Abstract
Absent pulmonary valve syndrome (APVS) is a rare congenital anomaly characterized by rudimentary PV tissue with variable degrees of PV stenosis and regurgitant pulmonary blood flow. In most cases, it is associated with tetralogy of Fallot. In a minority of APVS cases, with an unknown frequency, intact ventricular septum (IVS), patent ductus arteriosus, and possible tricuspid atresia are present. This condition is known as non-Fallot type APVS.
We describe a case of an antenatal diagnosis of APVS with IVS, a large patent ductus arteriosus, and ascending aorta dilatation. The mother was referred to our center at 32 weeks of gestation due to cardiomegaly on sonography. Fetal echocardiography revealed cardiomegaly, right atrial and ventricular enlargement, aneurysmal dilatation of the main pulmonary artery, and mild dilatation of the pulmonary artery branches. Also observed were IVS, rudimentary PV tissue with severe to-and-fro turbulence across the PV, a widely open ductus arteriosus, ascending aorta dilatation, and levorotation of the heart.
After childbirth, our diagnosis was confirmed by echocardiography and surgery. The baby experienced severe respiratory distress. At 15 days of life, surgical intervention in the form of pulmonary artery arterioplasty was performed, resulting in good outcomes. The patient underwent follow-up for 6 months and showed reasonable health.
Keywords: Pulmonary valve incompetence, Antenatal diagnosis, Pulmonary valve/abnormalities*
Introduction
Absent pulmonary valve syndrome (APVS) is a rare congenital anomaly of the outflow tract, with an unknown etiology. It is characterized by dysplastic or rudimentary PV tissue, resulting in varying degrees of pulmonary artery (PA) annular stenosis and stenotic and regurgitant pulmonic blood flow. These abnormalities lead to aneurysmal dilatation of the main PA and/or PA branches, causing tracheobronchial tree compression. As a result, severe respiratory distress shortly after birth is observed.1 In most cases, APVS is associated with tetralogy of Fallot (TF) (Fallot type) with a high frequency of patent ductus arteriosus (PDA) agenesis. This occurrence has been reported in 0.2%–0.4% of live-born infants with congenital heart disease (CHD) and 3%–6% of all TF cases. In a minority of APVS cases, with an unknown frequency, intact ventricular septum (IVS), PDA, and possible tricuspid atresia are present. This condition is also known as non–Fallot-type APVS.2–4
In this report, we describe a fetus with a non–Fallot-type APVS, presenting with an IVS, a large PDA, and ascending aorta dilatation. The fetus was referred to our center to undergo cardiomegaly evaluation via antenatal echocardiography.
Case Report
A 37-year-old woman, G2, P1, A0, was referred to our department at 32 weeks of gestation because of cardiomegaly and suspected cardiac anomaly in an anomaly scan. The pregnancy, followed up on in an in vitro fertilization trial, was unremarkable. The woman’s parents were distant relatives, and she had no family history of CHD.
Sonography showed cardiomegaly with the possibility of right ventricular enlargement with no significant extracardiac anomalies. In our ward, fetal echocardiography detected visceral-atrial situs solitus, levocardia, concordant atrioventricular and ventriculoarterial connection with IVS (Figure 1 & Figure 2), a large foramen ovale (3.35 mm) with a regular right-to-left shunt, mild tricuspid regurgitation, right atrial enlargement with the bowing of the interatrial septum toward the left atrium, right ventricular enlargement, right ventricular hypertrophy, right ventricular hypertrabeculation, mild pericardial effusion, marked cardiomegaly, and levorotation of the heart, resulting in relative left lung hypoplasia (Figure 1 & Figure 2).
Figure 1.

A & B) Five-chamber views
A) lateral 5-chamber and B) apical 5-chamber views, illustrating IVS, RVE, RVH, mild PE, dilatation of the AAO (diameter =1.1 cm), and levorotation of the heart
IVS, Intact ventricular septum; RVE, Right ventricular enlargement; RVH, Right ventricular hypertrophy; PE, Pericardial effusion; AAO, Ascending aorta
Figure 2.

