Abstract
The dynamic control of chiral (enantiomeric) responses in chiral host–guest complexes through external stimuli is a significant challenge in modern chemistry for developing smart stimuli-responsive materials. Herein, we report the (chir)optical properties and chiral recognition behavior of water-soluble chiral naphthotubes (1) under the influence of hydrostatic pressure as an external stimulus. The hydrostatic pressure spectral profiles compared to those obtained at normal pressure revealed the dynamic behavior of 1 under hydrostatic pressure, owing to the flexible linker. In chiral recognition experiments, hydrophilic amino acids such as phenylalanine (Phe) and tryptophan (Trp) exhibited reaction volume changes (ΔV°) of −0.9 cm3 mol–1 for d-Phe, −1.2 cm3 mol–1 for l-Phe, −5.6 cm3 mol–1 for d-Trp, and −7.0 cm3 mol–1 for l-Trp, with enantioselectivity ranging from 1.2 to 1.6. In contrast, hydrophobic chiral styrene oxide (2) showed ΔV° values of 1.5 cm3 mol–1 for R-2 and 3.5 cm3 mol–1 for S-2, with a relatively higher enantioselectivity of up to 7.6. These contrasting effects of hydrostatic pressure primarily originate from the dynamics of chiral naphthotubes.
Keywords: chiral naphthotube, hydrostatic pressure, chiral recognition, enantioselectivity, dynamic control
1. Introduction
The development of smart host molecules, such as artificial receptors and synthetic chemosensors, has garnered significant attention in multidisciplinary chemistry, particularly in the field of supramolecular chemistry.1−6 Such smart supramolecular materials are promising candidates for potential applications in molecular memories, logic gates, and drug delivery systems.7−9 In particular, enantiomeric responses induced by chiral guest molecule complexation are unique characteristics that can be harnessed in molecule-based devices such as 3D optical displays, security tags, and so on.10−13 Currently, the focus is shifting toward controlling such chiral recognition responses, which is expected to lead to the development of even smarter supramolecular systems.14−16 Therefore, in order to dynamically control chiral recognition in supramolecular complexation, a wide variety of external stimuli—such as solvent,17−19 temperature,20,21 electronic excitation,22,23 and mechanical forces24−26 (stress, strain, and pressure)—have been applied.
Recently, hydrostatic pressure or solution-state isotropic pressure has regained attention despite being an old topic, since many aspects, functions, and concepts in “mechano”-science are continually being discovered.25 Nevertheless, mysteries remain in mechanochemical27−29 and mechanobiological systems,30−32 i.e., how, to what extent, and where hydrostatic pressure stimuli affect these targets. Here, we exclude high-pressure solid chemistry using a diamond anvil cell at approximately GPa,33,34 which is beyond our target range of ca. MPa under hydrostatic pressure. Hydrostatic pressure effects in solution media have been investigated since the 1960s,35−45 wherein some host–guest supramolecular systems under hydrostatic pressure have been examined.46−53 However, so far, the hydrostatic pressure stimulus on chiral recognition upon supramolecular complexation has been little regarded as such a dynamic control effector. Very recently, we reported hydrostatic pressure-induced chiral responses upon the complexation of a chiral ion pair (guest) and a fluorescent anion receptor (host) with relatively effective reaction volume changes (ΔV°) of 2.3–9.7 cm3 mol–1.54 Hence, this finding encouraged us to newly explore an appreciable host-chiral guest combination that can be dynamically controlled by hydrostatic pressure. The good explanation of ΔV° in value and sign (instead of ΔG°) was illustrated in the previous host–guest system.25,47
In this study, to dynamically control chiral responses stimulated by hydrostatic pressure, we focused on a chiral water-soluble naphthotube.55,56 Naphthotubes are smart host molecules wherein naphthalene walls are connected by a flexible methylene linker with polar functional groups.56 Therefore, chiral naphthotubes (R2,S2-, and S2,R2-1, Figure 1 (bottom))55 provide a deeper hydrophobic cavity and a polar binding site, the combination of which plays an important role in the chiral discrimination ability in H2O. This cooperative binding motif differs from that operative in other water-soluble chiral hosts, e.g., cyclodextrins,57,58 chirally modified calixarenes,59 -cucurbiturils,60 -pillararenes,61 and other molecular receptors.62−66 Indeed, at an ambient pressure (0.1 MPa), the chiral naphthotube showed relatively good enantioselectivities (KS/KR or KR/KS) of up to 2.0 in H2O when using a series of chiral styrene oxide guests.55 More importantly, as shown in Figure 1 (top), achiral naphthotube derivatives (anti- and syn-isomers) exhibited good hydrostatic pressure effects on ΔV° as −6.3 (to anti) and 3.2 cm3 mol–1 (to syn) for 1,4-dioxane.67 These previous findings may provide us with a hint that the chiral naphthotube will function as an excellent candidate toward a pressure-responsive smart chiroptical material induced by chiral molecule complexation. Herein, we report the dynamic control of the chiral naphthotube (S2,R2-1) during chiral recognition induced by hydrostatic pressurization. For this purpose, we chose the enantiomeric pairs of phenylalanine (d/l-Phe), tryptophan (d/l-Trp), and styrene oxide (2) as hydrophilic guests for the former two and hydrophobic guests for the latter. The results obtained herein provide deeper insights into the factors governing the hydrostatic pressure effect of chiral naphthotubes.
Figure 1.
