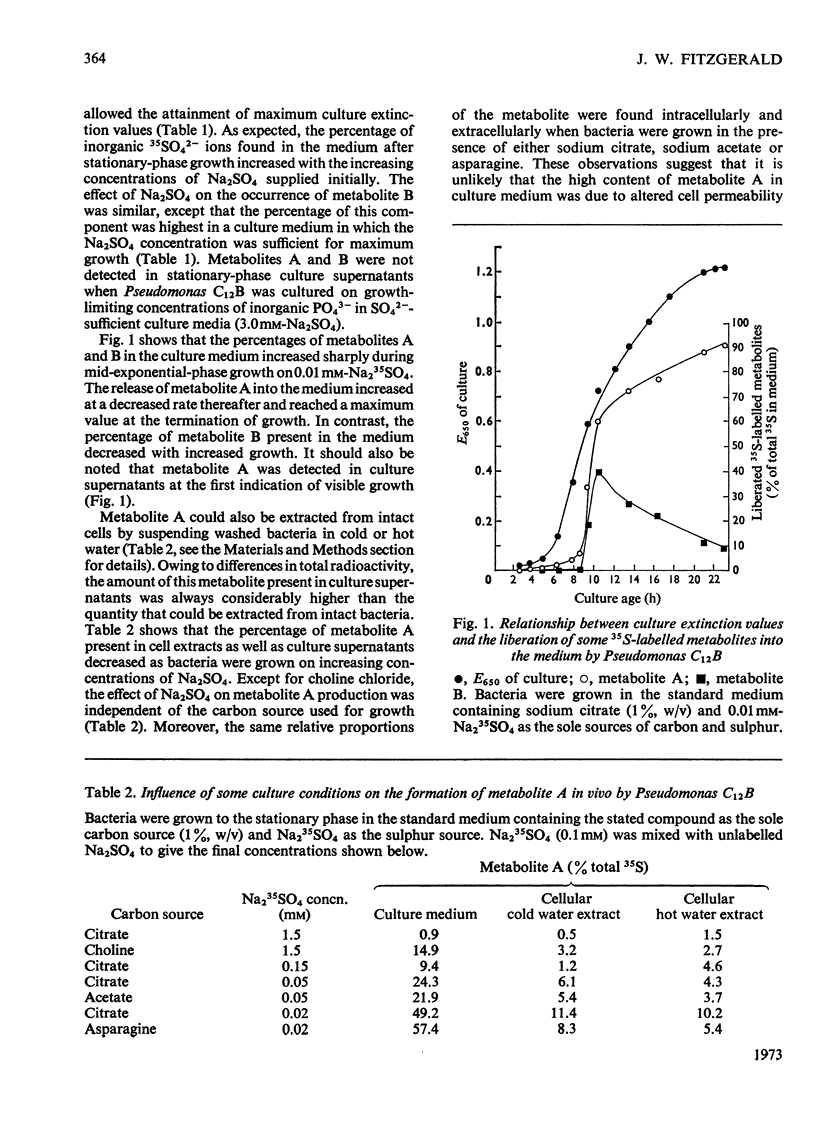
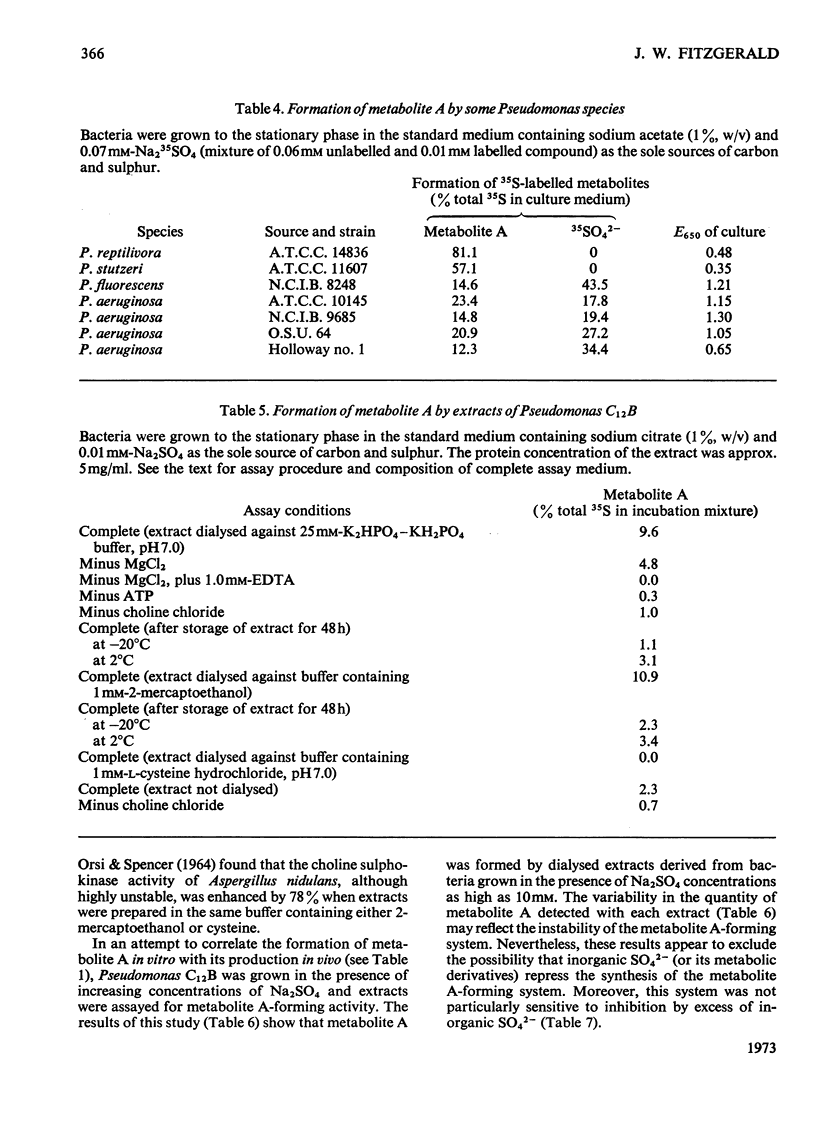
Pseudomonas C12B and other Pseudomonas species released larger amounts of a 35S-labelled metabolite into the medium when cultured on growth-limiting concentrations of Na2SO4 as opposed to growth in SO42−-sufficient media. The metabolite was found at all stages of the culture cycle of Pseudomonas C12B and maximum quantities occurred in stationary-phase culture supernatants. The metabolite was not detected when the bacterium was cultured on growth-limiting concentrations of potassium phosphate. The amount of the metabolite present in the medium greatly exceeded that which could be extracted from intact cells and, except for choline chloride, it was independent of the carbon source used for growth. If choline chloride was present in high concentration, then larger amounts of the metabolite were found in the culture medium. The metabolite was not detected extracellularly or intracellularly when the bacterium was grown in SO42−-deficient media containing 5mm-l-cysteine. The same metabolite was also synthesized in vitro only when Pseudomonas C12B extracts were incubated with choline chloride, ATP, MgCl2 and Na235SO4. The metabolite-forming system was not subject to repression by Na2SO4 and was completely inhibited by 0.5mm-l-cysteine and activated by Na2SO4 (up to 1.0mm). The metabolite was identified as choline O-sulphate by electrophoresis, chromatography and isotope-dilution analysis. Another 35S-labelled metabolite was also detected in culture supernatants, but was not identified.