Abstract
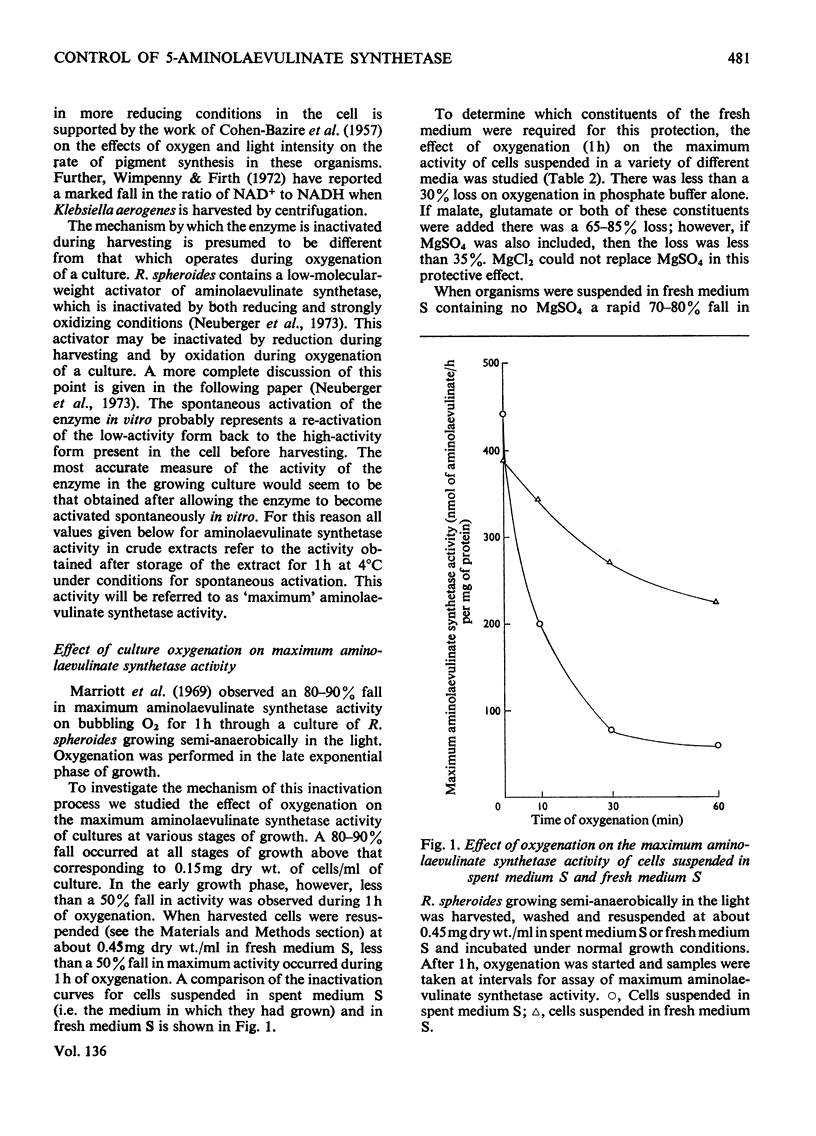
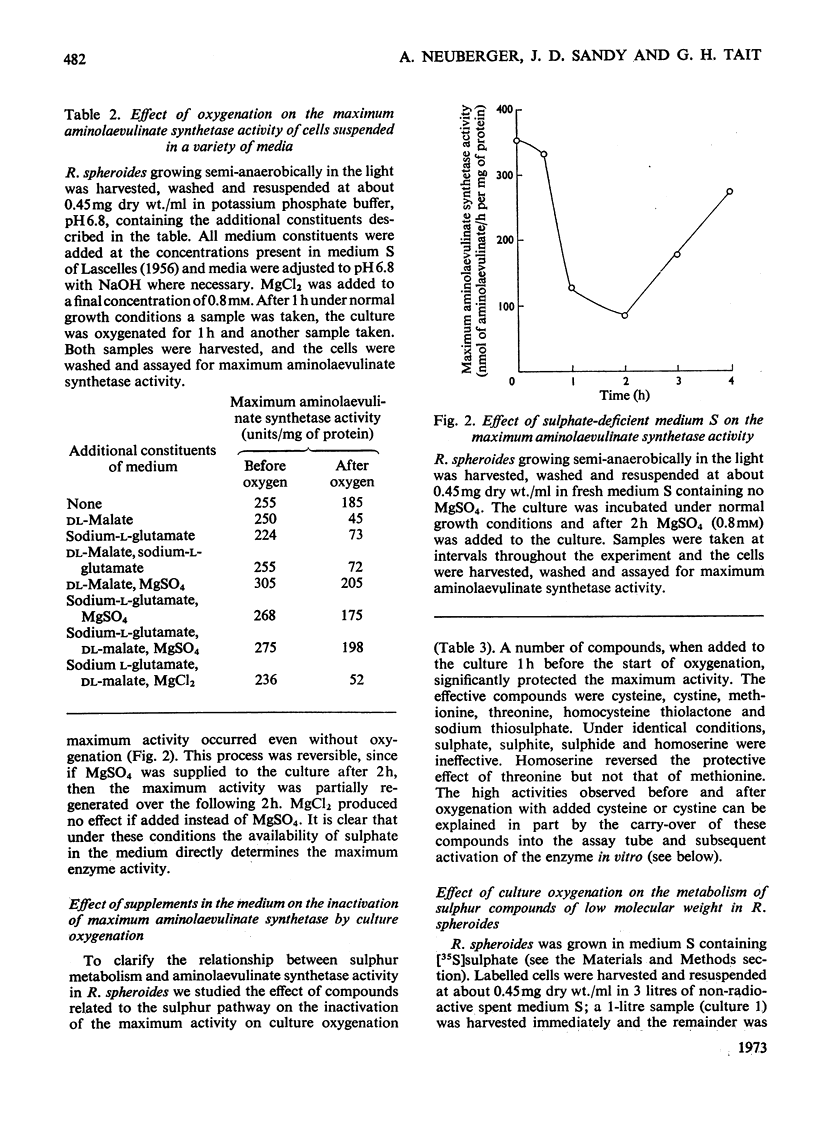
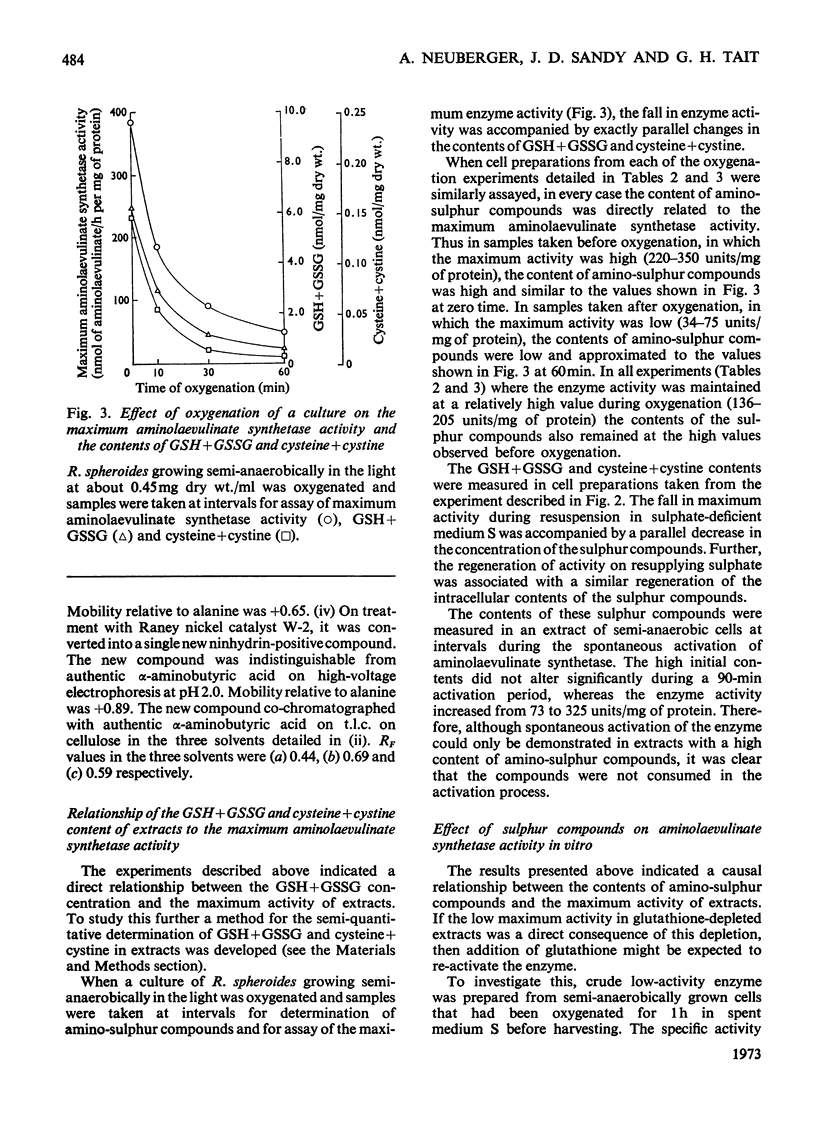
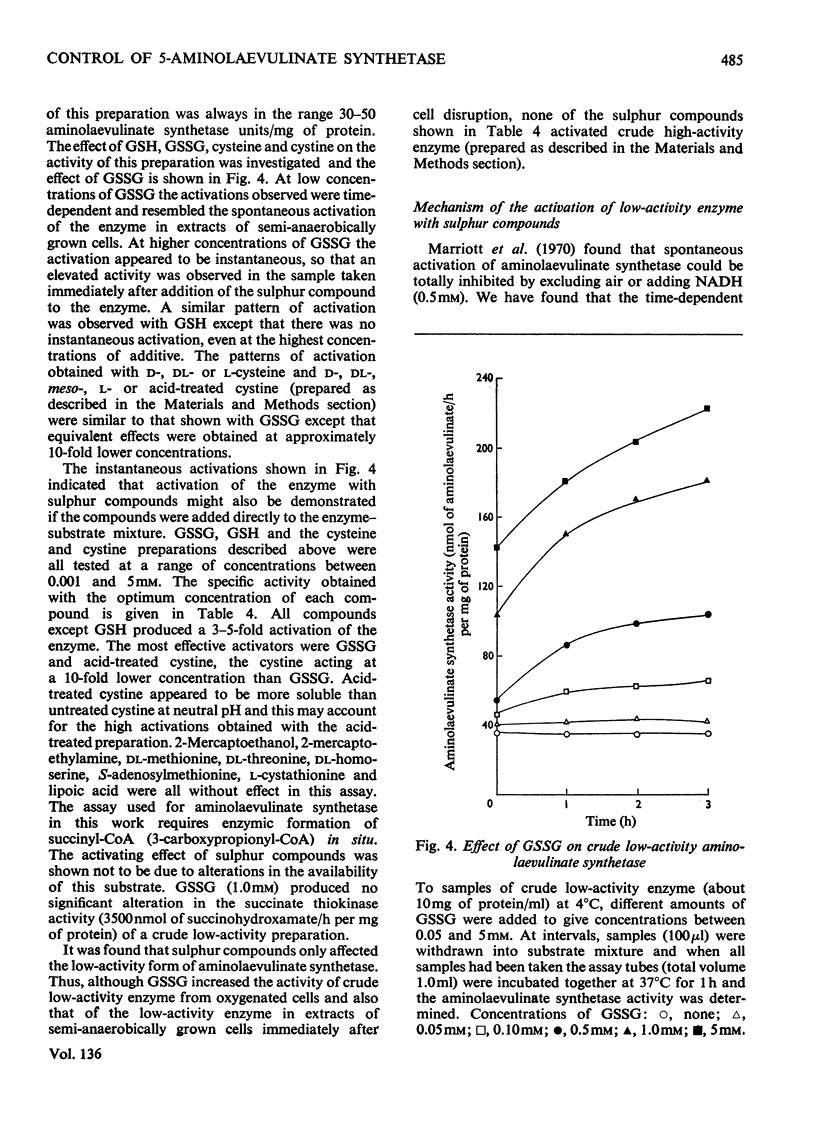
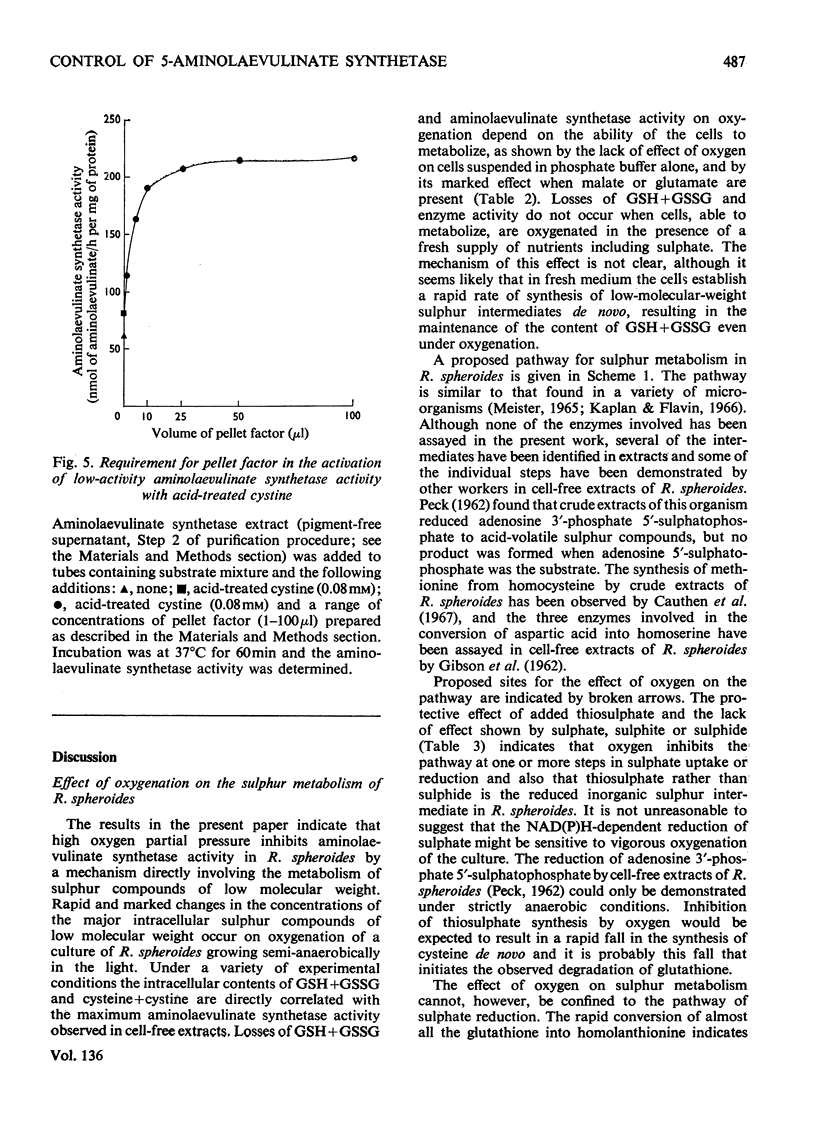
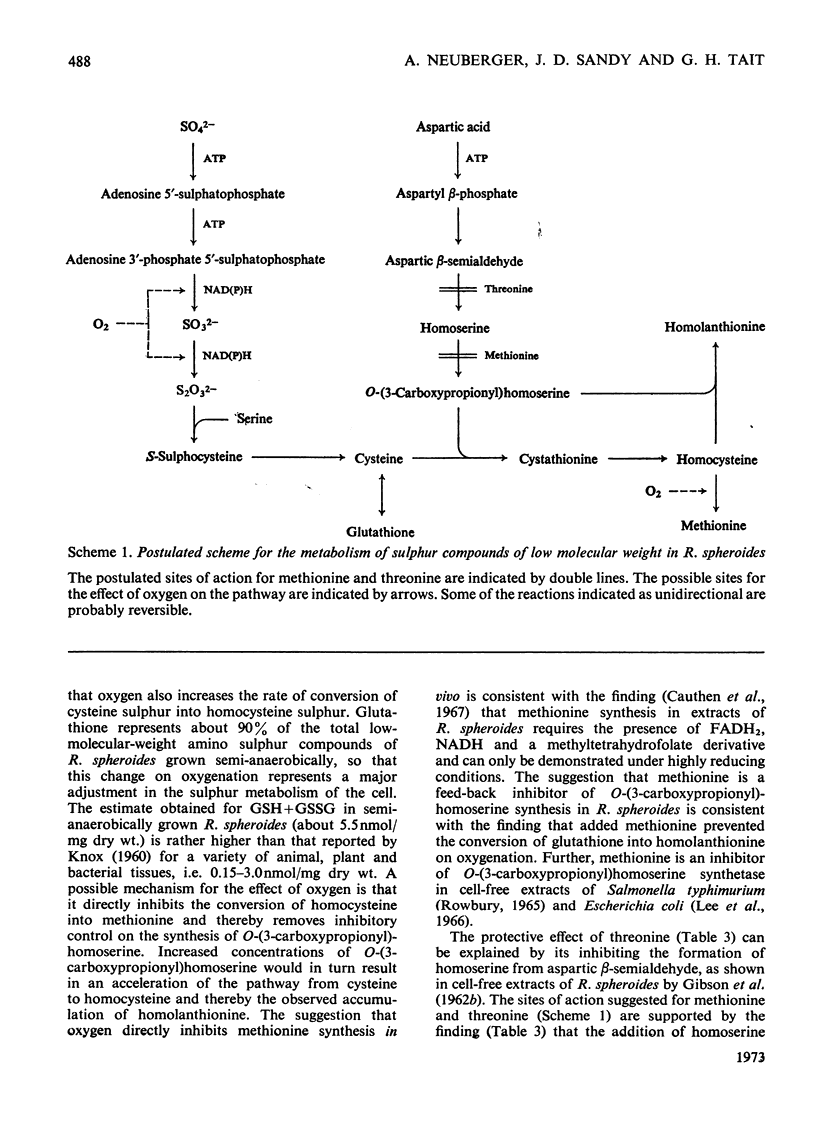
1. The `initial' 5-aminolaevulinate synthetase activity, that is the activity observed immediately after cell disruption, in extracts prepared from unharvested semianaerobically grown Rhodopseudomonas spheroides, was twice that observed under the same assay conditions in extracts prepared from harvested cells. 2. The effect of oxygenation of a culture on the `maximum' aminolaevulinate synthetase activity, that is the activity observed 1h after disruption of harvested cells, is markedly influenced by the contents of the growth medium. Oxygenation of organisms for 1h in the medium in which they have grown produces an 80–90% decrease in maximum activity, whereas similar treatment of organisms resuspended in fresh medium produces less than a 40% decrease. 3. This protective effect of fresh medium is absolutely dependent on the presence of sulphate. When cells are suspended in sulphate-deficient fresh medium, the maximum activity falls by 65–75% even without oxygenation. A high maximum activity is regenerated when sulphate is resupplied. 