Abstract
Background:
Fractional flow reserve (FFR) is crucial to evaluating coronary artery stenosis in patients diagnosed with chronic coronary syndrome (CCS). By assessing the severity of stenosis, FFR assists in determining whether percutaneous coronary intervention (PCI) is necessary.
Methods:
Conducted at Tehran Heart Center from 2013 through 2017, this cohort study involved 52,248 CCS patients who underwent coronary angiography. Among them, 598 symptomatic individuals, despite receiving comprehensive medical treatment, underwent FFR assessment. Subsequently, 225 patients with positive FFR (≤0.80) underwent PCI, while 373 patients received solely medical treatment. The patients were monitored for 3 years to evaluate primary and secondary endpoints.
Results:
After 3 years, the PCI group demonstrated a lower incidence of the primary composite endpoint, consisting of all-cause mortality, nonfatal myocardial infarction, repeat target vessel/lesion revascularization (TVR/TLR), and coronary artery bypass graft surgery, than the medical treatment group (HR, 0.85; 95% CI, 0.74 to 0.98; P=0.012). Additionally, urgent TVR/TLR significantly decreased in the PCI group (HR, 0.56; 95% CI, 0.42 to 0.74; P<0.001).
Conclusion:
FFR-guided PCI demonstrated effectiveness in reducing long-term major adverse cardiac events, primarily by lowering the incidence of TVR/TLR. The results emphasize the significance of FFR-guided PCI in addressing stenosis rather than alleviating ischemia.
Keywords: Coronary artery disease, Acute coronary syndrome, Fractional flow reserve, Myocardial, Percutaneous coronary intervention
Introduction
Coronary artery disease is the most common form of cardiovascular disease and is the third leading cause of death worldwide, accounting for 17.8 million deaths annually.1 This disease is caused by atherosclerotic plaque accumulation in the coronary arteries, leading to hypoxia and myocardial ischemia.2, 3 Myocardial ischemia causes angina, decreased functional capacity, and heart failure. Accurate selection of stenosis-induced ischemia is crucial for maximizing the benefits of revascularization.4 Revascularization always improves the outcome of acute patients with coronary syndrome. In contrast, the potential usefulness of revascularization in patients with chronic coronary syndrome (CCS) depends on the actuality and degree of myocardial ischemia, and revascularization on nonischemic stenoses can be harmful.5,6 Fractional flow reserve (FFR) is an indicator of the physiological importance of coronary artery stenosis.7,8
The significance of FFR in coronary artery disease lies in its ability to help guide treatment decisions. FFR can identify which blockages are responsible for ischemia or reduced blood flow to the heart muscle. This information can aid physicians in determining whether a particular blockage needs treatment with a stent or whether medication alone is sufficient.9
The present study aimed to evaluate the long-term outcome of patients with 50%–69% coronary artery stenosis undergoing PCI or medical treatment based on FFR.
Methods
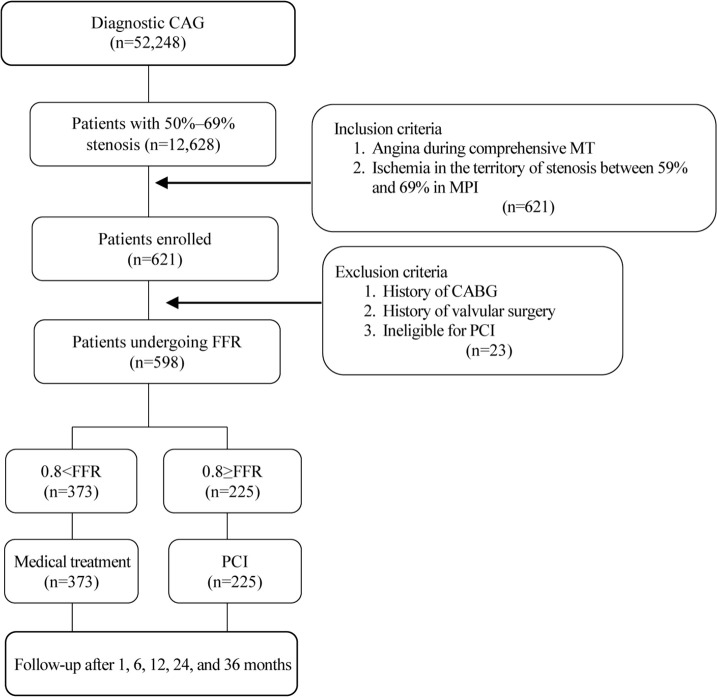
The present cohort study was conducted in accordance with the tenets of the Helsinki Declaration. The study protocol was approved by the Ethics Committee of Tehran Heart Center. All the studied patients granted informed written consent for the research. Between October 2013 and September 2017, a total of 52,248 patients with CCS underwent a diagnostic coronary angiography at Tehran Heart Center, Tehran, Iran. Of these, 12,628 patients with 50%–69% coronary artery stenosis underwent comprehensive medical treatment. FFR was performed on 598 patients without exclusion criteria due to the lack of response to comprehensive medical treatment and continued chest pain. Patients with one 50%–69% stenotic vessel underwent FFR directly, and those with multiple 50%–69% stenotic vessels underwent FFR after ischemia confirmation in the stenotic territory via myocardial perfusion imaging (MPI). Finally, 225 patients (37.7%) underwent PCI due to positive FFR (Figure 1).