Four-chamber view demonstrating RAE, RVE, RVH, mild PE, bowing of the IAS toward the LA, levorotation of the heart, and the length and width of LV and RV hypertrabeculation
RAE, Right atrial enlargement; RVE, Right ventricular enlargement; RVH, Right ventricular hypertrophy; PE, Pericardial effusion; IAS, Interatrial septum; LA, Left atrium; RV, Right ventricle
Outflow tract evaluation revealed normal related great arteries, a huge main PA (16 mm), mild right PA dilatation, mild PA annulus stenosis (8.6 mm), an aortic annulus of 6.5 mm, rudimentary PV tissue (Figure 1, Figure 2, Figure 3 & Figure 4) with severe to-and-fro turbulent flow across the PV by color flow mapping (Figure 5), significant pulmonary stenosis and insufficiency by pulsed-wave Doppler (pulmonary pressure gradient in pulmonary stenosis and insufficiency =35 mm Hg and 25 mm Hg) (Figure 6), a widely open DA, and ascending aorta dilatation (13 mm) (Figure 7). Furthermore, the ductus venosus was abnormal and showed a reverse A (atrial contraction) wave and an increased diastolic flow depth (Figure 8).
Figure 3.
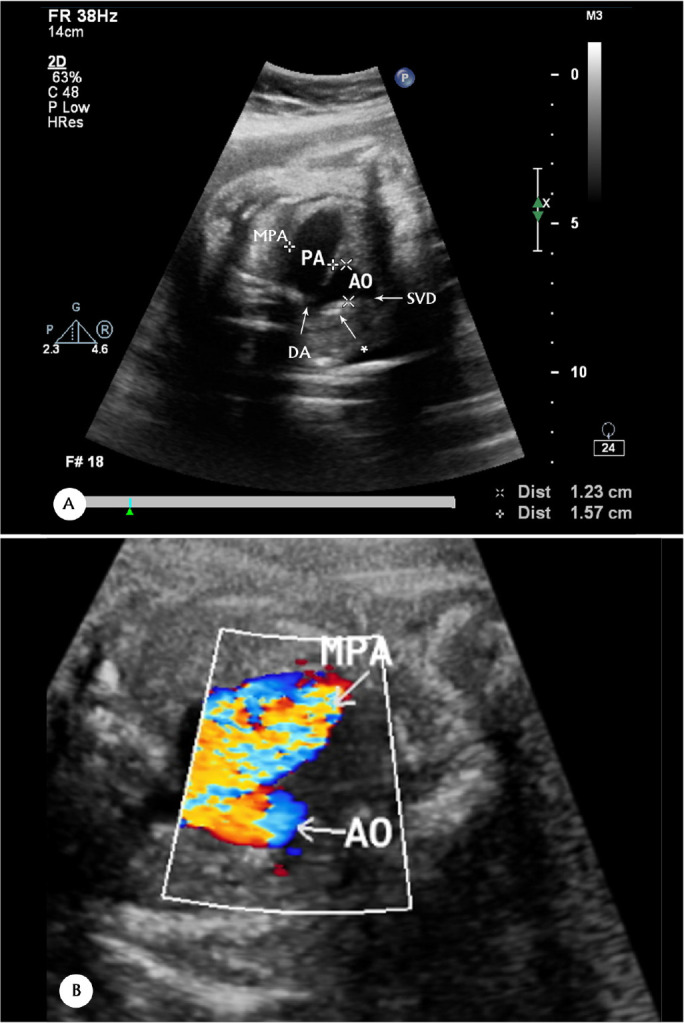
(A & B) Three-vessel or tracheal views, showing dilatation of the MPA and the transverse aortic arch in 2D (A) & CFM (B)
MPA=1.5 cm
The asterisk points to the transverse aortic arch.
