Abstract
Balloon aortic valvuloplasty (BAV) is a therapeutic option as palliative or bridging therapy in severe aortic stenosis, even though it is a risky procedure, especially in patients with concomitant left ventricular dysfunction. The use of percutaneous ventricular assist devices, such as the Impella CP, in this scenario provides optimal circulatory support and considerably reduces the risk of the procedure. Two patients with severe aortic stenosis and left ventricular dysfunction underwent BAV with the support of the Impella-CP. The Impella CP provided adequate support in both high-risk patients and safely allowed BAV.
Keywords: Aortic stenosis, Valvular heart disease, Percutaneous coronary intervention
Introduction
Balloon aortic valvuloplasty (BAV) can be considered a provisional treatment in hemodynamically unstable patients with severe aortic stenosis (AS) and as a bridging procedure to a definitive aortic valve replacement or transcatheter aortic valve implantation (TAVI).1 BAV is a high-risk procedure due to the temporary obstruction of blood flow through the aortic valve during balloon inflation. Consequently, the decision to perform BAV should be at the discretion of the heart team. The Impella (Abiomed, Danvers, Massachusetts, USA) is a percutaneous left ventricular assist device (PVAD) consisting of a miniaturized axial flow pump fitted onto a pigtail catheter, pumping the blood from the left ventricle (LV) into the ascending aorta and providing a cardiac output of 2.5 L/min (Impella 2.5) and up to 4 L/min (Impella CP).2 This additional output supply makes its use attractive during BAV in order to minimize the risk of the procedure. We describe 2 patients with AS in addition to LV dysfunction undergoing BAV via LV assistance with the Impella CP.
Case Report
Case #1
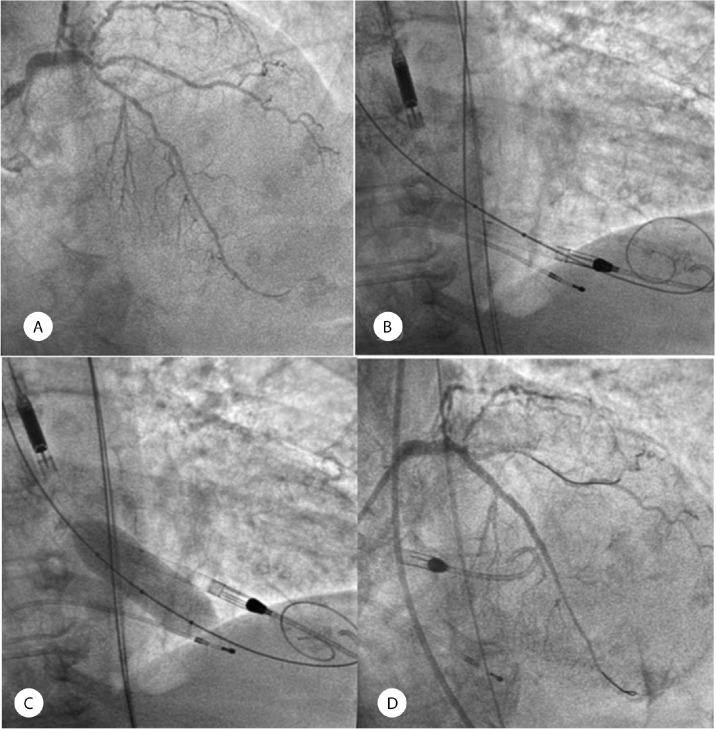
An 81-year-old woman with hypertension, type 2 diabetes mellitus, dyslipidemia, and ischemic cardiomyopathy with triple-vessel disease, including significant lesions in the proximal and mid-segments of the left anterior descending coronary artery (LAD), was referred to our institution (Figure 1A). Transthoracic echocardiography revealed a severely calcified aortic valve with a significant reduction in valve opening. However, aortic gradients were underestimated (the mean and maximum gradients were 17 and 27 mmHg, respectively) due to severe LV dysfunction with an ejection fraction of 28%. The LV-aorta peak-to-peak gradient assessed in the cath lab was 20 mmHg. BAV plus percutaneous coronary intervention with the support of the Impella CP was planned after informed consent had been obtained from the patient. First, a pre-shaped high-support wire was crossed through the aortic valve and positioned into the LV for a posterior BAV. Afterward, an Impella CP was inserted into the LV through the left common femoral artery (Figure 1B). The procedure was performed with a 20 mm Cristal balloon (BALT, Montmorency, France) (Figure 1C), achieving a post-intervention LV-aorta peak-to-peak gradient of 1 mm Hg. Percutaneous coronary intervention was performed on the proximal and mid-segments of the LAD via the implantation of 2 drug-eluting stents, yielding a successful outcome (Figure 1D). The Impella CP was removed in the cath lab since the patient remained hemodynamically stable. The left femoral artery was percutaneously closed with the ProStar XL system (Abbott Vascular).