Figure 1.
The image illustrates the sampling flowchart of the study for the evaluation of the long-term outcome of FFR-guided PCI.
CAG, Coronary angiography; PCI, Percutaneous coronary intervention; FFR, Fractional flow reserve; CABG, Coronary artery bypass graft surgery; MT, Medical treatment; MPI, Myocardial perfusion imaging.
For diagnostic coronary angiography, 6F catheters were used through the femoral or radial approach. Coronary lesion severity (%) was evaluated in the entire study population by 2 interventional cardiologists.
FFR was measured after an intracoronary administration of intravenous nitrates and the induction of hyperemia with intravenous adenosine (140 μg/kg/min). A pressure wire (St Jude Medical, USA) was advanced distal to the coronary lesion. An FFR value ≤0.80 was considered positive.10, 11
Patients treated with FFR-guided PCI or medical treatment underwent a 3-year follow-up (median=13 mon) with respect to primary and secondary endpoints. The primary endpoint was defined as a composite of death from any cause, nonfatal myocardial infarction (MI), repeat target vessel/lesion revascularization (TVR/TLR), and coronary artery bypass graft surgery (CABG). The secondary endpoints were composed of the individual components of the primary endpoint.
Survival analysis methods, including Kaplan-Meier analysis and Cox proportional hazards regression, were utilized to assess time-to-event outcomes. Additionally, traditional statistical methods, such as the Student t test for continuous variables and the Pearson χ2 test for categorical variables, were employed for group comparisons. All the analyses were performed with IBM SPSS Statistics for Windows, version 23.0 (Armonk, NY: IBM Corp), with the significance level set at 5%.
Results
Of the 598 patients who underwent FFR, 373 (62.3%) received medical treatment and 225 (37.7%) underwent PCI. Among the patients studied, 384 (64.2%) were male. The mean age of the study population was 61.67±9.88 years, which was not statistically significant between the PCI and medical groups. Among the initial laboratory findings, the mean high-density lipoprotein level was significantly higher in the PCI group than in the medical group (P=0.001). However, no significant differences existed concerning the other laboratory findings between the groups. Ejection fraction was higher in the PCI group, but this difference was not statistically significant (P=0.077) (Table 1).
Table 1.
Comparison of basic demographic, laboratory, and echocardiographic findings of the studied patients between the PCI and medical groups
| Variable | PCI Group | Medical Group | P |
|---|---|---|---|
|
|
|
||
| Mean (SD)/Median | Mean (SD)/Median | ||
|
|
|
|
|
| Age (y) | 61.80±9.58 | 61.54±10.21 | 0.135 |
| BMI (kg/m2) | 28.58±4.39 | 28.82±4.54 | 0.736 |
| FBS (mg/dL) | 103 (93–128) | 104 (94–133) | 0.580 |
| TCH (mg/dL) | 150.13±39.68 | 150.03±37.84 | 0.736 |
| TG (mg/dL) | 126.00 (95–172) | 134 (100–182) | 0.155 |
| LDL (mg/dL) | 82 (63–106) | 88 (69–109) | 0.095 |
| HDL (mg/dL) | 41.74±10.97 | 39.07±9.05 | 0.001 |
| Cr (μg/dL) | 0.98 (0.80–1.10) | 1 (0.80–1.10) | 0.888 |
| Hb (g/dL) | 14.18±1.63 | 14.69±1.68 | 0.667 |
| EF (%) | 50.48±7.93 | 49.23±7.68 | 0.077 |
PCI, Percutaneous coronary intervention; BMI, Body mass index; FBS, Fasting blood sugar; TCH, Total cholesterol; TG, Triglyceride; LDL, Low-density lipoprotein; HDL, High-density lipoprotein; Cr, Creatinine; Hb, Hemoglobin; EF, Ejection fraction
In both PCI and medical groups, there was a preponderance of men: 58.7% of the medical group and 73.7% of the PCI group. Additionally, the number of men was significantly higher in the PCI group than in the medical group (P<0.001). Among the risk factors, smoking was significantly different between the 2 groups: 17.7% in the group under medical treatment and 25.9% in the PCI group (P=0.012) (Table 2).