4. When organisms are oxygenated in the medium in which they have grown, the cellular contents of GSH+GSSG and cysteine+cystine fall very markedly and homolanthionine is formed. Both the fall in aminolaevulinate synthetase activity and the changes in sulphur metabolism are largely prevented by the addition of compounds which stimulate synthesis of cysteine de novo or inhibit the conversion of cysteine S into homocysteine S. 5. The maximum aminolaevulinate synthetase activity was directly proportional to the GSH+GSSG content of all cell preparations. In glutathione-depleted extracts the `low'-activity enzyme could be re-activated in vitro by the addition of GSH, GSSG, cysteine or cystine, whereas in extracts with a high glutathione content the `high'-activity enzyme was unaffected by these sulphur compounds. 6. The activation of low-activity enzyme with exogenous sulphur compounds was prevented by excluding air or by adding NADH. Studies with purified enzyme indicate that sulphur compounds do not interact directly with the enzyme, but that their effect is mediated by a number of other endogenous factors.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ATFIELD G. N., MORRIS C. J. Analytical separations by highvoltage paper electrophoresis. Amino acids in protein hydrolysates. Biochem J. 1961 Dec;81:606–614. doi: 10.1042/bj0810606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURNHAM B. F., PIERCE W. S., WILLIAMS K. R., BOYER M. H., KIRBY C. K. delta-aminolaevulate dehydratase from Rhodopseudomonas spheroides. Biochem J. 1963 Jun;87:462–472. doi: 10.1042/bj0870462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHEN-BAZIRE G., SISTROM W. R., STANIER R. Y. Kinetic studies of pigment synthesis by non-sulfur purple bacteria. J Cell Physiol. 1957 Feb;49(1):25–68. doi: 10.1002/jcp.1030490104. [DOI] [PubMed] [Google Scholar]
- Cauthen S. E., Pattison J. R., Lascelles J. Vitamin B(12) in photosynthetic bacteria and methionine synthesis by Rhodopseudomonas spheroides. Biochem J. 1967 Mar;102(3):774–781. doi: 10.1042/bj1020774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIBSON K. D., MATTHEW M., NEUBERGER A., TAIT G. H. Biosynthesis of porphyrins and chlorophylls. Nature. 1961 Oct 21;192:204–208. doi: 10.1038/192204a0. [DOI] [PubMed] [Google Scholar]
- GIBSON K. D., NEUBERGER A., TAIT G. H. Studies on the biosynthesis of prophyrin and bacteriochlorophyll by Rhodoseudomonas spheroides. 