Abstract
Terpios hoshinota Rützler & Muzik, 1993 is a poriferan species that competes with corals and is known as a coral-killing sponge. However, limited information is available on its biology, including morphological traits. This study aims to examine the arrangement and development of spicules in various body parts of the sponge, including settled larvae. Spicules were found to appear on the second day after settlement and were present in all individuals on the fifth day. The spicules in the thread-like tissue of the growth portion were oriented in the direction of growth, with their pointed tips facing forward to support the elongated pioneer tissue. Furthermore, the spicules in the surface layer of the sponge tissue were perpendicular, with outward-facing tips associated with collagens. The study indicates that the spicules of T. hoshinota are arranged to support both encrusting basal tissue and pioneering tissue to colonize corals.
Keywords: Coral, Cyanobacteriosponge, Defense, Sponge larvae, Structure
BACKGROUND
Sponges are the oldest metazoans and exhibit remarkable diversity (van Soest et al. 2012). They contribute significantly to coral reefs as water filters, substratum stabilizers and hosts of diverse symbionts (Wulff 2012). It is suggested that the regime will shift in coral reefs resulted in increasing sponge and/or macroalgae coverage and decreasing coral coverage. This shift also leads to a reduction in coral populations on a global scale due to ongoing climate change (HoeghGuldberg et al. 2007; Bruno et al. 2019). Concurrently, certain sponges known as ‘coral-killing sponges’ are spreading in the reefs, posing a significant threat to the corals and their associated organisms in the diverse reef ecosystems (Yamashiro et al. 2023).
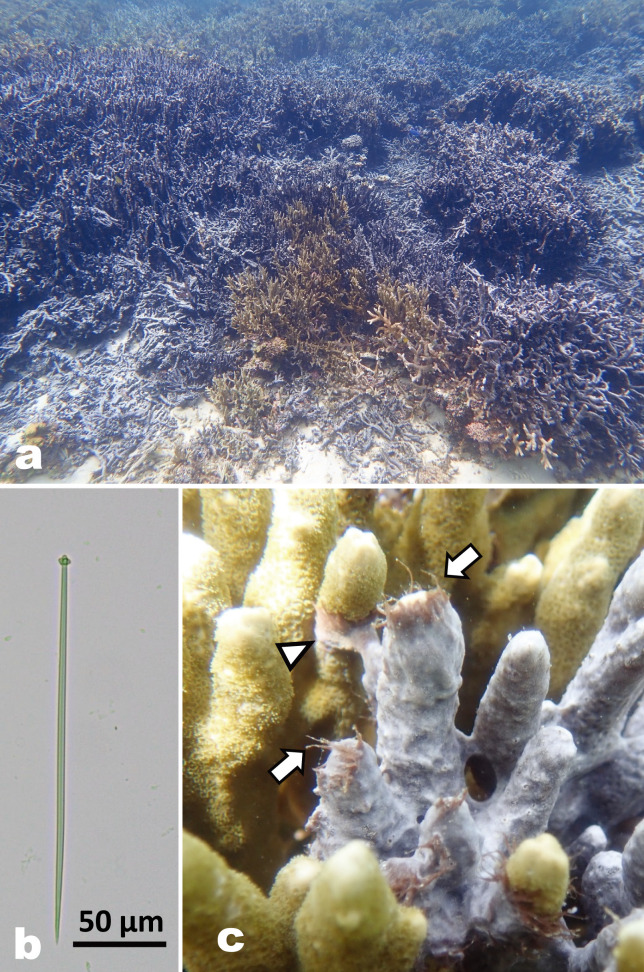
Since its initial report in 1973 (Bryan 1973) in Guam, the Mariana Islands, a blackish thin encrusting demosponge Terpios hoshinota has been observed widely in the Indo-Pacific Ocean, such as Taiwan (Liao et al. 2007; Wang et al. 2012b), Japan (Yamaguchi 1986; Reimer et al. 2011a b; Yomogida et al. 2017; Yamashiro et al. 2023), the Southeast China Sea (Shi et al. 2012; Yang et al. 2018), Indonesia (de Voogd et al. 2013; van der Ent et al. 2016), Australia (Fujii et al. 2011; Fromont et al. 2019), the Maldives (Montano et al. 2015), and Papua New Guinea (Ekins et al. 2017). The sponge harbors numerous cyanobacteria as a symbiont and overgrows live corals serving as substrates (PlucerRosario 1987; Hirose and Murakami 2011; Syue et al. 2021; Aini and Yamashiro 2022). It appears as if there is a competitive interaction between the sponge and corals in terms of the cyanobacteria and zooxanthellae, the symbiotic algae of corals. T. hoshinota is found thriving in patches patches in certain reefs in Okinawa Island, Japan (Reimer et al. 2011a; Aini et al. 2021; Hirose et al. 2021). In the northern part of Okinawa Island, particularly in Nakijin and Ogimi, the sponge extensively covers hard corals (e.g., branching Montipora digitata, the dominant coral species in the shallow reefs), shells, and even plastics such as abandoned fishing gear. Due to the high coverage of sponges, these shallow reefs have transformed into blackish fields (Fig. 1a). Understanding the potential impact of this sponge outbreak upon interactions between sponges, corals, and associated organisms is essential for coral conservation and the health of the reef ecosystem. However, limited knowledge of the sponge, combined with the impracticality of maintaining it in an aquarium for the necessary length of time, has hindered the acquisition of crucial biological information including morphological traits, especially at each life stage of its life cycle.