Table 2.
Comparison of risk factors and comorbidities between the PCI and medical groups
| Variable | PCI Group n (%) | Medical Group n (%) | P |
|---|---|---|---|
|
|
|
|
|
| Sex (male) | 165 (73.7) | 219 (58.7) | <0.001 |
| Positive FH | 48 (21.4) | 80 (21.4) | 0.542 |
| DLP | 135 (60.3) | 229 (61.4) | 0.425 |
| HTN | 112 (50.0) | 216 (57.9) | 0.036 |
| DM | 87 (38.8) | 151 (40.5) | 0.379 |
| Current CS | 58 (25.9) | 66 (17.7) | 0.012 |
| Opium addiction | 24 (10.7) | 35 (9.4) | 0.347 |
| CVA | 3 (1.3) | 9 (2.4) | 0.549 |
| COPD | 2 (0.9) | 6 (1.6) | 0.368 |
| CHF | 2 (0.9) | 5 (1.3) | 0.474 |
| CKD | 2 (0.9) | 6 (1.6) | 0.368 |
| Previous PCI | 58 (25.9) | 77 (20.2) | 0.157 |
| Previous CABG | 3 (1.3) | 9 (2.4) | 0.549 |
FH, Family history; DLP, Dyslipidemia; HTN, Hypertension; CS, Cigarette smoke; CVA, Cerebral vascular accident; COPD, Chronic obstructive pulmonary disease; CHF, Congestive heart failure; PCI, Percutaneous coronary intervention; CABG, Coronary artery bypass graft surgery
At the 3-year follow-up, 23 patients (10.2%) in the PCI group and 53 (14.2%) in the medical group developed major adverse cardiac events (MACE) (HR, 0.85; 95% CI, 0.74 to 0.98; P=0.012) (Figure 2).
Figure 2.
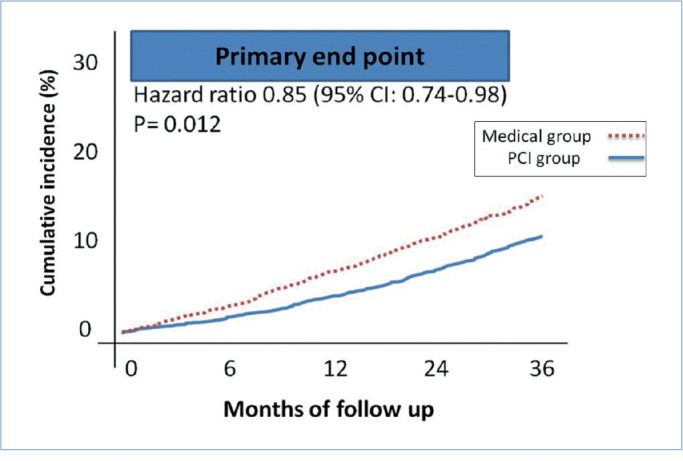
The image depicts the Kaplan–Meier curve for the primary endpoints in the patients treated with FFR-guided PCI or medical treatment (3 years of follow-up).