3. The effect of threonine on the biosynthesis of homoserine and methionine. Biochem J. 1962 Sep;84:483–490. doi: 10.1042/bj0840483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorchein A. Magnesium protoporphyrin chelatase activity in Rhodopseudomonas spheroides. Studies with whole cells. Biochem J. 1972 Mar;127(1):97–106. doi: 10.1042/bj1270097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorchein A., Neuberger A., Tait G. H. Adaptation of Rhodopseudomonas spheroides. Proc R Soc Lond B Biol Sci. 1968 Aug 13;171(1022):111–125. doi: 10.1098/rspb.1968.0060. [DOI] [PubMed] [Google Scholar]
- HIGUCHI M., GOTO K., FUJIMOTO M., NAMIKI O., KIKUCHI G. EFFECT OF INHIBITORS OF NUCLEIC ACID AND PROTEIN SYNTHESES ON THE INDUCED SYNTHESES OF BACTERIOCHLOROPHYLL AND DELTA-AMINOLEVULINIC ACID SYNTHETASE BY RHODOPSEUDOMONAS SPHEROIDES. Biochim Biophys Acta. 1965 Jan 11;95:94–110. doi: 10.1016/0005-2787(65)90215-7. [DOI] [PubMed] [Google Scholar]
- HUANG H. T. Accumulation of 1-homolanthionine by an Escherichia coli mutant. Biochemistry. 1963 Mar-Apr;2:296–298. doi: 10.1021/bi00902a018. [DOI] [PubMed] [Google Scholar]
- Kaplan M. M., Flavin M. Cystathionine gamma-synthetase of Salmonella. Catalytic properties of a new enzyme in bacterial methionine biosynthesis. J Biol Chem. 1966 Oct 10;241(19):4463–4471. [PubMed] [Google Scholar]
- LASCELLES J. The synthesis of enzymes concerned in bacteriochlorophyll formation in growing cultures of Rhodopseudomonas spheroides. J Gen Microbiol. 1960 Dec;23:487–498. doi: 10.1099/00221287-23-3-487. [DOI] [PubMed] [Google Scholar]