MPA, Main pulmonary artery; AO, Transverse aortic arch; SVC, Superior vena cava; DA, Ductus arteriosus; CFM, Color flow mapping
Figure 4.

A & B) Ductal arch 2D view, illustrating dilated RVOT, MPA, stenotic pulmonary valve, and diminutive PA leaflets (PA valve opening =3.91 mm and MPA=15.2 mm)
RVOT, Right ventricular outflow tract; MPA, Main pulmonary artery; PA, Pulmonary artery; AO, Aorta; LA, Left atrium
The white arrows are for better visualization.
Figure 5.

A) Ductal arch view, demonstrating severe turbulent flow toward the RVOT due to PI by CFM
B) CFM at the same view, showing severe turbulent flow toward the MPA owing to PS
RVOT, Right ventricular outflow tract; PI, Pulmonary insufficiency; PS, Pulmonary stenosis; MPA, Main pulmonary artery; PA, Pulmonary artery;
DA, Ductus arteriosus; RPA, Right pulmonary artery; CFM, Color flow mapping
The white arrows are for better visualization.
Figure 6.

Pulsed-wave Doppler, showing marked PS (upper Doppler flow) and PI (lower Doppler flow) during systole (PS) and diastole (PI), respectively
PS, Pulmonary stenosis; PI, Pulmonary insufficiency
Figure 7.

A) Ductal arch 2D view, showing a widely open DA and a large MPA, B) Aortic arch 2D view, demonstrating a dilated AAO (=1.17cm), C) CFM, demonstrating dilated AAO and DA (DA=6.28 mm), D) CFM, showing dilated AAO (1.2cm), AAO, Ascending aorta; DAO, Descending aorta; DA; Ductus arteriosus; MPA, Main pulmonary artery; CFM, Color flow mapping
The white arrows are for better visualization.
Figure 8.


Abnormal ductus venosus flow in the Doppler view, showing reverse A (atrial contraction) wave and an increased end-diastolic flow depth
Karyotyping for chromosomal disorders turned out negative.
Delivery was conducted at a tertiary care hospital. A male baby was delivered prematurely at 36 weeks of gestation with a birth weight of 2700 g. The baby, a product of C/S with no extracardiac anomalies, was admitted to the neonatal intensive care unit owing to respiratory distress. Shortly afterward, with cyanosis and progressing respiratory distress, he was transferred to an equipped pediatric cardiologic center. The neonatal diagnosis was APVS with an IVS and a large PDA, associated with ascending aorta dilatation, as well as secundum atrial septal defect (ASD) and mild right ventricular dysfunction, which generally confirmed our antenatal diagnosis.
The baby underwent surgery 15 days after birth in another hospital. An aortic homograft was applied between the right ventricular outflow tract and the PA bifurcation. Additionally, ASD was closed, the ascending aorta was repaired, and the large PDA was divided and ligated. The surgical result was acceptable, without residual defects. Subsequently, the patient underwent chest physiotherapy for the collapse of the left lung with regressing right ventricular size. He demonstrated significant weight gain and overall good health until 6 months of age during the most recent assessment.
Discussion
APVS is a rare congenital anomaly of unknown etiology predominantly seen in TF patients presenting soon after birth owing to tracheobronchial tree compression by aneurysmal main PA and PA branches.1,5,6
Some reasons for massive PA enlargement have been offered, but none completely conforms to this phenomenon since the agenesis of ductus arteriosus is ruled out by the accompaniment of APVS with PDA in some patients.7 Ventricular septal defect in association with mild pulmonary stenosis cannot explain the reason accurately because of the occurrence of TF with severe pulmonary stenosis and APVS concomitantly.8 Emman et al7 proposed a histologic abnormality resembling connective tissue disorders like Marfan syndrome. This theory better aligns with our patient’s condition since the coexistence of ascending aorta dilatation with APVS is not adequately explained by other abnormalities.