This study examines the arrangement and development of spicules of T. hoshinota to deepen our understanding of the sponge’s fundamental morphology. In T. hoshinota, the spicules are pin-shaped monoaxon spicules with characteristic lobed heads (tylostyle type) (see Fig. 1b) (Rützler and Muzik 1993; Fujii et al. 2011). These spicules are produced in a thin (< 1 mm) sponge tissue, and their length ranges from 129 to 329 µm (Rützler and Muzik 1993; Hoeksema et al. 2014; Ekins et al. 2017; Aini and Yamashiro 2022). We conducted two experiments to investigate spicule arrangements of mature bodies (thread tissue and base tissue) and the development of spicules in juveniles, specifically examining their absence/presence over time.
Fig. 1.
a) Aggregates of branching coral Montipora digitata are being replaced by the sponge Terpios hoshinota in Ogimi, Okinawa Island. b) Tylostyle spicule with a lobed head and a sharpened end of T. hoshinota. c) T. hoshinota overgrowing M. digitata in Nakijin. Thread tissue (arrow) and a thread reaching a neighboring coral branch (arrowhead).
Thread tissues extending from the base tissue are commonly observed at the growing front (Fig. 1c), and serve as a means to detect new substrates, similar to a sensor (Soong et al. 2009). The interactions between T. hoshinota and corals at the growing front of the sponge vary and are not uniformly species-specific when the sponge aggressively overgrows corals (Wang et al. 2012a 2015). Examining the arrangements in the thread and base tissues can help to assess their role in interactions between the sponge and other organisms. Hsu et al. (2013) reported the presence of spicules in juvenile T. hoshinota on the fifth day after settlement, although the number of individuals was not specified (Hsu et al. 2013). Detailed information regarding culture conditions, including photographic data, still remains unknown. Therefore, the present study aims to explore the spicule development in juveniles obtained during the larval release period (Hirose et al. 2022). The depiction of spicule development at varying life stages from swimming larvae to attached juveniles, and the organization of spicules in mature individuals, would advance the comprehension of the essential elements and functions of T. hoshinota.
MATERIALS AND METHODS
Sample collection
Individuals of Terpios hoshinota were collected from the shallow moat of Nakijin (26°42'30.9"N, 127°56'59.2"E) in the northern area of Okinawa Island, Japan, where there is a dense population of T. hoshinota (Hirose et al. 2022). Sampling was conducted from September 2019 to July 2020. Individuals of T. hoshinota overgrowing on the branching coral Montipora digitata were collected using diagonal nippers. Sponge samples were brought to the marine laboratory, Sesoko Station, University of the Ryukyus, Sesoko Island, Japan (26°39'07.82"N, 127°51'23.26"E), 12 km from Nakijin, and kept in an outdoor aquarium (28.7 L in volume, shaded with a screen, 25% light intensity) supplied with running seawater at a flow rate of 7.5 L/min.
Arrangement of spicules in sponge tissue
To compare the arrangement of spicules in sponge tissues, 10 sponge tissues were divided into 3 parts: (1) the tip of the thread tissue, (2) the middle of the thread tissue arising from the base tissue, and (3) the base tissue covering the coral skeletons (Fig. 2). To observe the spicules of the tip and middle of the thread, the thread tissues were cut with scissors from the growth front and divided into tip and middle parts, and subsequently placed on a microscope slide glass. By carefully adding a drop of 0.5% sodium hypochlorite solution under a cover glass to bleach colored sponge tissues due to numerous cyanobacteria, this procedure allowed the sponge tissue to be cleared so that transparent silicate spicules could be observed. The spicules were observed under the light microscope (Eclipse Ci, Nikon Co.) equipped with a digital camera (I-J1, Nikon Co.). To observe the spicules in the basal tissues, the sponge tissues were separated from the coral skeleton by decalcification treatment. Coral branches were fixed in 10% formalin seawater for 8 hours, and coral skeletons were kept in the decalcifying solution containing 12% acetic acid and 6% formalin. After decalcification, the sponge tissues were washed with tap water, cut to appropriate size, placed on a microscope slide glass, and bleached as described above.
Fig. 2.
a) Coral branch overgrown with T. hoshinota showing many threads coming from the base tissue. b) Enlarged image showing a tip and a middle part of the thread (th). The lower part is covered by numerous tiny white sand particles.
For histologic observation, 10 sponge individuals from overgrown coral branches were fixed in 10% formalin-seawater for 8 hours and preserved in 80% ethanol until use. Sponge samples were decalcified to remove the coral skeleton for 14 to 21 days depending on fragment size. Sponge tissues were washed with tap water, dehydrated through a graded series of ethanolxylene solutions, embedded in paraffin, sectioned vertically and/or horizontally at 7 µm thickness, stained with a Picro-sirius red staining kit (Scy TeK Laboratories, Inc.) to stain collagen, and observed under a light microscope. Spicule length was measured using ImageJ (NIH 1.52a, imagej.nih.gov/ij).
Assessment of sponge spicules on fishing lines
During the field survey, T. hoshinota growing on discarded fishing lines were frequently observed at the sampling site, which had a depth of less than 2 meters. These fishing lines were collected from Nakijin reef on September 17, 2019 to observe the arrangement of spicules on a thin linear substrate. After confirming the efficiency of observing the arrangement of spicules on the discarded fishing lines, new nylon fishing lines (0.2 mm in diameter and 1m long) were placed on the T. hoshinota patches in the same reef on September 26, 2019. T. hoshinota eventually covered and grew on the fishing lines, and these fishing lines were collected on two separated dates, on December 29, 2019 and May 27, 2020. Another set of fishing lines discarded in a patch of T. hoshinota was found by chance in Nakijin and collected on June 30, 2020. These four fishing lines covered with sponge were kept in the outdoor tank of the marine laboratory and supplied with running seawater until observation. The diameter of these fishing lines ranged from 0.1 mm to 2 mm.