- LASCELLES J. The synthesis of porphyrins and bacteriochlorophyll by cell suspensions of Rhodopseudomonas spheroides. Biochem J. 1956 Jan;62(1):78–93. doi: 10.1042/bj0620078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lascelles J., Altschuler T. Mutant strains of Rhodopseudomonas spheroides lacking delta-aminolevulinate synthase: growth, heme, and bacteriochlorophyll synthesis. J Bacteriol. 1969 May;98(2):721–727. doi: 10.1128/jb.98.2.721-727.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lascelles J. The regulation of haem and chlorophyll synthesis. Biochem Soc Symp. 1968;28:49–59. [PubMed] [Google Scholar]
- Lee L. W., Ravel J. M., Shive W. Multimetabolite control of a biosynthetic pathway by sequential metabolites. J Biol Chem. 1966 Nov 25;241(22):5479–5480. [PubMed] [Google Scholar]
- MAUZERALL D., GRANICK S. The occurrence and determination of delta-amino-levulinic acid and porphobilinogen in urine. J Biol Chem. 1956 Mar;219(1):435–446. [PubMed] [Google Scholar]
- Marriott J., Neuberger A., Tait G. H. Activation of delta-aminolaevulate synthetase in extracts of Rhodopseudomonas spheroides. Biochem J. 1970 Apr;117(3):609–613. doi: 10.1042/bj1170609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marriott