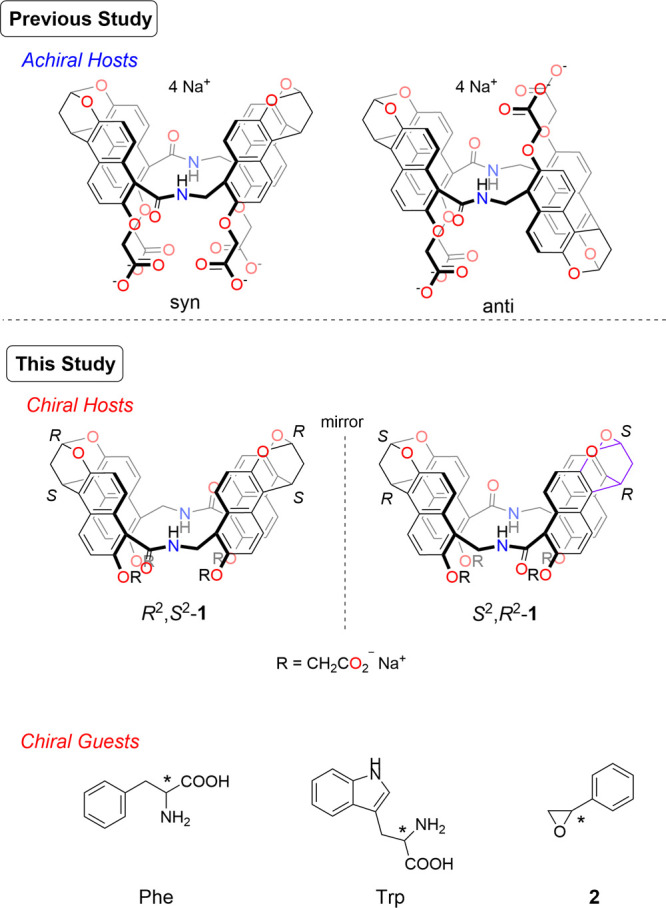
Chemical structures: achiral naphthotubes (top), chiral naphthotubes, and chiral guests used in this study (bottom).
2. Materials and Methods
2.1. Materials
All commercial reagents were used as received without further purification. Fluorescence-free grade water (Milli-Q) was used for spectroscopy. Chiral naphthotubes (R2,S2-, and S2,R2-1) were synthesized according to a literature procedure.55
2.2. Instruments
The UV/vis spectra were measured by using a JASCO V-650 spectrometer. Fluorescence spectra were measured by using a JASCO FP-8500 instrument. The fluorescence lifetime decay profiles were obtained by using a Hamamatsu Quantaurus-Tau single-photon counting apparatus fitted with an LED light source. Circular dichroism (CD) spectra were obtained by using a JASCO J-720WI instrument.
2.3. Hydrostatic Pressure Spectroscopy
Spectroscopic experiments under hydrostatic pressures were conducted using a custom-built high-pressure apparatus; the details are summarized in our previous study.25 Concisely, a quartz inner cell (2 mm path length) with a Teflon tube was filled with an H2O solution of the sample. The cell was then placed into the outer cell, wherein sapphire windows were fitted. The tightly packed outer cell was placed in the spectrometers and hydrostatically pressurized in the range of 0.1–400 MPa. The photographs are shown in Figure S1 in the Supporting Information (SI).25,68
3. Results and Discussion
3.1. (Chir)optical Properties and Molecular Recognition Behavior of 1
Before subjecting the compound to hydrostatic pressurization experiments, we investigated the (chir)optical properties and molecular recognition behavior of 1 in H2O at 0.1 MPa. Although we previously measured circular dichroism (CD) spectra,55 we did not focus on the main band in detail. Here, we report the detailed chiroptical properties of 1. As shown in Figure 2a, at the main band based on the long axis (1Bb transition) of the naphthalene chromophore, a strong bisignate couplet was observed in the anisotropy (g) factor profiles; g239 nm = −0.0024, g226 nm = 0.0011. According to the exciton chirality theory,69 the observed negative exciton coupling suggests that the four naphthalene walls in S2,R2-1 were aligned in a left-handed manner. In the fluorescence spectra (Figure 2b), a slightly large Stokes shift of 3280 cm–1 was observed despite the naphthalene chromophore, indicating excited-state flexibility or relaxation in the chiral naphthotube. As shown in Figure 2c, fluorescence lifetime decays (λem: 403, 450, and 525 nm) were reasonably fitted to a sum of two exponential functions to afford τ1 as 0.4 and τ2 as 3.5 ns, respectively, as listed in Table 1; all decay fitting data are shown in Figures S2–S4 in SI. By shifting the observed wavelength from 403 to 525 nm, τ1 species was preferred (A1: 0.81–0.94), thus indicating that the short-lived species are located in the longer wavelength region; in contrast to τ2 at the shorter wavelength. Therefore, the longer-lived species τ2 was ascribed to monomer-state naphthalene. In addition, the short-lived τ1 can be assigned to the intramolecular ground-state stacked species, according to the promoted radiationless deactivation process.70 In particular, based on the structural features observed in the X-ray single crystal,55 this may occur in the bis(naphthalene) cleft connected by the flexible cycloalkoxy group (purple moiety in Figure 1a (bottom)); the packing structure was given in the previous report.55 The fluorescence quantum yield (ΦF) was 0.07, for which the major stacked species in H2O is highly likely responsible.
Figure 2.
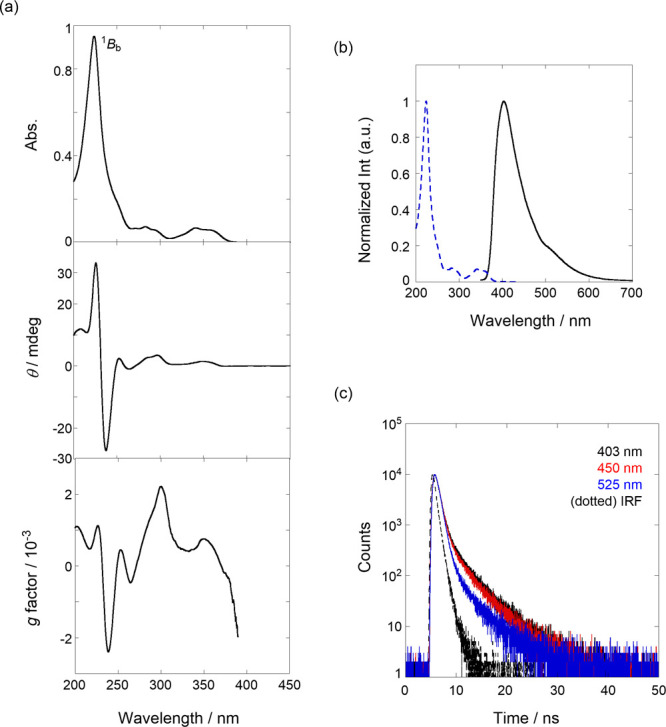
(a) UV/vis (top), CD (middle), and g factor spectra (bottom) of 1 (9.01 μM) in H2O at 25 °C. (b) Fluorescence spectrum (λex: 300 nm, black solid line) of 1 (9.01 μM) in H2O at 25 °C; the blue dotted line represents the normalized UV/vis spectrum. (c) Fluorescence lifetime decays (λex 280 nm) of 1 (9.01 μM), monitored at 403 (black), 450 (red), and 525 nm (blue) at room temperature. All spectra were measured in a 1 cm cell.