To observe the arrangement of spicules on a fine fishing line, sponge cyanobacteria were bleached with organic solvent (ethanol or methanol) and sponge tissue was digested with the enzyme trypsin (EC.3.4.21.4). Ten samples of nylon fishing lines covered with T. hoshinota were cut into 5 cm long pieces, then placed in a 5 ml tube filled with 100% methanol and kept for more than 1 day to extract and remove the pigments of the sponge cyanobacteria. Another treatment was tested in which the samples were first fixed in 10% formalin-seawater for 8 hours and then transferred to 80% ethanol. After removal of the pigments by both treatments, the samples were placed in a 0.1% trypsin solution in a tissue culture plate (6-well type, Becton Dickinson Labware, Co.). After soaking in 0.1% trypsin solution for 2 to 3 days in an incubator at 35°C (IC-150MA, As One Co. Japan), all samples were air dried. Both treatments with methanol or 80% ethanol prior to soaking in trypsin solution were effective in observing the arrangement of spicules on fishing lines. The arrangement of spicules growing on the fishing lines was observed under a stereomicroscope (SMZ 1000, Nikon Co.).
Spicule formation and development in juveniles
Larvae released from parent sponge individuals, reared in an aquarium at Sesoko Station, were collected in September and October 2020(Hirose et al. 2022). They were then transferred to a Petri dish (90 × 15 mm, As One Co., Japan) or a 6-well tissue culture plate (9.6 cm2/well, Becton Dickinson Co., USA), made of sterile polystyrene and maintained under laboratory conditions (room temperature at about 25°C, under 12L/12D light regime, 50 µmol m-2 sec-1 under LED light, ISL-150X, CCS Co. Japan). Filtered seawater (< 0 µm) in a Petri dish/6-well tissue culture plate was changed daily for the culture of swimming larvae and settled juveniles. To confirm the presence/absence of spicules in larvae, 15 pre-settlement (day 0) larvae were used. The development of spicules in juveniles was observed daily from day 1 to day 21 after settlement. After day 8, observations were made every 3 days. A successful settlement was defined as a larva with its anterior pole attached to the substrate and its body flattened (Whalan et al. 2008). The total number of tested larvae and juveniles by days varied from 2 to 17. The settled juveniles on a Petri dish or a 6-well tissue culture plate were carefully removed and placed on a microscope slide glass treated with 0.5% sodium hypochlorite solution to bleach the sponge tissue. The length of the spicules was measured using ImageJ.
RESULTS
Arrangement of spicules in sponge tissue
Figure 3 shows the arrangement of spicules from tip to base of the tissues of T. hoshinota. The threads were composed of stems and surrounding tissues (Fig. 3a). In the stem, spicules were bundled together to form a bony framework (Fig. 3a, b). In the tip, the spicules were always oriented in the direction of growth, with the pointed side facing forward (Fig. 3c). In the middle part of the thread, two types of spicule arrangement were observed. One is a stem/stalk type consisting of densely packed bundles of spicules, oriented with the pointed side forward (Fig. 3b). The other type of arrangement consists of dispersed spicules, some of which are tilted outward. In the basal tissues covering the coral skeletons, protruding spicules were observed in living tissue before treatment with sodium hypochlorite solution, but most spicules were randomly crossed in the sponge bodies (Fig. 3d).
Fig. 3.
Arrangement of spicules in the thread and basal tissues of T. hoshinota. Tissues were treated with bleach (sodium hypochlorite) and trypsin to show the arrangement of spicules. Particles (p) were trapped by the sponge from the surrounding seawater. a) Spicules forming a bundled stream at the tip of the thread tissue. b) Middle part of the thread tissue showing a stem of bundled spicules and surrounding sparse spicules. c) Enlarged view of the tip. Spicules are oriented in the direction of growth. d) Spicules in the base tissue are randomly arranged (crisscrossed) compared to the thread tissue.
Spicule length ranged from 129 to 253 µm (201 ± 32 µm, mean ± SD, n = 126) in basal tissue, and from 148 to 256 µm (213 ± 24 µm, mean ± SD, n = 104) at the terminal end of the threads. There was a significant difference between the two groups (p < 0.05, Welch’s t-test). The number of shorter spicules (< 200 µm) in the tips of the thread and base tissues was 24 (23%) and 46 (37%), respectively.
Histological observation of spicule arrangement
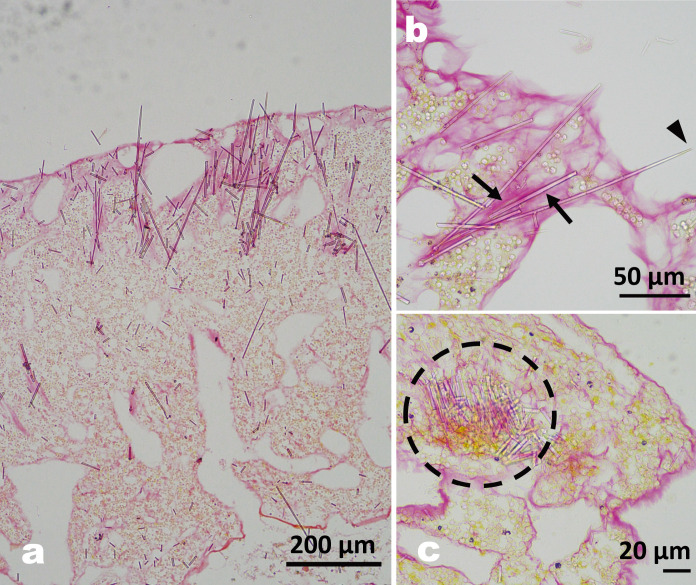
Figure 4a shows a longitudinal section through the soft tissue of T. hoshinota. Both coral skeleton and calcium carbonate particles trapped by the sponge near the outer layer were dissolved during the decalcification process. Sections stained with Picro-sirius red for collagenous tissue showed that mesophyl (amorphous substance in the sponge, stained red) accumulated where spicules had accumulated in the surface layer (Fig. 4a, b). Some spicules arranged their pointed side to protrude through the outer layer (Fig. 4a, b). The cross section of the thread tissue (Fig. 4c) shows a bundle of spicules near the center of the thread.