A retrospective single-center study on 12 cases with a diagnosis of APVS in the fetal period during 10 years observed 3 subtypes: 1) TF with no DA (n=10; 83%), 2) isolated APVS with IVS and large PDA (n=1; 8%), and 3) tricuspid atresia, right ventricular dysplasia, and restricted DA (n=1; 8%).9 In another study by Alpana et al10 in 2010 on 2 cases of antenatal diagnosis of APVS, 1 fetus had the TF type of APVS with ductal agenesis, and the other had APVS, IVS, and PDA associated with functional tricuspid atresia. In a study by Gottschalk et al,4 out of 40 cases of APVS diagnosed prenatally, 37 (92.5%) were associated with TF, and 3 (7.5%) had IVS. According to their findings, DA was detected in 17 out of 37 (45.9%) TF types, with all 3 cases having IVS.
The severity of clinical manifestations in APVS with IVS and PDA relates to the size of PDA and the degree of pulmonary stenosis. A lesser degree of pulmonary stenosis and a larger PDA size result in more pulmonary congestion and more left-to-right shunting over the PDA, respectively.1 A recently published case report of isolated APVS noted that the pulmonary artery was dilated, but the branches were not dilated to the massive size often seen in Fallot type. Additionally, the case report noted that PDA, as well as hypertrabeculation, was a common finding in isolated APVS relative to Fallot type.11 These findings chime with our presented case; however, we observed the coexistence of ascending aorta dilatation. The cited case report also mentioned that, according to a comprehensive literature review, that more than two-thirds of fetuses survive the neonatal period, underscoring the paucity of associated disorders.11
The management of APVS with TF or IVS is not well-defined, with some investigations suggesting patient follow-up once every few months as long as the symptoms are tolerable and right ventricular function is acceptable.12 A reason for a delay in surgery is to permit lower pulmonary vascular resistance to diminish pulmonary insufficiency. Nonetheless, PDA ligation is the first option to improve respiratory distress without hesitation. PA arterioplasty should be reserved only when severe respiratory distress is bothering.1
In our case, prenatal diagnosis was at 32 weeks of gestation, so the mother was referred to an equipped tertiary obstetric center for delivery. Everything was ready to obtain a better outcome for the baby, which was effective: the surgical outcome was excellent, with reasonable weight gain and well-being up to the last follow-up period at 6 months of life.
We wish to emphasize the role of echocardiography as an ever-expanding field in the early diagnosis of CHD. Precise and careful echocardiography via its different modalities, such as spectral and color Doppler, assisted us in establishing the correct diagnosis. In fetal echocardiography, similar to other fields of echocardiography (transthoracic and transesophageal echocardiography, cardiac function estimation by deformation study, Doppler, and tissue Doppler), a thorough knowledge regarding ultrasound physics and the normal growth and development of the heart during the fetal period would be beneficial. In this case, our evaluation of anatomic details via echocardiography helped us select the best management plan and the most appropriate surgery.13–15
Conclusion
APVS with IVS and PDA associated with aneurysmal ascending aorta dilatation is a rare congenital anomaly. This cardiac anomaly might be successfully managed clinically and surgically with a reasonable outcome if diagnosed accurately and promptly. Establishing an accurate diagnosis requires careful and detailed fetal echocardiography.
Notes:
This paper should be cited as: Rashidighader F, Mirzaaghayan MR. A Case of Antenatal Diagnosis of Absent Pulmonary Valve Syndrome with Intact Ventricular Septum, Large Patent Ductus Arteriosus, and Ascending Aorta Dilatation. J Teh Univ Heart Ctr 2024;19(1):60-65.