Table 1. Fluorescence Lifetimes of 1 in the Absence and Presence of 1,4-Dioxane in H2O at Room Temperaturea.
| compd. | λemb (nm) | τ1 (ns) | A1 | τ2 (ns) | A2 | χ2 |
|---|---|---|---|---|---|---|
| 1c | 403 | 0.4 | 0.81 | 3.5 | 0.19 | 1.3 |
| 450 | 0.4 | 0.85 | 3.5 | 0.15 | 1.2 | |
| 525 | 0.4 | 0.94 | 3.5 | 0.06 | 1.1 | |
| 1 + 1,4-dioxaned | 403 | 1.6 | 1.00 | 1.3 | ||
| 450 | 1.6e | 0.99 | 3.6 | 0.01 | 1.3 | |
| 525 | 1.6 | 0.95 | 3.9 | 0.05 | 1.3 |
Fluorescence lifetime (τi) and relative abundance (Ai) of each excited species, determined by the single-photon counting method in nondegassed solution.
Monitoring wavelength.
[1] = 9.01 μM.
[1] = 9.63 μM, [1,4-dioxane] = 5.56 mM.
Fixed.
3.2. Complexation-Induced Optical Properties of 1: Dynamic Flapping
Second, we investigated the optical properties affected by the complexation of a guest molecule at 0.1 MPa. As shown in Figure 3a,b, the gradual addition of 1,4-dioxane, which was used as a guest molecule for achiral naphthotubes with a binding constant (K) of 103–104 M–1,67 caused a steady increase in the fluorescence intensity, despite negligible changes in the UV/vis spectra. According to the previous binding stoichiometry,67Figure 3c shows the nonlinear least-squares fitting of the fluorescence titration data, assuming a 1:1 stoichiometry, which afforded K as 2930 ± 30 M–1, comparable to that obtained in the achiral naphthotube. To gain deeper mechanistic insights, we measured the fluorescence lifetime decay, as shown in Figure 3d. The decay profiles with and without 1,4-dioxane were also reasonably fitted to two exponentials of τ1 as 1.6 and τ2 as 3.6–3.9 ns (the fitting data are shown in Figures S2 and S3 in SI). The extended τ1 (0.4 → 1.6 ns) can be applicably accounted in terms that the intramolecular stack in the bis(naphthalene) cleft was canceled out due to the inclusion of the bulky guest. The plausible dynamic behavior of compound 1 is illustrated in Figure 3e. If the cleft works as a hinge, 1 is most likely to induce butterfly-like flapping upon guest addition. Eventually, this caused the gradual turn-on of fluorescence signals upon the stepwise addition of hydrophobic guests. At a host occupancy >99.9%, based on the addition of 1,4-dioxane (3.51 mM) to an H2O solution of 1 (9.01 μM), the ΦF value improved to 0.20, supporting the turn-on mechanism.
Figure 3.
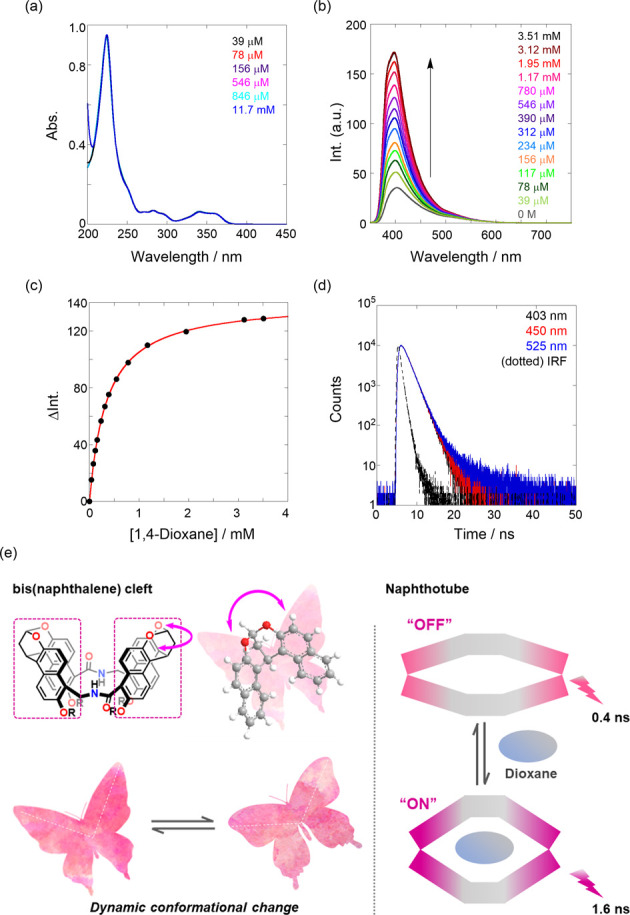
(a) UV/vis and (b) fluorescence spectra (λex 300 nm) of 1 (9.01 μM) upon the addition of 1,4-dioxane (0–3.51 mM, colored lines) in H2O at 25 °C. (c) Nonlinear least-squares fitting, assuming a 1:1 stoichiometry, to determine the binding constant (K) of 1,4-dioxane with 1. (d) Fluorescence lifetime decays (λex = 280 nm) of 1 (9.63 μM) and 1,4-dioxane (5.56 mM), monitored at 403 (black), 450 (red), and 525 nm (blue) at room temperature. All spectra were measured in a 1 cm cell. (e) Schematic illustration of the (left) dynamic flapping and (right) turn-on mechanism of 1.