Fig. 4.
a) Longitudinal section of T. hoshinota stained with Picro-sirius red. b) Enlarged section showing that the spicules are fixed by collagenous tissue (stained pink, arrows), and a protruding spicule (arrowhead). c) Cross section of the thread showing a cluster of spicules in the center (dashed circle) forming a stem-like structure with collagenous tissue.
Assessment of sponge spicules on fishing lines
Thin fishing lines covered with T. hoshinota were bleached and then digested to reveal the arrangement of spicules. Before digestion, the upright spicules were observed under the microscope (Fig. 5a). Figure 5a shows that the spicules are erect through the outermost layer of pinacoderms, with the sharp tips pointing outward. Upright spicules surrounded the surface of a fishing line like a cleaning brush (Fig. 5b). There was no clustering of spicules in fishing line.
Fig. 5.
T. hoshinota covering an abandoned fishing line. a) Pointed tips of spicules protruding from the sponge tissue (before bleaching treatment). b) Spicules showing a brush-like structure all over the surface of the calcareous alga covering the fishing line (after bleaching/digesting treatment).
Spicule formation and development in juveniles
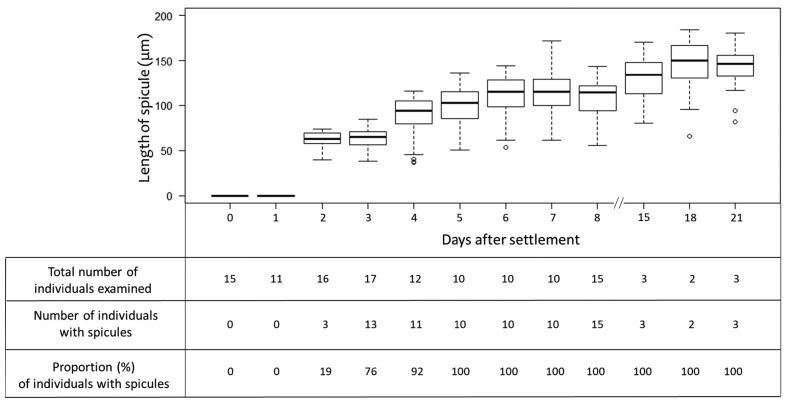
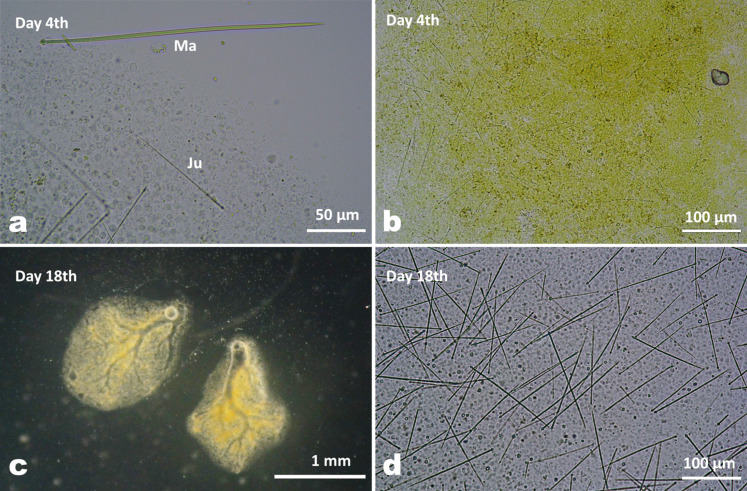
When the larvae settled with their anterior end on the substrate, telescopic movements were observed, the larvae expanded and flattened on the substrate, and then successful settlement was confirmed. Figure 6 shows that no larvae or juveniles had spicules in their bodies on the day of release (day 0) and the next day (day 1). On the 2nd day after settlement, few juveniles (3 out of 16 juveniles) produced tiny spicules with obscure heads compared to the spicules of mature individuals (Fig. 7a, b). A gradual development of spicules in terms of length was confirmed between the 2 and 6 days after settlement (Fig. 6). The mean length of spicules measured in juveniles (n = 60) was 62.3 ± 8 µm (mean ± SD, day 2), 63.4 ± 11 µm (day 3) and 89.9 ± 20 µm (day 4). Spicules were found in all juveniles examined after day 5 with a mean length of 100.5 ± 20 µm (mean ± SD, day 5), while the beginning of the formation of ostia and canal systems was observed, with canals appearing in a small number of juveniles on day 4 after settlement. The size range of spicules increased from day 2 (39.4 to 70.7 µm) to day 8 (55.5 to 133.3 µm). In addition, the density of spicules was visually different on day 4 compared to day 18, with more spicules observed in the latter juveniles that had developed canal systems with oscula (Fig. 7 c, d). In juveniles, spicules were randomly arranged in their tiny bodies. Spicules protruding from the surface layer were also observed in a dried juvenile body (not shown).
Fig. 6.
Box plot showing the sequential changes in the spicule length of juvenile T. hoshinota. The lower and upper boundaries of the box indicate the 25th and 75th percentiles, respectively. The horizontal line inside the box indicates the median. Error bars above and below the box indicate the 10th and 90th percentiles, respectively. Dots indicate outliers.
Fig. 7.