Figure 1.
A) The images depict significant lesions in the proximal and mid-segments of the left anterior descending coronary artery. B) A high-support pre-shaped wire was introduced into the left ventricle for balloon aortic valvuloplasty. Subsequently, an Impella CP was inserted into the left ventricle over a second dedicated wire. C) Balloon aortic valvuloplasty was performed with the support of an Impella CP. D) The image shows the final result after the implantation of 2 drug-eluting stents in the proximal and mid-segments of the left anterior descending coronary artery.
Case #2
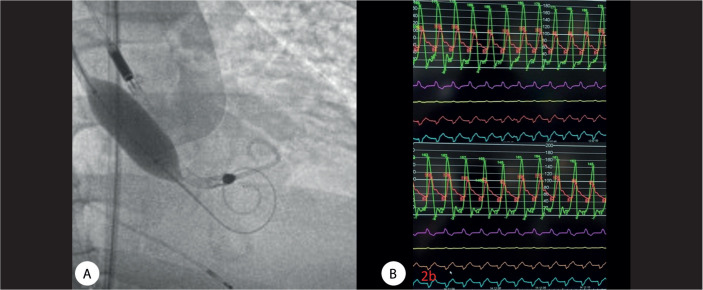
A 64-year-old man with hypertension, obesity, type 2 DM, hypercholesterolemia, former smoking habits, and chronic kidney disease (creatinine=1.4 mg/dL) was diagnosed with severe AS without coronary artery disease. While on the waiting list for aortic valve replacement, the patient presented with cardiogenic shock and atrial fibrillation. His hemoglobin level was initially measured at 7.8 g/dL and subsequently increased to 9.9 g/dL following a blood transfusion. Sinus rhythm was recovered after electrical cardioversion. He developed a progressive respiratory deterioration, requiring orotracheal intubation. Afterward, he presented with electromechanical dissociation, needing cardiopulmonary resuscitation for 6 minutes. A temporary pacemaker was inserted through the jugular vein due to a complete atrioventricular block. Because of hemodynamic instability and after obtaining informed consent from the patient’s guardian, BAV with the Impella CP support, as a bridging procedure, was scheduled. Transthoracic echocardiography showed an ejection fraction of 40% under dobutamine infusion therapy. The LV-aorta peak-to-peak gradient was 70 mmHg before BAV. An Impella CP device was inserted via the left common femoral artery, and BAV was sequentially performed with 18, 20, and 23 mm balloons (Figure 2A). The post-intervention LV-aorta peak-to-peak gradient decreased to 55 mmHg (Figure 2B), and the Impella CP was removed in the cath lab. The patient experienced a gradual clinical improvement and 2 weeks later underwent TAVI with an Edwards SAPIEN 3 (29 mm), yielding a successful outcome.
Figure 2.
A) The image shows balloon aortic valvuloplasty with the Impella CP support. B) The image shows a moderate reduction in the left ventricle-aorta peak-to-peak gradient from 70 mmHg to 55 mmHg after balloon aortic valvuloplasty.
Discussion
The salient finding of this case series is the feasibility of BAV in 2 high-risk patients with AS in addition to LV dysfunction with the support of the Impella CP as a PVAD. The introduction of TAVI has renewed the interest in BAV with a substantial improvement in the technique over time, although the procedure carries a significant risk of complications.3 The procedure is associated with a high risk of adverse events owing to pressure overload of the LV during BAV, particularly in patients with LV dysfunction and hemodynamic compromise.4,5 Indications for BAV in this scenario include palliation of symptoms, bridge to definitive valve replacement, and evaluation of the response.6
Prior studies have reported the use of Impella 2.5 as a supportive device during BAV, with or without additional percutaneous coronary intervention, in patients with cardiogenic shock. These reports have shown positive immediate outcomes, highlighting the potential benefits of using the Impella 2.5 device in such high-risk scenarios.7 The Impella CP offers superior hemodynamic support with a peak flow of 4 L/min, compared with the Impella 2.5. Accordingly, in higher-risk patients, utilizing the Impella during BAV provides additional stability to the balloon during inflation, which may be attributed to a scoring effect.8 In our second case, we directly observed the benefit of this facilitating effect since multiple inflations with various balloon sizes were required.