3.3. Hydrostatic Pressure Effects on 1
Next, we measured the hydrostatic pressure spectroscopy of 1 in the absence of chiral guests. As shown in Figure 4a, a gradual increase in absorbance upon hydrostatic pressurization was observed simply because of the increase in the effective concentration by pressurization. Interestingly, in Figure 4b, the fluorescence intensities gradually decreased, although in general, the intensity of fluorophores increased upon hydrostatic pressurization owing to the inhibition of solvent attack in the excited state, based on the increasing viscosity of the solution used.45 This contrasting fluorescence behavior in 1 vs other fluorophores is well-understood by normalizing the fluorescence spectra (Figure 4c), indicating an increasing amount of intramolecular stacked species in the longer wavelength region. To confirm this further, the hydrostatic pressure lifetime decay (Figure 4d) was measured. As listed in Table 2 and Figure S4, the short-lived τ1 was further decreased from 0.42 to 0.35 ns with increasing hydrostatic pressure, indicating that the intramolecular stacking was further promoted (more stacked). The long-lived τ2 was also decreased from 3.2 to 2.3 ns with elevating pressure, which may be originated from gradual deactivation by pressure-induced solvent attack in the naphthalene chromophore moiety (see Figure 6c, left). Again, this suggests that 1, particularly at the bis(naphthalene) cleft, is flexible or dynamic upon hydrostatic pressurization. In addition, the depressurized fluorescence spectrum (0.1 from 280 MPa (Figure 4b, sky blue line)) was superimposable on the original spectrum of 0.1 MPa (Figure 4b, black line), indicating that dynamic stacking is a reversible process.
Figure 4.
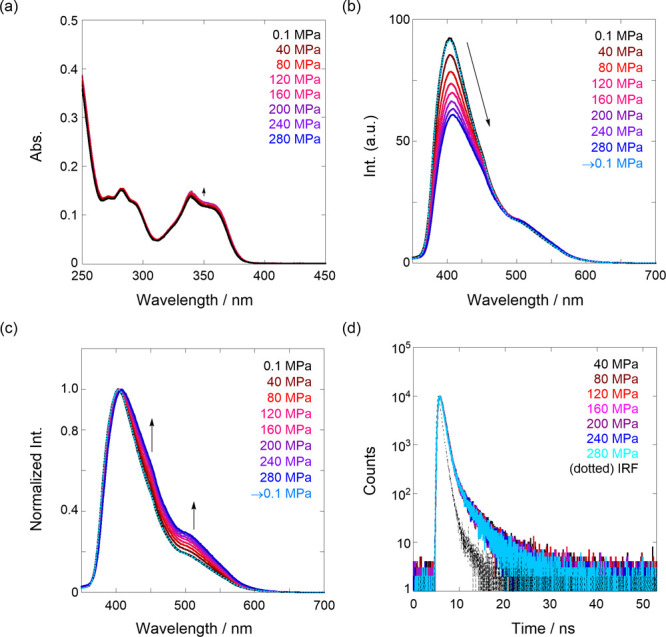
Pressure-dependent UV/vis (a) (90.1 μM), fluorescence (b) (9.63 μM, λex 300 nm), normalized fluorescence spectra (c), and fluorescence lifetime decays (d) (87.9 μM, λex 280 nm, λem 525 nm) of 1 in H2O at room temperature over the range of 0.1–280 MPa. All spectra were measured in a high-pressure cell.
Table 2. Fluorescence Lifetimes of 1 upon the Hydrostatic Pressurization in H2O at Room Temperaturea.
| pressure (MPa) | τ1 (ns) | A1 | τ2 (ns) | A2 | χ2 |
|---|---|---|---|---|---|
| 40 | 0.42 | 0.93 | 3.2 | 0.07 | 1.1 |
| 80 | 0.40 | 0.92 | 3.0 | 0.08 | 1.0 |
| 120 | 0.39 | 0.92 | 2.8 | 0.08 | 1.2 |
| 160 | 0.38 | 0.92 | 2.9 | 0.08 | 1.2 |
| 200 | 0.36 | 0.90 | 2.4 | 0.10 | 1.3 |
| 240 | 0.36 | 0.90 | 2.3 | 0.10 | 0.9 |
| 280 | 0.35 | 0.90 | 2.3 | 0.10 | 1.1 |
Fluorescence lifetime (τi) and relative abundance (Ai) of each excited species, determined by the single-photon counting method in nondegassed solution; [1] = 87.9 μM, λem 525 nm.
Figure 6.
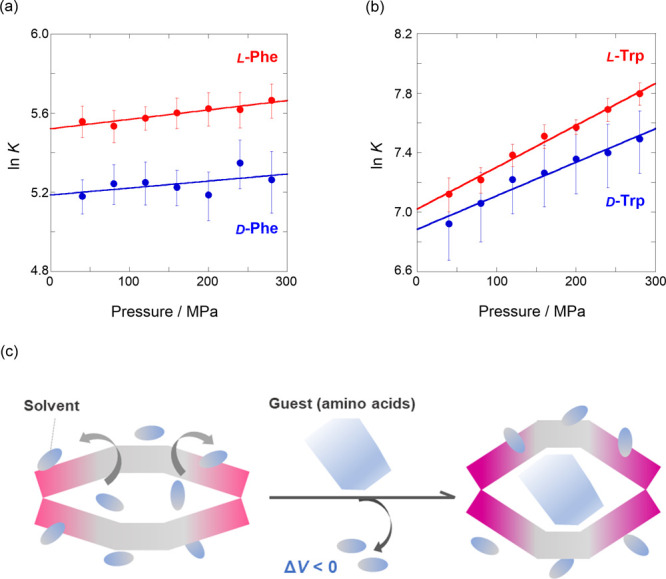
Pressure dependence of the binding constant (K) upon the complexation of (a) Phe (r = 0.541 for d, r = 0.938 for l) and (b) Trp (r = 0.981 for d, r = 0.995 for l) with 1 in H2O in a hydrostatic pressure range of 40–280 MPa at room temperature. (c) Schematic illustration of the dynamic stretch of 1 stimulated by guest inclusion upon hydrostatic pressurization.