Spicules of settled juveniles of T. hoshinota. a) Spicules on the 4th day after settlement, and a spicule from a mature sponge tissue for comparison. Ma: a spicule from a mature individual (242.7 µm). Ju: a spicule from a juvenile (105.3 µm). b) Bleaching status of larval tissue on day 4 after settlement using 0.5% sodium hypochlorite solution. Soft tissue is in the process of decomposition. Short spicules were sparsely present. c) Juveniles 18 days after settlement. The canal system with oscula is fully developed. d) Spicules in juveniles 18 days after settlement.
The number of juveniles carrying calcium carbonate particles originating from filtered sea water was 55% on day 1 (settlement day), 25% on day 2, 76% on day 3, 58% on day 4, 60% on day 5, 50% on day 6 and 50% on day 7. The number of particles in each juvenile was not abundant, and one to three particles were determined.
DISCUSSION
The present study investigated the arrangement of spicules in the body tissue and juveniles of Terpios hoshinota. Spicules of the sponge have a direction with a tylostyle lobed head and a spear-like end. The role of spicules has been suggested mainly as a framework for body plans and as a deterrent to predators (Uriz et al. 2003). In the class Demospongiae, most sponges produce spicules from siliceous elements, which are classified into six basic types with intermediate forms: hymedesmoid, plumose, axial, radiate, reticulate, or arranged in strength confusion (Boury-Esnault and Rützler 1997). The hymedesmoid type, in which spicules are arranged singly with their heads attached to the basal plate and their tips pointing upward, is found in thinly encrusting sponges (Boury-Esnault and Rützler 1997; Uriz et al. 2003). Plumose is a type of skeletal construction consisting of primary fibers or spicule tracts from which skeletal elements radiate obliquely (Boury-Esnault and Rützler 1997).
In the thread tissue of T. hoshinota, the spicules were oriented like a stream from the base part of the sponge to the growing front. In the center of the threads, the spicules formed a stem-like bundle, which allowed for a bone-like, robust structure. Wang et al. (2012a) observed the diverse interactions between coral and T. hoshinota. They described a high density of cyanobacteria and spicules at the front of the sponge, which they referred to as the “hairy tip”. It is possible that the cyanobacteria play a role in supporting the stemlike bundle, eventually forming a robust structure of the thread. In addition to the stem-like bundle of spicules, the orientation of the spicules surrounding the stem was outwardly oriented in the thread tissues. Rützler and Muzik (1993) noted that spicules were organized into radiating bundles near the ectosomal region when this sponge was first identified. Threads with stem-like bundles of spicules inside may originate from where these radiating bundles occur at the ectosomal layer, which was described as plumose (Boury-Esnault and Rützler 1997). Histological sections of the stem-like structure in the thread tissue showed that collagenous substance functions as a cement to interlock the spicules and support this bony framework. This mass of spicules may serve as a scaffold to support the thread tissue, which acts as a pioneer tissue to find suitable substrates as observed by Soong et al. (2009).
In the freshwater demosponge Ephydatia fluvatilis, spicule movement was recorded by time-lapse imaging using fluorescence techniques, suggesting that spicules are transported from the site of production and carried by spicule-transporting cells (EflSoxB1-expressing cells) (Nakayama et al. 2015; Funayama 2019). In T. hoshinota, the mean lengths of spicules showed a significant difference between two tissue parts. In the thread, spicules were longer (mean length was 213 µm) than those of the base (201 µm), suggesting that threads had fully grown spicules that were transported from the base to the thread tissues, rather than being produced in the growing anterior tissues.
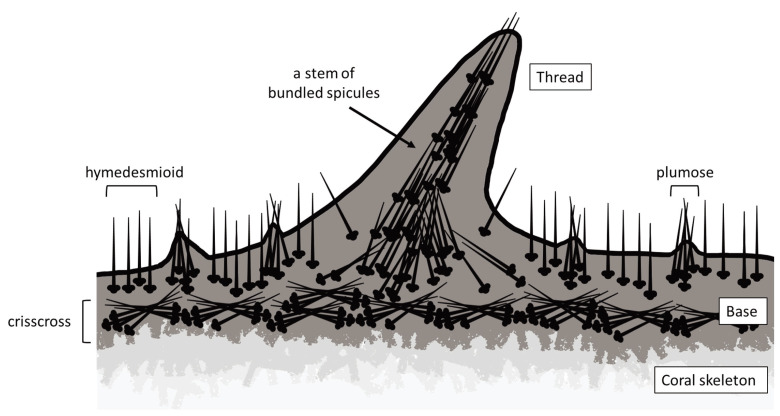
On fishing lines covered with T. hoshinota, spicule heads were fixed in basal tissues and their tips were directed upward all around the periphery as hymedesmioid arrangement like a brush. Histological observation of the choanosome cross section indicated that T. hoshinota probably belonged to a hymedesmioid spicule type like other members of the family Suberitidae, such as Suberites luna (Giraldes et al. 2020) and S. diversicolor (Becking and Lim 2009). In basal tissues, spicules were randomly crossed, which may help thin encrusting sponge tissues to grow firmly on various substrates. Crisscrossing in choanosomes as well as brush-like structure observed in this study were consistent with the observation in the previous study (Rützler and Muzik 1993). In the same genus of Terpios, tylostyle spicules in T. fugax, T. manglaris, and T. belindae were also arranged in strands traversing the choanosome and in brush-like structures protruding through the ectosome (Rützler and Smith 1993). Figure 8 summarizes the arrangement of spicules determined in the present study in T. hoshinota.
Fig. 8.
The schematic drawing of the spicules in T. hoshinota, based on the observations made in the present study. The spicules’ arrangement and direction are drawn in various parts of the sponge tissue.