From a technical standpoint, a crucial initial step in performing BAV is to cross the aortic valve and establish a pre-shaped wire within the LV. Following this, the Impella device should be positioned in the LV to provide hemodynamic support throughout the procedure. It is vital to exercise caution during the procedure to avoid inadvertently introducing the wire into the Impella pump system, as this could potentially lead to damage or compromise the device’s functionality. We believe that the use of the Impella CP as a PVAD in our 2 high-risk patients was crucial to reducing the risk of BAV as much as possible. The Impella CP assistance provided hemodynamic stability during the procedure and enabled us to plan a staged and definitive therapy thereafter.
Conclusion
BAV assisted by the Impella CP in patients with severe AS and LV depression is feasible and provides suitable support during the procedure, reducing the risk of intervention.
Notes:
This paper should be cited as: Mohandes M, Fuertes M, Pernigotti A, Zambrano D. Impella CP-Assisted Balloon Aortic Valvuloplasty in 2 High-Risk Patients. J Teh Univ Heart Ctr 2024;19(1):70-73.
References
- 1.Vahanian A, Beyersdorf F, Praz F, Milojevic M, Baldus S, Bauersachs J, Capodanno D, Conradi L, De Bonis M, De Paulis R, Delgado V, Freemantle N, Gilard M, Haugaa KH, Jeppsson A, Jüni P, Pierard L, Prendergast BD, Sádaba JR, Tribouilloy C, Wojakowski W, ESC/EACTS Scientific Document Group . 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J 2022;43:561–632. [DOI] [PubMed] [Google Scholar]
- 2.Meyns B, Stolinski J, Leunens V, Verbeken E, Flameng W. Left ventricular support by catheter-mounted axial flow pump reduces infarct size. J Am Coll Cardiol 2003;41:1087–1095. [DOI] [PubMed] [Google Scholar]
- 3.Alkhouli M, Zack CJ, Sarraf M, Bashir R, Nishimura RA, Eleid MF, Nkomo VT, Sandhu GS, Gulati R, Greason KL, Holmes DR, Rihal CS. Morbidity and Mortality Associated With Balloon Aortic Valvuloplasty: A National Perspective. Circ Cardiovasc Interv 2017;10:e004481. [DOI] [PubMed] [Google Scholar]
- 4.Hamid T, Eichhöfer J, Clarke B, Mahadevan VS. Aortic balloon valvuloplasty: is there still a role in high-risk patients in the era of percutaneous aortic valve replacement? J Interv Cardiol 2010;23:358–361. [DOI] [PubMed] [Google Scholar]
- 5.Buchwald AB, Meyer T, Scholz K, Schorn B, Unterberg C. Efficacy of balloon valvuloplasty in patients with critical aortic stenosis and cardiogenic shock--the role of shock duration. Clin Cardiol 2001;24:214–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ford TJ, Nguyen K, Brassil J, Kushwaha V, Friedman D, Allan R, Pitney M, Jepson N. Balloon Aortic Valvuloplasty in the Transcatheter Valve Era: Single Centre Indications and Early Safety Data in a High Risk Population. Heart Lung Circ 2018;27:595–600. [DOI] [PubMed] [Google Scholar]
- 7.Karatolios K, Chatzis G, Luesebrink U, Markus B, Ahrens H, Tousoulis D, Schieffer B. Impella support following emergency percutaneous balloon aortic valvuloplasty in patients with severe aortic valve stenosis and cardiogenic shock. Hellenic J Cardiol 2019;60:178–181. [DOI] [PubMed] [Google Scholar]
- 8.Megaly M, Jones P. Impella CP-assisted balloon aortic valvuloplasty. J Cardiol Cases 2016;14:49–51. [DOI] [PMC free article] [PubMed] [Google Scholar]