3.4. Achiral Guest Complexation of 1 upon Hydrostatic Pressurization
For the hydrostatic pressure-binding behavior, the pressure effect of 1 upon complexation was first investigated by using achiral 1,4-dioxane. As shown in Figure S5, the gradual addition of 1,4-dioxane to an H2O solution of 1 at different pressures (40–200 MPa) caused a steady increase in fluorescence intensity. Therefore, this “turn-on” signaling based on the guest complexation under hydrostatic pressures is most originated from the dynamic flexibility of the bis(naphthalene) cleft (stacking on/off), which is supported by the ambient and hydrostatic pressure fluorescence lifetime measurements (vide supra). Similar to the K value obtained at 0.1 MPa, nonlinear least-squares fitting of the fluorescence increase at each pressure yielded K values, as listed in Table 3. To further evaluate the hydrostatic pressure effect of 1 upon complexation more quantitatively, we calculated ΔV° according to eq 1:
| 1 |
Table 3. Binding Constants (K), Enantioselectivity, and Reaction Volume Changes (ΔV°) for 1:1 Complexation of Achiral and Chiral Guests with 1 in H2O under Hydrostatic Pressure at Room Temperaturea.
| guest | pressure (MPa) | Kb (M–1) | KL/KD | ΔV° (cm3 mol–1) |
|---|---|---|---|---|
| KS/KR | ||||
| 1,4-dioxane | 0.1 | 2902 ± 245 | c | 1.2 ± 0.3 |
| 40 | 2924 ± 213 | |||
| 80 | 2739 ± 230 | |||
| 120 | 2699 ± 237 | |||
| 160 | 2662 ± 241 | |||
| 200 | 2675 ± 267 | |||
| l-Phe | 40 | 260 ± 20 | 1.5 | –1.2 ± 0.2 |
| 80 | 253 ± 21 | 1.3 | ||
| 120 | 264 ± 15 | 1.4 | ||
| 160 | 271 ± 21 | 1.5 | ||
| 200 | 277 ± 23 | 1.6 | ||
| 240 | 276 ± 25 | 1.3 | ||
| 280 | 289 ± 25 | 1.5 | ||
| d-Phe | 40 | 178 ± 15 | c | –0.9 ± 0.6 |
| 80 | 189 ± 19 | |||
| 120 | 191 ± 21 | |||
| 160 | 186 ± 16 | |||
| 200 | 178 ± 22 | |||
| 240 | 210 ± 26 | |||
| 280 | 193 ± 30 | |||
| l-Trp | 40 | 1241 ± 141 | 1.2 | –7.0 ± 0.3 |
| 80 | 1364 ± 115 | 1.2 | ||
| 120 | 1610 ± 119 | 1.2 | ||
| 160 | 1828 ± 146 | 1.3 | ||
| 200 | 1943 ± 94 | 1.2 | ||
| 240 | 2189 ± 168 | 1.3 | ||
| 280 | 2435 ± 180 | 1.4 | ||
| d-Trp | 40 | 1016 ± 223 | c | –5.6 ± 0.5 |
| 80 | 1164 ± 266 | |||
| 120 | 1367 ± 281 | |||
| 160 | 1428 ± 293 | |||
| 200 | 1568 ± 327 | |||
| 240 | 1637 ± 344 | |||
| 280 | 1795 ± 370 | |||
| S-2 | 40 | 12877 ± 991 | 6.8 | 3.5 ± 0.5 |
| 80 | 13618 ± 1142 | 7.6 | ||
| 120 | 12168 ± 944 | 6.7 | ||
| 160 | 12044 ± 1256 | 7.2 | ||
| 200 | 10878 ± 1541 | 6.4 | ||
| 240 | 10159 ± 1555 | 6.2 | ||
| 280 | 9649 ± 1553 | 5.9 | ||
| R-2 | 40 | 1903 ± 348 | c | 1.5 ± 0.3 |
| 80 | 1786 ± 315 | |||
| 120 | 1812 ± 330 | |||
| 160 | 1673 ± 259 | |||
| 200 | 1689 ± 365 | |||
| 240 | 1648 ± 368 | |||
| 280 | 1648 ± 424 |
All titration experiments were performed in a high-pressure cell.
For convenience, we reported values to the nearest whole number for the lnK-P plots.
Not applicable.
As shown in Figure 5, the natural logarithm of each K value obtained for 1,4-dioxane was plotted against pressure with a good linear relationship (r = 0.903), indicating that a single mechanism operated in the range of pressures studied. The ΔV° value obtained from the slope in the plot was 1.2 ± 0.3 cm3 mol–1, which is relatively small but positive. This is highly likely preferable for the tighter stacking of flexible naphthalene walls rather than the complexation-induced extension of the walls, resulting in a slight inhibition of supramolecular complexation upon hydrostatic pressurization. Therefore, the value and sign of ΔV° can provide us with the degree of dynamism in the naphthotube skeleton.
Figure 5.