In addition to scaffolding, the defensive role of spicules are suggested in previous studies. In coral reefs, many organisms are known to be spongivorous such as wrasses, angelfish, sea urchins, and other generalists (Chanas and Pawlik 1996; Hill and Hill 2002; Ferguson and Davis 2008). Hill and Hill (2002) demonstrated the changes in spicule concentrations in tropical sponge bodies in response to simulated predation events, suggesting that spicules may be effective in antipredator defense. In contrast, Chanas and Pawlik (1996) showed that three common Caribbean demosponges were predated upon by reef fishes regardless of spicule orientation, whether they were oriented perpendicularly or in tracts parallel to the surface, suggesting that spicule orientation may be arranged for structural rather than anti-predator purposes. While the deterrent effect of spicules in sponges has been discussed, it is difficult to demonstrate the efficiency of feeding deterrents on this sponge because it is still a mystery whether any organisms feed on T. hoshinota. The previous study examined the physiological performance of the sponge-cyanobacteria association in its interaction with corals. It was suggested that both physiological outperformance and morphological response play a role in competing with corals (Wang et al. 2015). Further research is needed to answer the remaining questions, such as whether there are predators that actually feed on T. hoshinota, to understand the functions of the brushlike structures or the pointed orientation of the siliceous spicules, and to assess how these arrangements are controlled by the sponge, which may be involved in competition with other organisms on coral reefs.
Hsu et al. (2013) found that T. hoshinota juveniles produced spicules on the fifth day after settlement. In the present study, we showed the variation of spicule length over time and verified that the fastest spicule production occurred on the second day through continuous observation. In this study, two types of spicule arrangements were observed in juveniles under the microscope: a hymedesmioid structure in dried samples and a random arrangement in bleached sponge tissue. Compared to adults, a significantly lower number of spicules were observed to be arranged in a brush-like configuration, extending from their tips. Consequently, the majority of spicules appeared to be randomly arranged immediately after their development, which could potentially influence specific roles as they develop and expand. Spicule growth rates in the freshwater sponge Ephydatia muelleri (Lieberkühn 1856) were measured under different environmental culture conditions. (Uriz 2006). The results indicate that growth rates increased with temperature, especially in the range of 10 to 25°C (Uriz 2006). Investigating the development of spicules in early-stage juveniles under various temperature conditions is essential due to the long period of larval release for T. hoshinota (Nozawa et al. 2016; Hirose et al. 2022). In addition to temperature conditions, light availability may also affect juvenile growth and spicule formation, given that larvae harbor cyanobacteria (Wang et al. 2012b). Further research is essential to understand the configuration, role, and growth of spicules at different developmental stages of settled juveniles.
Aini and Yamashiro (2022) measured the particle
size of T. hoshinota and found a mean diameter of 22.3 µm. The number of calcium carbonate particles in juveniles was few and stable. These particles were trapped from the sea water by the juveniles and low particle count may be attributed to the use of 10 µm filtered seawater in the juvenile culture.
CONCLUSIONS
Various observations on wet/dried specimens and histological analysis corroborate that the sponge has different arrangements of spicules. These diverse arrangements may be related to specific functions in each part of their body: interlocking as a bony framework, especially in thread tissues, and standing upright in the tips of thread or thin tissues near the surface layer. These flexible arrangements suggest that the spicules of T. hoshinota may have roles as scaffolds supporting encrusting basal tissues, as well as pioneer tissues exploring new suitable substrates, which may contribute to their wide distribution in coral reefs.
Acknowledgments
We are grateful to Dr. Y. Ise for his valuable comments on the morphology of the sponge. Comments from an anonymous reviewer greatly improved the manuscript. This work was supported by JSPS (Japan Society for the Promotion of Science) KAKENHI (19K06091). We thank the staff of Sesoko Station, Tropical Biosphere Research Center, University of the Ryukyus for their kind support. Coral sampling was carried out with the permission of the authorities of Okinawa Prefecture, Japan (No. 30-87, 2-2).
Footnotes
Authors’ contributions: All authors contributed to the study conception and design. Sampling was done by YH and HY. All authors read and approved the final manuscript. Sampling and field studies:All necessary permits for sampling and observational field studies were obtained from the appropriate authorities and are mentioned in the acknowledgments section.
Competing interests: The authors declare that they have no conflicts of interest.
Availability of data and materials: The datasets generated or analyzed during the current study are available from the corresponding author on reasonable request.
Consent for publication: Not applicable.
Ethics approval consent to participate: Not applicable.
References
- Aini SN, Yamashiro H. 2022. Densities of cyanobacterial cells, spicules, and particles in the coral-killing sponge Terpios hoshinota in Sesoko Island, Okinawa, Japan. Plankton Benthos Res 17:263–270. doi:10.3800/pbr.17.263.
- Aini SN, Tang S-L, Yamashiro H. 2021. Monthly progression rates of the coral-killing sponge Terpios hoshinota in Sesoko Island, Okinawa, Japan. Coral Reefs 40: 973–981. doi:10.1007/s00338 021-02099-6.
- Becking LE, Lim SC. 2009. A new Suberites (Hadromerida: Suberitidae) species from the tropical Indo West-Pacific. Zool Meded 83:853–862.
- Boury-Esnault N, Rützler K. 1997. Thesaurus of sponge morphology. Smithsonian Contrib Zool No.596, 55pp.
- Bruno JF, Cote IM, Toth LT. 2019. Climate change, coral loss, and the curious case of the parrotfish paradigm: Why don’t marine protected areas improve reef resilience? Ann Rev Mar Sci 11:307–334. doi:10.1146/annurev-marine-010318-095300. [DOI] [PubMed]
- Bryan PG. 1973. Growth rate, toxicity and distribution of the encrusting sponge Terpios sp. (Hadromerida: Suberitidae) in Guam, Mariana Islands. Micronesica 9:237–242.