FFR, Fractional flow reserve; PCI, Percutaneous coronary intervention
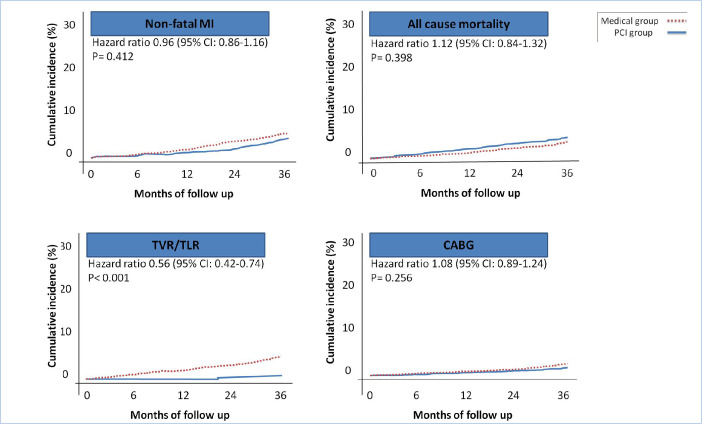
A comparison of the secondary endpoints showed that only urgent TVR/TLR varied significantly between the groups (HR, 0.56; 95% CI, 0.42 to 0.74; P<0.001) (Figure 3). The frequencies of the secondary endpoints are shown in Table 3.
Figure 3.
The images present the Kaplan–Meier curves for the secondary endpoints in the patients treated with FFR-guided PCI or medical treatment (3 years of follow-up).
FFR, Fractional flow reserve; PCI, Percutaneous coronary intervention; MACE, Major adverse cardiac events; MI, Myocardial infarction; CABG, Coronary artery bypass graft surgery; TVR/TLR, Target vessel/lesion revascularization
Table 3.
Comparison of the frequencies of the secondary endpoints between the PCI and medical groups
| Variable n (%) | Total (n=598) | PCI Group (n=225) | Medical Group (n=373) | P |
|---|---|---|---|---|
|
|
|
|
|
|
| Nonfatal MI | 26 (4.3) | 9 (4) | 17 (4.6) | 0.412 |
| All-cause mortality | 22 (3.6) | 9 (4) | 13 (3.5) | 0.398 |
| TVR/TLR | 18 (3) | 1 (0.4) | 17 (4.5) | < 0.001 |
| CABG | 11 (1.8) | 4 (1.3) | 7 (1.8) | 0.256 |
MI, Myocardial infarction; TVR/TLR, Target lesion/vessel revascularization; CABG, Coronary artery bypass graft surgery
Discussion
In this study, patients with single-vessel disease (50%–69% stenosis) failing to respond to comprehensive medical treatment and those with multivessel disease (50%–69% stenosis) and positive MPI findings failing to respond to comprehensive medical treatment underwent FFR. FFR is a pressure-wire–based index used during a coronary angiography to evaluate coronary stenosis to induce myocardial ischemia.12, 13 Almost 38% of the patients included in the study had a positive FFR, defined as an FFR of 0.8 or less.9, 10 A negative FFR (underestimated FFR) despite chest pain or positive MPI findings can have several causes, including microvascular dysfunction, endothelial dysfunction, and technical problems during FFR in the absence of maximum hyperemia.14–16 In the present study, FFR was conducted appropriately under the observation of 2 interventional cardiologists.
In the FGTHC cohort study, we found that FFR-guided PCI failed to reduce nonfatal MI, all-cause mortality, and CABG. Rather, by lessening the need for urgent revascularization, it diminishes the need for TVR/TLR. These findings were similar to previous studies (the FAME2 and COURAGE studies).17, 18 Nonfatal MI can occur for reasons other than coronary artery stenosis, such as plaque rupture and thrombosis in mild lesions, which may not be addressed by FFR-guided PCI. Furthermore, compared with techniques like intravascular ultrasound or optical coherence tomography, FFR-guided PCI may not be as effective in identifying and treating all significant stenoses in a patient’s coronary arteries, potentially leaving some stenoses that could cause nonfatal MI untreated. In conclusion, while FFR-guided PCI can reduce MACE, its impact on diminishing nonfatal MI may be limited due to nonstenotic causes of MI and the limitations of the procedure.19
Studies comparing FFR-guided PCI with medical therapy over a lengthier period (5 y) have also clarified that FFR-guided PCI cannot decrease the overall rate of mortality and nonfatal MI.20 In the COURAGE study,18 PCI did not reduce overall mortality and MI. Our findings confirmed that PCI cannot reduce mortality in patients with CCS.