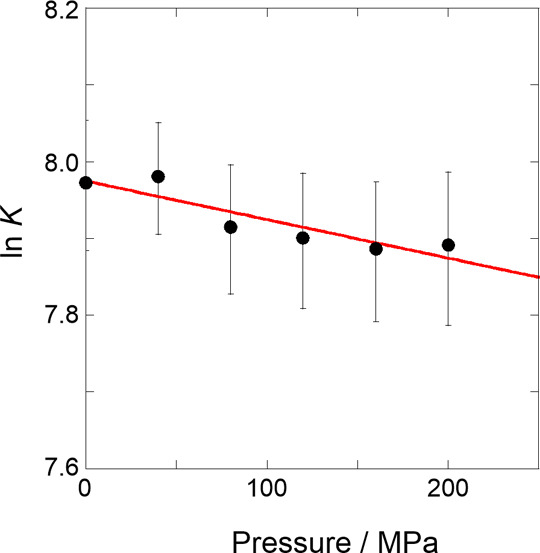
Pressure dependence of the binding constant (K) upon the complexation of 1,4-dioxane with 1 in H2O under hydrostatic pressures (0.1–200 MPa) at room temperature (correlation coefficient r = 0.903).
3.5. Amino Acids Complexation of 1 upon Hydrostatic Pressurization
Next, we investigated the hydrostatic pressure effects on 1 by using chiral guests. First, hydrophilic enantiomeric pairs (D/L) of Phe and Trp were investigated in a similar manner. In Figures S6 and S7, a similar trend for the complexation of Phe is observed, enabling the formation of K at each pressure (see Table 3). The enantioselectivity (KL/KD) of Phe ranged from 1.3 to 1.6, suggesting that the diastereomeric energy difference (1 ⊂ d or l-Phe) had little effect on hydrostatic pressure stimulation. Interestingly, the obtained ΔV° values in the lnK-P plot (Figure 6a) were −0.9 ± 0.6 for d and −1.2 ± 0.2 cm3 mol–1 for l, which are relatively small and similar to those of 1,4-dioxane, but negative. This behavior can be reasonably explained by the preferential opening of the flexible naphthalene walls, causing a slight promotion of supramolecular complexation stimulated by hydrostatic pressure. This is most likely because the desolvation of H2O molecules around the hydrophilic functional groups (COO– and NH3+) in Phe occurred upon complexation, leading to a decrease of ΔV° (negative sign), as illustrated in Figure 6c. A similar investigation of hydrophilic Trp provided stronger evidence of the dynamic behavior in naphthotubes. The routine hydrostatic pressure fluorescence titration of Trp (Figures S8 and S9) exhibited similar turn-on signaling to afford KD and KL at each pressure; the enantioselectivity varied in the range of 1.2–1.4 (see Table 3). More importantly, the lnK-P plot (Figure 6b) gave ΔV° as −5.6 ± 0.5 cm3 mol–1 for d and −7.0 ± 0.3 cm3 mol–1 for l, the larger value of which indicates the more dynamic open-up of the naphthalene walls upon hydrostatic pressurization. As is the case with Phe, this can be reasonably explained in terms of the much greater desolvation of hydrated H2O around the larger hydrophilic Trp moiety than that of Phe, which is most likely the origin.
3.6. Hydrophobic Chiral Guest Complexation of 1 upon Hydrostatic Pressurization: Toward Higher Enantioselectivity
Finally, the extent to which the hydrophobic chiral guest (2) affected the dynamics of the naphthotube was investigated. A similar trend was observed when 2 was added to an H2O solution of 1 under high pressure (Figures S10 and S11, Table 3). Interestingly, the addition of the S enantiomer showed a larger turn-on signal in the lower concentration range than the R enantiomer, indicating stronger binding of the S enantiomer. Surprisingly, the enantioselectivity (KS/KR) varied in the range of 5.9–7.6, which is significantly higher than those obtained in other previous chiral hosts.57−66 This improved enantioselectivity, compared to that observed with hydrophilic amino acids, may be attributed to the dynamic naphthotube tightly conforming to the size and shape of the hydrophobic guest in an induced-fit manner. This was further supported by the ΔV° values obtained from the lnK-P plot: 3.5 ± 0.5 cm3 mol–1 for S (Figure 7a) and 1.5 ± 0.3 cm3 mol–1 for R (Figure 7b). Based on the characteristics of the dynamic naphthotube, the positive ΔV° can be easily explained by the strong solvation around hydrophobic guest 2, which promotes the closing of the naphthalene walls. In other words, both 2 and the solvated H2O molecules strongly bind to the cavity in the naphthotubes, suggesting that such cosolvation plays a critical role in achieving higher chiral discrimination in a dynamic chiral host.
Figure 7.
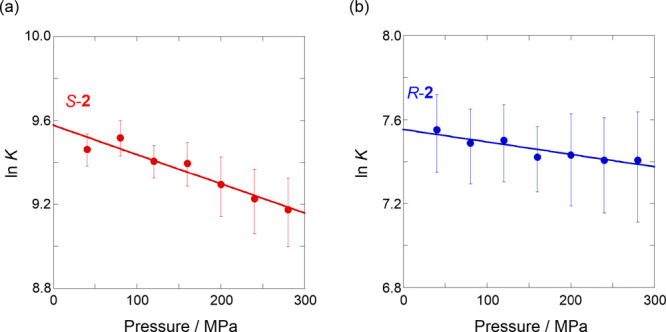
Pressure dependence of the binding constant (K) upon the complexation of (a) S-2 (r = 0.955) and (b) R-2 (r = 0.918) with 1 in H2O in a hydrostatic pressure range of 40–280 MPa at room temperature.
4. Conclusions
In conclusion, we demonstrated hydrostatic pressure-induced chiral recognition of both hydrophilic and hydrophobic guests using water-soluble chiral naphthotubes. The chiral naphthotube used in this study was relatively flexible and dynamic upon hydrostatic pressure stimulation due to its flexible linker. This dynamism critically determines the contrasting complexation of hydrophilic amino acids (negative ΔV°) and hydrophobic 2 (positive ΔV°), with the desolvation/solvation of water likely playing a significant role. Notably, a high enantioselectivity of up to 7.6 was achievable using a hydrostatic pressure control approach. Hence, this study provides valuable guidelines for the development of smart chiral chemosensors, materials, and devices.