- Chanas B, Pawlik JR. 1996. Does the skeleton of a sponge provide a defense against predatory reef fish? Oecologia 107:225–231. doi:10.1007/BF00327906. [DOI] [PubMed]
- Ekins M, Willis B, Bridge T, Srinivasan M, Rowley S, Hooper JNA. 2017. The coral killing sponge Terpios hoshinota in Kimbe Bay, Papua New Guinea. Mem Queensl Mus Nature 60:174–175. doi:10.17082/j.2204-1478.60.2017.2017-02.
- Ferguson AM, Davis AR. 2008. Heart of glass: spicule armament and physical defense in temperate reef sponges. Mar Ecol Prog Ser 372:77–86. doi:10.3354/meps07680.
- Fromont J, Richards ZT, Wilson NG. 2019. First report of the coralkilling sponge Terpios hoshinota Rützler and Muzik, 1993 in Western Australia: A new threat to Kimberley coral reefs? Diversity 11:184. doi:10.3390/d11100184.
- Fujii T, Keshavmurthy S, Zhou W, Hirose E, Chen CA, Reimer JD. 2011. Coral-killing cyanobacteriosponge (Terpios hoshinota) on the Great Barrier Reef. Coral Reefs 30: 483. doi:10.1007/s00338 011-0734-6.
- Funayama N. 2019. Produce, carry/position, and connect: morphogenesis using rigid materials. Curr Opin Genet Dev 57:91–97. doi:10.1016/j.gde.2019.08.001. [DOI] [PubMed]
- Giraldes BW, Goodwin C, Al-Fardi NAA, Engmann A, Leitão A, Ahmad AA, Ahmed KO, Abudulkader HA, Al-Korbi HA, Easa HSS, Eltai NOA, Hanifi-Moghaddam P. 2020. Two new sponge species (Demospongiae: Chalinidae and Suberitidae) isolated from hyperarid mangroves of Qatar with notes on their potential antibacterial bioactivity. PLoS ONE 15: e0232205. doi:10.1371/journal.pone.0232205. [DOI] [PMC free article] [PubMed]
- Hill MS, Hill AL. 2002. Morphological plasticity in the tropical sponge Anthosigmella varians: response to predators and wave energy. Biol Bull 202:86–95. doi:10.2307/1543225. [DOI] [PubMed]
- Hirose E, Murakami A. 2011. Microscopic anatomy and pigment characterization of coral-encrusting black sponge with cyanobacterial symbiont, Terpios hoshinota. Zool Sci 28:199–205. doi:10.2108/zsj.28.199. [DOI] [PubMed]
- Hirose Y, Aini SN, Yamashiro H. 2021 Contact reactions between individuals of the coral-killing sponge Terpios hoshinota. Zool Stud 60:41. doi:10.6620/ZS.2021.60-41. [DOI] [PMC free article] [PubMed]
- Hirose Y, Aini SN, Yamashiro H. 2022. Coral-killing sponge Terpios hoshinota releases larvae at midnight. Coral Reefs 41:149–160. doi:10.1007/s00338-021-02210-x.
- Hoegh-Guldberg O, Mumby PJ, Hooten AJ, Steneck RS, Greenfield P, Gomez E, Harvell CD, Sale PF, Edwards AJ, Caldeira K, Knowlton N, Eakin CM, Iglesias-Prieto R, Muthiga N, Bradbury RH, Dubi A, Hatziolos ME. 2007. Coral reefs under rapid climate change and ocean acidification. Science 318:1737–1742. doi:10.1126/science.1152509. [DOI] [PubMed]
- Hoeksema BW, Waheed Z, de Voogd NJ. 2014. Partial mortality in corals overgrown by the sponge Terpios hoshinota at Tioman Island, Peninsular Malaysia (South China Sea). Bull Mar Sci 90:989–990. doi:10.5343/bms.2014.1047.
- Hsu C-M, Wang J-T, Chen CA. 2013. Larval release and rapid settlement of the coral-killing sponge, Terpios hoshinota, at Green Island, Taiwan. Mar Biodiv 43: 259–260. doi:10.1007/s12526-013-0176-1.
- Liao M-H, Tang S-L, Hsu C-M, Wen K-C, Wu H, Chen W-M, Wang J-T, Meng P-J, Twan W-H, Lu C-K, Dai C-F, Soong K, Chen CA. 2007. The “black disease” of reef-building corals at Green Island, Taiwan -outbreak of a cyanobacteriosponge, Terpios hoshinota (Suberitidae; Hadromerida). Zool Stud 46:520.
- Montano S, Chou WH, Chen CA, Galli P, Reimer JD. 2015. First record of the coral-killing sponge Terpios hoshinota in the Maldives and Indian Ocean. Bull Mar Sci 91:97–98. doi:10.5343/bms.2014.1054.
- Nakayama S, Arima K, Kawai K, Mohri K, Inui C, Sugano W, Koba H, Tamada K, Nakata YJ, Kishimoto K, Arai-Shindo M, Kojima C, Matsumoto T, Fujimori T, Agata K, Funayama N. 2015. Dynamic transport and cementation of skeletal elements build up the pole-and-beam structured skeleton of sponges. Curr Biol 25:2549–2554. doi:10.1016/j.cub.2015.08.023. [DOI] [PubMed]
- Nozawa Y, Huang Y, Hirose E. 2016. Seasonality and lunar periodicity in the sexual reproduction of the coral-killing sponge, Terpios hoshinota . Coral Reefs 35: 1071–1081. doi:10.1007/s00338-016 1417-0.
- Plucer-Rosario G. 1987. The effect of substratum on the growth of Terpios, an encrusting sponge which kills corals. Coral Reefs 5:197–200. doi:10.1007/BF00300963.