In the FAME2 study,17 a group of patients with an FFR value below 0.8 received medical treatment, and in the current study, a group of patients with an FFR value exceeding 0.8 received medical treatment. In both groups, the need for urgent PCI did not decrease compared with the PCI group. The above findings show that the amount of TVR/TLR does not depend on the numerical value of FFR (whether it is >0.8 or <0.8), but the existence of a specific stenosis determines the amount of TVR/TLR in the future. Therefore, we can conclude that FFR-guided PCI reduces the incidence of TVR/TLR by resolving stenosis rather than ischemia. FFR-guided PCI does not necessarily increase regional blood flow. As shown in previous studies, there is no clear change in absolute blood flow before and after PCI in patients with CCS. In fact, microvascular dysfunction is the culprit in this scenario. This finding is compatible with the assumption that FFR-guided PCI positively affects TVR/TLR by eliminating stenosis rather than ischemia.21
Patients with ST-segment-elevation MI and multivessel disease can benefit from complete revascularization, guided by FFR measurements. This approach has the potential to reduce the risk of future events when compared with not performing additional invasive interventions following primary PCI. The principal reason for this reduction is that fewer repeat revascularizations are needed since there is no significant difference in all-cause mortality and nonfatal reinfarction between PCI and medical therapy groups.22, 23
Previous findings have shown that more than half of MI cases occur in lesions with moderate stenosis.15–17 Of course, rather than indicate these lesions as the main culprit, the findings suggest the high prevalence of moderate lesions. Furthermore, the higher the degree of stenosis, the greater the likelihood of MI.18, 24 Another noteworthy point is that the number of lesions and their vulnerability can play a critical role in the occurrence of MI occurrence, an issue not addressed in our study and other investigations on FFR-guided PCI.11, 12
According to our findings, for the occurrence of MI, the presence of stenosis is more important than the ischemic phenomenon, and MI occurs even in moderate lesions with an FFR value above 0.8. Considering the presence of chest pain or positive MPI findings in our entire study population, microvascular dysfunction or endothelial dysfunction in moderate lesions with an FFR value exceeding 0.8 may play a role in the occurrence of MI and TVR/TLR in the future. We, therefore, suggest that specific studies be conducted on this quandary in the future.
Conclusion
FFR-guided PCI lessens long-term MACE by reducing the incidence of TVR/TLR. This beneficial effect is through the elimination of stenosis rather than ischemia. FFR-guided PCI fails to diminish all-cause mortality, nonfatal MI, and CABG compared with medical treatment.
Acknowledgments
This study was approved and supported by Tehran Heart Center, Tehran University of Medical Sciences.
Notes:
This paper should be cited as: Khederlou H, Mohajeri M, Bavandpour Karvane H, Jalali A, Salarifar M. Long-Term Outcomes of Fractional Flow Reserve-Guided Percutaneous Coronary Intervention of Moderate Lesions in Patients with Chronic Coronary Syndrome. J Teh Univ Heart Ctr 2024;19(1):54-59.
References
- 1.Motedayen M, Khederlou H. ST-Segment Elevation Myocardial Infarction with Normal Coronary Arteries Angiography 2020;11:e104397. [Google Scholar]