Acknowledgments
G.F. appreciates the generous support provided by Grants-in-Aid (Nos. 23H04020 and 24K01536) from the Japan Society for the Promotion of Science (JSPS) and Ajinomoto Co., Inc.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsnanoscienceau.4c00052.
Hydrostatic pressure apparatus, fluorescence lifetime decays, and titration data (DOCX)
Author Contributions
∥ This paper is dedicated to Prof. Wei Jiang, who passed away during this research. CRediT: Junnosuke Motoori data curation, formal analysis, investigation, writing - original draft, writing - review & editing; Tomokazu Kinoshita data curation, formal analysis, investigation, writing - original draft, writing - review & editing; Hongxin Chai investigation, writing - original draft, writing - review & editing; Ming-Shuang Li data curation, formal analysis, investigation, writing - original draft, writing - review & editing; Song-Meng Wang data curation, formal analysis, investigation, writing - original draft, writing - review & editing; Wei Jiang conceptualization, resources; Gaku Fukuhara conceptualization, data curation, formal analysis, funding acquisition, project administration, supervision, validation, writing - original draft, writing - review & editing.
The authors declare no competing financial interest.
Supplementary Material
References
- Shinkai S.; Ikeda M.; Sugasaki A.; Takeuchi M. Positive Allosteric Systems Designed on Dynamic Supramolecular Scaffolds: Toward Switching and Amplification of Guest Affinity and Selectivity. Acc. Chem. Res. 2001, 34, 494–503. 10.1021/ar000177y. [DOI] [PubMed] [Google Scholar]
- You L.; Zha D.; Anslyn E. V. Recent Advances in Supramolecular Analytical Chemistry Using Optical Sensing. Chem. Rev. 2015, 115, 7840–7892. 10.1021/cr5005524. [DOI] [PubMed] [Google Scholar]
- Schroeder V.; Savagatrup S.; He M.; Lin S.; Swager T. M. Carbon Nanotube Chemical Sensors. Chem. Rev. 2019, 119, 599–663. 10.1021/acs.chemrev.8b00340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuhara G. Analytical supramolecular chemistry: Colorimetric and fluorimetric chemosensors. J. Photochem. Photobiol. C: Photochem. Rev. 2020, 42, 100340 10.1016/j.jphotochemrev.2020.100340. [DOI] [Google Scholar]
- Rivera-Tarazona L. K.; Campbell Z. T.; Ware T. H. Stimuli-responsive engineered living materials. Soft Matter 2021, 17, 785–809. 10.1039/D0SM01905D. [DOI] [PubMed] [Google Scholar]
- Hua B.; Shao L.; Li M.; Liang H.; Huang F. Macrocycle-Based Solid-State Supramolecular Polymers. Acc. Chem. Res. 2022, 55, 1025–1034. 10.1021/acs.accounts.2c00011. [DOI] [PubMed] [Google Scholar]
- Molecular Memory and Processing Devices in Solution and on Surfaces; Shipway A. N.; Karz E.; Willner I.; Springer-Verlag: Berlin Heidelberg, 2001. [Google Scholar]
- de Silva A. P.; Gunaratne H. Q. N.; McCoy C. P. A molecular photoionic AND gate based on fluorescent signalling. Nature 1993, 364, 42–44. 10.1038/364042a0. [DOI] [Google Scholar]
- Yang J.; Wang X.; Wang B.; Park K.; Wooley K.; Zhang S. Challenging the fundamental conjectures in nanoparticle drug delivery for chemotherapy treatment of solid cancers. Adv. Drug Delivery Rev. 2022, 190, 114525 10.1016/j.addr.2022.114525. [DOI] [PubMed] [Google Scholar]
- Pu L. Fluorescence of Organic Molecules in Chiral Recognition. Chem. Rev. 2004, 104, 1687–1716. 10.1021/cr030052h. [DOI] [PubMed] [Google Scholar]
- David A. H. G.; Casares R.; Cuerva J. M.; Campaña A. G.; Blanco V. A [2]Rotaxane-Based Circularly Polarized Luminescence Switch. J. Am. Chem. Soc. 2019, 141, 18064–18074. 10.1021/jacs.9b07143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan X.; Xu F.-F.; Zhou Z.; Yan Y.; Yao J.; Zhao Y. S. 3D Laser Displays Based on Circularly Polarized Lasing from Cholesteric Liquid Crystal Arrays. Adv. Mater. 2021, 33, 2104418. 10.1002/adma.202104418. [DOI] [PubMed] [Google Scholar]
- MacKenzie L. E.; Pal R. Circularly polarized lanthanide luminescence for advanced security inks. Nat. Rev. Chem. 2021, 5, 109–124. 10.1038/s41570-020-00235-4. [DOI] [PubMed] [Google Scholar]
- Shen J.; Okamoto Y. Efficient Separation of Enantiomers Using Stereoregular Chiral Polymers. Chem. Rev. 2016, 116, 1094–1138. 10.1021/acs.chemrev.5b00317. [DOI] [PubMed] [Google Scholar]
- Zhang L.; Wang H.-X.; Li S.; Liu M. Supramolecular chiroptical switches. Chem. Soc. Rev. 2020, 49, 9095–9120. 10.1039/D0CS00191K. [DOI] [PubMed] [Google Scholar]
- Zhang C.; Zhao S.; Zhang M.-M.; Li B.; Dong X.-Y.; Zang S.-Q. Emergent induced circularly polarized luminescence in host-guest crystalline porous assemblies. Coord. Chem. Rev. 2024, 514, 215859 10.1016/j.ccr.2024.215859. [DOI] [Google Scholar]
- Fujiki M. Supramolecular Chirality: Solvent Chirality Transfer in Molecular Chemistry and Polymer Chemistry. Symmetry 2014, 6, 677–703. 10.3390/sym6030677. [DOI] [Google Scholar]