- Reimer JD, Mizuyama M, Nakano M, Fujii T, Hirose E. 2011a. Current status of the distribution of the coral-encrusting cyanobacteriosponge Terpios hoshinota in southern Japan. Galaxea 13:35–44. doi:10.3755/galaxea.13.35.
- Reimer JD, Nozawa Y, Hirose E. 2011b. Domination and disappearance of the black sponge: A quarter century after the initial Terpios outbreak in southern Japan. Zool Stud 50:394.
- Rützler K, Muzik K. 1993. Terpios hoshinota, a new cyanobacteriosponge threatening Pacific reefs. Sci Mar 57:395–403.
- Rützler K, Smith KP. 1993. The genus Terpios (Suberitidae) and new species in the «Lobiceps» complex*. Sci Mar 57:381–393.
- Shi Q, Liu G-H, Yan H-Q, Zhang H-L. 2012. Black disease (Terpios hoshinota): A probable cause for the rapid coral mortality at the northern reef of Yongxing Island in the South China Sea. AMBIO 41:446–455. doi:10.1007/s13280-011-0245-2. [DOI] [PMC free article] [PubMed]
- Soong K, Yang S-L, Chen CA. 2009. A novel dispersal mechanism of a coral-threatening sponge, Terpios hoshinota (Suberitidae, Porifera). Zool Stud 48:596.
- Syue S-T, Hsu C-H, Soong K. 2021. Testing of how and why the Terpios hoshinota sponge kills stony corals. Sci Rep 11:7661. doi:10.1038/s41598-021-87350-4. [DOI] [PMC free article] [PubMed]
- Uriz MJ, Turon X, Becerro MA, Agell G. 2003. Siliceous spicules and skeleton frameworks in sponges: origin, diversity, ultrastructural patterns, and biological functions. Microsc Res Tech 62:279–299. doi:10.1002/jemt.10395. [DOI] [PubMed]
- Uriz MJ. 2006. Mineral skeletogenesis in sponges. Can J Zool 84:322–356. doi:10.1139/Z06-032.
- van Soest RWM, Boury-Esnault N, Vacelet J, Dohrmann M, Erpenbeck D, de Voogd NJ, Santodomingo N, Vanhoorne B, Kelly M, Hooper JNA. 2012. Global diversity of sponges (Porifera). PLoS ONE 7:e35105. doi:10.1371/journal.pone.0035105. [DOI] [PMC free article] [PubMed]
- van der Ent E, Hoeksema BW, de Voogd NJ. 2016. Abundance and genetic variation of the coral-killing cyanobacteriosponge Terpios hoshinota in the Spermonde Archipelago, SW Sulawesi, Indonesia. J Mar Biol Assoc UK 96: 453–463. doi:10.1017/ S002531541500034X.
- de Voogd NJ, Cleary DFR, Dekker F. 2013. The coral-killing sponge Terpios hoshinota invades Indonesia. Coral Reefs 32:755. doi:10.1007/s00338-013-1030-4.
- Wang J-T, Chen Y-Y, Meng P-J, Sune Y-H, Hsu C-M, Wei K-Y, Chen CA. 2012a. Diverse interactions between corals and the coralkilling sponge, Terpios hoshinota (Suberitidae: Hadromerida). Zool Stud 51:150–159.
- Wang J-T, Hirose E, Hsu C-M, Chen Y-Y, Meng P-J, Chen CA. 2012b. A coral-killing sponge, Terpios hoshinota, releases larvae harboring cyanobacterial symbionts: an implication of dispersal. Zool Stud 51:314–320.
- Wang J-T, Hsu C-M, Kuo C-Y, Meng P-J, Kao JK, Chen CA. 2015. Physiological outperformance at the morphologicallytransformed edge of the cyanobacteriosponge Terpios hoshinota (Suberitidae: Hadromerida) when confronting opponent corals. PLoS ONE 10:e0131509. doi:10.1371/journal.pone.0131509. [DOI] [PMC free article] [PubMed]
- Whalan S, Ettinger-Epstein P, Battershill C, de Nys R. 2008. Larval vertical migration and hierarchical selectivity of settlement in a brooding marine sponge. Mar Ecol Prog Ser 368:145–154. doi:10.3354/meps07573.
- Wulff J. 2012. Ecological interactions and the distribution, abundance, and diversity of sponges. Adv Mar Biol 61:273–344. doi:10.1016/B978-0-12-387787-1.00003-9. . [DOI] [PubMed]
- Yamaguchi M. 1986. Introduction to the study of coral reefs 4 coralreef sponges (1) Sponges as destroyers of reef-building corals. Aquabiology 8:88–92. (in Japanese)
- Yamashiro H, Wada N, Tang SL. 2023. Succession and spread of coral diseases and coral-killing sponges with special reference to microbes in Southeast Asia and adjacent waters. In: I Takeuchi and Yamashiro H (eds), Coral reefs of Eastern Asia under anthropogenic impacts. Coral reefs of the World. Vol. 17, Springer. pp.73–96. doi:10.1007/978-3-031-27560-9.
- Yang S-Y, Chen H-J, Ho M-J, Chen Y-J, Huang Y-Y, Chow W-S, Tang S-L, Jeng M-S, Chen AC. 2018. Outbreak of coral-killing cyanobacteriasponge, Terpios hoshinota in Taiping Island (Itu Aba), Spratlys, South China Sea. Bull Mar Sci 94:1543–1544. doi:10.5343/bms.2018.0023.
- Yomogida M, Mizuyama M, Kubomura T, Reimer JD. 2017. Disappearance and return of an outbreak of the coral-killing cyanobacteriosponge Terpios hoshinota in southern Japan. Zool Stud 56:7. doi:10.6620/ZS.2017.56-07. [DOI] [PMC free article] [PubMed]