- 2.Hassanzadeh-Makoui R, Jamei M, Hassanzadeh-Makoui M, Khederlou H. Effects of Vitamin D on Left Ventricular Ejection Fraction in Patients with Systolic Heart Failure: A Double-Blind Randomized Clinical Trial. Int J Endocrinol Metab 2020;18:e103528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Makoui RH, Khederlou H, Rasouli A, Shojaei FS, Ramezanpour N. Relationship between the ABO Blood Groups and Acute Coronary Syndrome; Zanjan Mousavi Hospital 2017–2020. Clin Lab 2022;65. [DOI] [PubMed] [Google Scholar]
- 4.Marzilli M, Crea F, Morrone D, Bonow RO, Brown DL, Camici PG, Chilian WM, DeMaria A, Guarini G, Huqi A, Merz CNB, Pepine C, Scali MC, Weintraub WS, Boden WE. Myocardial ischemia: From disease to syndrome. Int J Cardiol. 2020. Sep 1;314:32–35. [DOI] [PubMed] [Google Scholar]
- 5.Pham V, Moroni A, Gall E, Benedetti A, Zivelonghi C, Picard F. Revascularization and Medical Therapy for Chronic Coronary Syndromes: Lessons Learnt from Recent Trials, a Literature Review. J Clin Med. 2023;12:2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sels JW, Tonino PA, Siebert U, Fearon WF, Van't Veer M, De Bruyne B, Pijls NH. Fractional flow reserve in unstable angina and non-ST-segment elevation myocardial infarction experience from the FAME (Fractional flow reserve versus Angiography for Multivessel Evaluation) study. JACC Cardiovasc Interv 2011;4:1183–1189. [DOI] [PubMed] [Google Scholar]
- 7.Bruno F, D'Ascenzo F, Marengo G, Manfredi R, Saglietto A, Gallone G, Franchin L, Piroli F, Angelini F, De Filippo O, Conrotto F, Omedè P, Montefusco A, Pennone M, Boffini M, Pocar M, Rinaldi M, De Ferrari GM. Fractional flow reserve guided versus angiographic guided surgical revascularization: A meta-analysis. Catheter Cardiovasc Interv 2021;98:E18–E23. [DOI] [PubMed] [Google Scholar]
- 8.Scoccia A, Tomaniak M, Neleman T, Groenland FTW, Plantes ACZD, Daemen J. Angiography-Based Fractional Flow Reserve: State of the Art. Curr Cardiol Rep 2022;24:667–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zimmermann FM, Ferrara A, Johnson NP, van Nunen LX, Escaned J, Albertsson P, Erbel R, Legrand V, Gwon HC, Remkes WS, Stella PR, van Schaardenburgh P, Bech GJ, De Bruyne B, Pijls NH. Deferral vs. performance of percutaneous coronary intervention of functionally non-significant coronary stenosis: 15-year follow-up of the DEFER trial. Eur Heart J 2015;36:3182–3188. [DOI] [PubMed] [Google Scholar]
- 10.Koo BK, Hu X, Kang J, Zhang J, Jiang J, Hahn JY, Nam CW, Doh JH, Lee BK, Kim W, Huang J, Jiang F, Zhou H, Chen P, Tang L, Jiang W, Chen X, He W, Ahn SG, Yoon MH, Kim U, Lee JM, Hwang D, Ki YJ, Shin ES, Kim HS, Tahk SJ, Wang J, FLAVOUR Investigators . Fractional Flow Reserve or Intravascular Ultrasonography to Guide PCI. N Engl J Med 2022;387:779–789. [DOI] [PubMed] [Google Scholar]
- 11.Cavallari LH, Lee CR, Duarte JD, Nutescu EA, Weitzel KW, Stouffer GA, Johnson JA. Implementation of inpatient models of pharmacogenetics programs. Am J Health Syst Pharm 2016;73:1944–1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pijls NH, van Son JA, Kirkeeide RL, De Bruyne B, Gould KL. Experimental basis of determining maximum coronary, myocardial, and collateral blood flow by pressure measurements for assessing functional stenosis severity before and after percutaneous transluminal coronary angioplasty. Circulation 1993;87:1354–1367. [DOI] [PubMed] [Google Scholar]
- 13.Pijls NH, De Bruyne B, Peels K, Van Der Voort PH, Bonnier HJ, Bartunek J Koolen JJ, Koolen JJ. Measurement of fractional flow reserve to assess the functional severity of coronary-artery stenoses. N Engl J Med 1996;334:1703–1708. [DOI] [PubMed] [Google Scholar]
- 14.Murai T, Lee T, Yonetsu T, Isobe M, Kakuta T. Influence of microvascular resistance on fractional flow reserve after successful percutaneous coronary intervention. Catheter Cardiovasc Interv 2015;85:585–592. [DOI] [PubMed] [Google Scholar]