J., Neuberger A., Tait G. H. Control of delta-aminolaevulate synthetase activity in Rhodopseudomonas spheroides. Biochem J. 1969 Feb;111(4):385–394. doi: 10.1042/bj1110385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marriott J. Regulation of porphyrin synthesis. Biochem Soc Symp. 1968;28:61–74. [PubMed] [Google Scholar]
- Neuberger A., Sandy J. D., Tait G. H. Control of 5-aminolaevulinate synthetase activity in Rhodopseudomonas spheroides. The purification and properties of an endogenous activator of the enzyme. Biochem J. 1973 Nov;136(3):491–499. doi: 10.1042/bj1360491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PECK H. D., Jr Enzymatic basis for assimilatory and dissimilatory sulfate reduction. J Bacteriol. 1961 Dec;82:933–939. doi: 10.1128/jb.82.6.933-939.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry T. L., Hansen S., MacDougall L. Homolanthionine excretion in homocystinuria. Science. 1966 Jun 24;152(3730):1750–1752. doi: 10.1126/science.152.3730.1750. [DOI] [PubMed] [Google Scholar]
- Rowbury R. J. Resistance to norleucine and control of methionine synthesis in Escherichia coli. Nature. 1965 May 29;206(987):962–963. doi: 10.1038/206962a0. [DOI] [PubMed] [Google Scholar]