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DODGSON K. S. Determination of inorganic sulphate in studies on the enzymic and non-enzymic hydrolysis of carbohydrate and other sulphate esters. Biochem J. 1961 Feb;78:312–319. doi: 10.1042/bj0780312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DODGSON K. S., LLOYD A. G., TUDBALL N. O-sulphate esters of L-serine, L-threonine and L-hydroxyproline. Biochem J. 1961 Apr;79:111–117. doi: 10.1042/bj0790111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald J. W., Dodgson K. S. Carbon and sulphur utilization during growth of Pseudomonas fluorescens on potassium D-glucose 6-O-sulphate as the sole sulphur source. Biochem J. 1971 Apr;122(3):277–283. doi: 10.1042/bj1220277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald J. W., Dodgson K. S. Sulphur utilization during growth of pseudomonas fluorescens on potassium D-glucose 6-O-sulphate. Biochem J. 1971 Feb;121(3):521–528. doi: 10.1042/bj1210521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald J. W., Payne W. J. The regulation of arylsulphatase formation in Pseudomonas C 12 B. Microbios. 1972 Sep-Oct;6(22):147–156. [PubMed] [Google Scholar]
- GUNSALUS I. C., HORECKER B. L., WOOD W. A. Pathways of carbohydrate metabolism in microorganisms. Bacteriol Rev. 1955 Jun;19(2):79–128. doi: 10.1128/br.19.2.79-128.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goren M. B. Mycobacterial sulfolipids: spontaneous desulfation. Lipids. 1971 Jan;6(1):40–46. doi: 10.1007/BF02536373. [DOI] [PubMed] [Google Scholar]
- Goren M. B. Sulfolipid I of Mycobacterium tuberculosis, strain H37Rv. I. Purification and properties. Biochim Biophys Acta. 1970 Jun 9;210(1):116–126. doi: 10.1016/0005-2760(70)90067-6. [DOI] [PubMed] [Google Scholar]