- 15.Melikian N, De Bondt P, Tonino P, De Winter O, Wyffels E, Bartunek J, Heyndrickx GR, Fearon WF, Pijls NH, Wijns W, De Bruyne B. Fractional flow reserve and myocardial perfusion imaging in patients with angiographic multivessel coronary artery disease. JACC Cardiovasc Interv 2010;3:307–314. [DOI] [PubMed] [Google Scholar]
- 16.Jenei C, Tar B, Ágoston A, Sánta P, Sánta J, Csippa B, Wéber R, Gyürki D, Halász G, Szabó GT, Czuriga D, Kőszegi Z. Novel Method to Detect Pitfalls of Intracoronary Pressure Measurements by Pressure Waveform Analysis. J Pers Med 2022;12:2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Bruyne B, Pijls NH, Kalesan B, Barbato E, Tonino PA, Piroth Z, Jagic N, Möbius-Winkler S, Rioufol G, Witt N, Kala P, MacCarthy P, Engström T, Oldroyd KG, Mavromatis K, Manoharan G, Verlee P, Frobert O, Curzen N, Johnson JB, Jüni P, Fearon WF, FAME 2 Trial Investigators . Fractional flow reserve-guided PCI versus medical therapy in stable coronary disease. N Engl J Med 2012;367:991–1001. [DOI] [PubMed] [Google Scholar]
- 18.Boden WE, O'Rourke RA, Teo KK, Hartigan PM, Maron DJ, Kostuk WJ, Knudtson M, Dada M, Casperson P, Harris CL, Chaitman BR, Shaw L, Gosselin G, Nawaz S, Title LM, Gau G, Blaustein AS, Booth DC, Bates ER, Spertus JA, Berman DS, Mancini GB, Weintraub WS, COURAGE Trial Research Group . Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med 2007;356:1503–1516. [DOI] [PubMed] [Google Scholar]
- 19.Koo BK, Hu X, Kang J, Zhang J, Jiang J, Hahn JY, Nam CW, Doh JH, Lee BK, Kim W, Huang J, Jiang F, Zhou H, Chen P, Tang L, Jiang W, Chen X, He W, Ahn SG, Yoon MH, Kim U, Lee JM, Hwang D, Ki YJ, Shin ES, Kim HS, Tahk SJ, Wang J, FLAVOUR Investigators . Fractional Flow Reserve or Intravascular Ultrasonography to Guide PCI. N Engl J Med 2022;387:779–789. [DOI] [PubMed] [Google Scholar]
- 20.Xaplanteris P, Fournier S, Pijls NHJ, Fearon WF, Barbato E, Tonino PAL, Engstrøm T, Kääb S, Dambrink JH, Rioufol G, Toth GG, Piroth Z, Witt N, Fröbert O, Kala P, Linke A, Jagic N, Mates M, Mavromatis K, Samady H, Irimpen A, Oldroyd K, Campo G, Rothenbühler M, Jüni P, De Bruyne B, FAME 2 Investigators . Five-Year Outcomes with PCI Guided by Fractional Flow Reserve. N Engl J Med 2018;379:250–259. [DOI] [PubMed] [Google Scholar]
- 21.Hamaya R, Kanaji Y, Usui E, Hoshino M, Murai T, Yonetsu T, Kakuta T. Improvement of Fractional Flow Reserve after Percutaneous Coronary Intervention Does Not Necessarily Indicate Increased Coronary Flow. Eur Cardiol 2019;14:10–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smits PC, Abdel-Wahab M, Neumann FJ, Boxma-de Klerk BM, Lunde K, Schotborgh CE, Piroth Z, Horak D, Wlodarczak A, Ong PJ, Hambrecht R, Angerås O, Richardt G, Omerovic E, Compare-Acute Investigators . Fractional Flow Reserve-Guided Multivessel Angioplasty in Myocardial Infarction. N Engl J Med 2017;376:1234–1244. [DOI] [PubMed] [Google Scholar]
- 23.Engstrøm T, Kelbæk H, Helqvist S, Høfsten DE, Kløvgaard L, Holmvang L, Jørgensen E, Pedersen F, Saunamäki K, Clemmensen P, De Backer O, Ravkilde J, Tilsted HH, Villadsen AB, Aarøe J, Jensen SE, Raungaard B, Køber L, DANAMI-3—PRIMULTI Investigators . Complete revascularisation versus treatment of the culprit lesion only in patients with ST-segment elevation myocardial infarction and multivessel disease (DANAMI-3—PRIMULTI): an open-label, randomised controlled trial. Lancet 2015;386:665–671. [DOI] [PubMed] [Google Scholar]
- 24.Frøbert O, van't Veer M, Aarnoudse W, Simonsen U, Koolen JJ, Pijls NH. Acute myocardial infarction and underlying stenosis severity. Catheter Cardiovasc Interv 2007;70:958–965. [DOI] [PubMed] [Google Scholar]