- Tallan H. H., Pascal T. A., Schneidman K., Gillam B. M., Gaull G. E. Homolanthionine synthesis by human liver cystathionase. Biochem Biophys Res Commun. 1971 Apr 16;43(2):303–310. doi: 10.1016/0006-291x(71)90753-4. [DOI] [PubMed] [Google Scholar]
- Tuboi S., Hayasaka S. Control of -aminolevulinate synthetase activity in Rhodopseudomonas spheroides. II. Requirement of a disulfide compound for the conversion of the inactive form of fraction I to the active form. Arch Biochem Biophys. 1972 Jun;150(2):690–697. doi: 10.1016/0003-9861(72)90087-2. [DOI] [PubMed] [Google Scholar]
- Tuboi S., Hayasaka S. Partial purification of an enzyme which is required for the conversion of the inactive form to the active form of -aminolevulinate synthetase from Rhodopseudomonas spheroides. J Biochem. 1972 Jul;72(1):219–222. doi: 10.1093/oxfordjournals.jbchem.a129893. [DOI] [PubMed] [Google Scholar]
- Warnick G. R., Burnham B. F. Regulation of prophyrin biosynthesis. Purification and characterization of -aminolevulinic acid synthase. J Biol Chem. 1971 Nov 25;246(22):6880–6885. [PubMed] [Google Scholar]
- Wimpenny J. W., Firth A. Levels of nicotinamide adenine dinucleotide and reduced nicotinamide adenine dinucleotide in facultative bacteria and the effect of oxygen. J Bacteriol. 1972 Jul;111(1):24–32. doi: 10.1128/jb.111.1.24-32.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]