- HARADA T., SPENCER B. Choline suphate in fungi. J Gen Microbiol. 1960 Apr;22:520–527. doi: 10.1099/00221287-22-2-520. [DOI] [PubMed] [Google Scholar]
- KAJI A., McELROY W. D. Enzymic formation of choline sulfate. Biochim Biophys Acta. 1958 Oct;30(1):190–191. doi: 10.1016/0006-3002(58)90260-9. [DOI] [PubMed] [Google Scholar]
- Kates M., Palameta B., Perry M. P., Adams G. A. A new glycolipid sulfate ester in Halobacterium cutirubrum. Biochim Biophys Acta. 1967 Feb 14;137(1):213–216. doi: 10.1016/0005-2760(67)90034-3. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Nissen P., Benson A. A. Choline Sulfate in Higher Plants. Science. 1961 Dec 1;134(3492):1759–1759. doi: 10.1126/science.134.3492.1759. [DOI] [PubMed] [Google Scholar]
- ORSI B. A., SPENCER B. CHOLINE SULPHOKINASE (SULPHOTRANSFERASE). J Biochem. 1964 Jul;56:81–91. doi: 10.1093/oxfordjournals.jbchem.a127963. [DOI] [PubMed] [Google Scholar]
- PAYNE W. J., FEISAL V. E. Bacterial utilization of dodecyl sulfate and dodecyl benzene sulfonate. Appl Microbiol. 1963 Jul;11:339–344. doi: 10.1128/am.11.4.339-344.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEGAL I. H., JOHNSON M. J. Hydrolysis of choline-O-sulfate by cell-free extracts from Penicillium. Biochim Biophys Acta. 1963 Feb 5;69:433–434. doi: 10.1016/0006-3002(63)91287-3. [DOI] [PubMed] [Google Scholar]
- Spencer B., Harada T. The role of choline sulphate in the sulphur metabolism of fungi. Biochem J. 1960 Nov;77(2):305–315. doi: 10.1042/bj0770305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer B., Hussey E. C., Orsi B. A., Scott J. M. Mechanism of choline O-sulphate utilization in fungi. Biochem J. 1968 Jan;106(2):461–469. doi: 10.1042/bj1060461. [DOI] [PMC free article] [PubMed] [Google Scholar]