References
- 1.Grotenhuis HB, Nijveld A, Backx A. Absent pulmonary valve syndrome with intact ventricular septum and patent ductus arteriosus: report of two cases and a short review of the literature. Ann Thorac Surg 2003;75:280–282. [DOI] [PubMed] [Google Scholar]
- 2.Podzimkova J, Hickey MS, Slavik Z, Leanage R, Chan KC. Absent pulmonary valve syndrome with intact ventricular septum: role of ductus arteriosus revisited. Int J Cardiol 1997;61:109–112. [DOI] [PubMed] [Google Scholar]
- 3.Rabinovitch M, Grady S, David I, Van Praagh R, Sauer U, Buhlmeyer K, Castaneda AR, Reid L. Compression of intrapulmonary bronchi by abnormally branching pulmonary arteries associated with absent pulmonary valves. Am J Cardiol 1982;50:804–813. [DOI] [PubMed] [Google Scholar]
- 4.Gottschalk I, Jehle C, Herberg U, Breuer J, Brockmeier K, Bennink G, Hellmund A, Strizek B, Gembruch U, Geipel A, Berg C. Prenatal diagnosis of absent pulmonary valve syndrome from first trimester onwards: novel insights into pathophysiology, associated conditions and outcome. Ultrasound Obstet Gynecol 2017;49:637–642. [DOI] [PubMed] [Google Scholar]
- 5.Karimi A, Peiravian F, Amirghofran AA, Kariminejad A. Absent pulmonary valve, intact interventricular septum, rudimentary aortic non-coronary cusp and ascending aortic aneurysm in a single patient. Interact Cardiovasc Thorac Surg 2010;10:636–638. [DOI] [PubMed] [Google Scholar]
- 6.Zucker N, Rozin I, Levitas A, Zalzstein E. Clinical presentation, natural history, and outcome of patients with the absent pulmonary valve syndrome. Cardiol Young 2004;14:402–408. [DOI] [PubMed] [Google Scholar]
- 7.Emmanoulides GC, Thanopoulos B, Siassi B, Fishbein M. “Agenesis” of ductus arteriosus associated with the syndrome of tetralogy of Fallot and absent pulmonary valve. Am J Cardiol 1976;37:403–409. [DOI] [PubMed] [Google Scholar]
- 8.Pachirat O, Seward JB, O'leary PW. Absent Pulmonary Valve: Echocardiographic Features. Echocardiography 1997;14:129–134. [DOI] [PubMed] [Google Scholar]
- 9.Wertaschnigg D, Jaeggi M, Chitayat D, Shannon P, Ryan G, Thompson M, Yoo SJ, Jaeggi E. Prenatal diagnosis and outcome of absent pulmonary valve syndrome: contemporary single-center experience and review of the literature. Ultrasound Obstet Gynecol 2013;41:162–167. [DOI] [PubMed] [Google Scholar]
- 10.Joshi AN, Rane HS, Kamble RC, Mestry PJ, Maniar H, Shah Y. Prenatal diagnosis of absent pulmonary valve syndrome: report of 2 cases, most common and most rare presentations. J Ultrasound Med 2010;29:823–829. [DOI] [PubMed] [Google Scholar]
- 11.Rakha S, Alkhushi N. Fetal diagnosis of isolated absent pulmonary valve with intact interventricular septum: How to counsel the parents? Ann Pediatr Cardiol 2020;13:136–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Favilli S, Lapi E, Pollini I, Calabri GB, Bini RM. Prenatal diagnosis and postnatal outcome in patients with absent pulmonary valve syndrome not associated with tetralogy of Fallot: report of one case and review of the literature. J Cardiovasc Med (Hagerstown) 2008;9:1127–1129. [DOI] [PubMed] [Google Scholar]
- 13.Moradian M. Diagnostic errors in echocardiography: review of five interesting pediatric cases. J Tehran Heart Cent 2012;7:33–36. [PMC free article] [PubMed] [Google Scholar]
- 14.Moradian M, Rashidighader F, Golchinnaghash F, Meraji M, Ghaemi HR. Impact of pulmonary valve replacement on left and right ventricular function using strain analysis, in children with repaired tetralogy of Fallot. Egypt Heart J 2023;75:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ghader FR, Abaskhanian ZA. Influence of metoprolol on systolic and diastolic function in children with heart failure. Pak J Biol Sci 2009;12:451–4. [DOI] [PubMed] [Google Scholar]