- Yashima E.; Ousaka N.; Taura D.; Shimomura K.; Ikai T.; Maeda K. Supramolecular Helical Systems: Helical Assemblies of Small Molecules, Foldamers, and Polymers with Chiral Amplification and Their Functions. Chem. Rev. 2016, 116, 13752–13990. 10.1021/acs.chemrev.6b00354. [DOI] [PubMed] [Google Scholar]
- García F.; Gómez R.; Sánchez L. Chiral supramolecular polymers. Chem. Soc. Rev. 2023, 52, 7524–7548. 10.1039/D3CS00470H. [DOI] [PubMed] [Google Scholar]
- Kulkarni C.; Meijer E. W.; Palmans A. R. A. Cooperativity Scale: A Structure-Mechanism Correlation in the Self-Assembly of Benzene-1,3,5-tricarboxamides. Acc. Chem. Res. 2017, 50, 1928–1936. 10.1021/acs.accounts.7b00176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht M.; Würthner F. Supramolecularly Engineered J-Aggregates Based on Perylene Bisimide Dyes. Acc. Chem. Res. 2021, 54, 642–653. 10.1021/acs.accounts.0c00590. [DOI] [PubMed] [Google Scholar]
- Vallavoju N.; Sivaguru J. Supramolecular photocatalysis: combining confinement and non-covalent interactions to control light initiated reactions. Chem. Soc. Rev. 2014, 43, 4084–4101. 10.1039/c3cs60471c. [DOI] [PubMed] [Google Scholar]
- Ji J.; Wei X.; Wu W.; Yang C. Asymmetric Photoreactions in Supramolecular Assemblies. Acc. Chem. Res. 2023, 56, 1896–1907. 10.1021/acs.accounts.3c00234. [DOI] [PubMed] [Google Scholar]
- Zhou X.; Fang S.; Leng X.; Liu Z.; Baughman R. H. The Power of Fiber Twist. Acc. Chem. Res. 2021, 54, 2624–2636. 10.1021/acs.accounts.1c00112. [DOI] [PubMed] [Google Scholar]
- Mizuno H.; Fukuhara G. Solution-State Hydrostatic Pressure Chemistry: Application to Molecular, Supramolecular, Polymer, and Biological Systems. Acc. Chem. Res. 2022, 55, 1748–1762. 10.1021/acs.accounts.2c00176. [DOI] [PubMed] [Google Scholar]
- Goldup S. M. The End of the Beginning of Mechanical Stereochemistry. Acc. Chem. Res. 2024, 57, 1696–1708. 10.1021/acs.accounts.4c00195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagara Y.; Kato T. Mechanically induced luminescence changes in molecular assemblies. Nat. Chem. 2009, 1, 605–610. 10.1038/nchem.411. [DOI] [PubMed] [Google Scholar]
- Pucci A.; Bizzarri R.; Ruggeri G. Polymer composites with smart optical properties. Soft Matter 2011, 7, 3689–3700. 10.1039/c0sm01038c. [DOI] [Google Scholar]
- Xue P.; Ding J.; Wang P.; Lu R. Recent progress in the mechanochromism of phosphorescent organic molecules and metal complexes. J. Mater. Chem. C 2016, 4, 6688–6706. 10.1039/C6TC01503D. [DOI] [Google Scholar]
- Meersman F.; Dobson C. M.; Heremans K. Protein unfolding, amyloid fibril formation and configurational energy landscapes under high pressure conditions. Chem. Soc. Rev. 2006, 35, 908–917. 10.1039/b517761h. [DOI] [PubMed] [Google Scholar]
- Murthy S. E.; Dubin A. E.; Patapoutian A. Piezos thrive under pressure: mechanically activated ion channels in health and disease. Nat. Rev. Mol. Cell Biol. 2017, 18, 771–783. 10.1038/nrm.2017.92. [DOI] [PubMed] [Google Scholar]
- Mohammed D.; Versaevel M.; Bruyère C.; Alaimo L.; Luciano M.; Vercruysse E.; Procès A.; Gabriele S. Innovative Tools for Mechanobiology: Unraveling Outside-In and Inside-Out Mechanotransduction. Front. Bioeng. Biotechnol. 2019, 7, 162–179. 10.3389/fbioe.2019.00162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee R.; Howard J. A. K.; Probert M. R.; Steed J. W. Structure of Organic Solids at Low Temperature and High Pressure. Chem. Soc. Rev. 2014, 43, 4300–4311. 10.1039/C4CS00046C. [DOI] [PubMed] [Google Scholar]
- O′Bannon E. F. III; Jenei Z.; Cynn H.; Lipp M. J.; Jeffries J. R. Contributed Review: Culet Diameter and the Achievable Pressure of a Diamond Anvil Cell: Implications for the Upper Pressure Limit of a Diamond Anvil Cell. Rev. Sci. Instrum. 2018, 89, 111501. 10.1063/1.5049720. [DOI] [PubMed] [Google Scholar]
- Bovey F. A.; Yanari S. S. Effect of Solvent Polarizability on the Ultra-Violet Spectral Shifts of Aromatic Compounds. Nature 1960, 186, 1042–1044. 10.1038/1861042a0. [DOI] [Google Scholar]
- Johnson P. C.; Offen H. W. Effect of Pressure on Pyrene Excimer Fluorescence in Toluene. J. Chem. Phys. 1972, 56, 1638–1642. 10.1063/1.1677418. [DOI] [Google Scholar]
- Rollinson A. M.; Drickamer H. G. High Pressure Study of Luminescence from Intramolecular CT Compounds. J. Chem. Phys. 1980, 73, 5981–5996. 10.1063/1.440132. [DOI] [Google Scholar]
- Hara K.; Yano H. High-Pressure Study on Intramolecular Excimer Formation of 1,3-Di-1-pyrenylpropane in Various Solvents. J. Am. Chem. Soc. 1988, 110, 1911–1915. 10.1021/ja00214a040. [DOI] [Google Scholar]
- Hara K.; Rettig W. Effect of Pressure on the Fluorescence of TICT States in (N,N-Dimethylamino)benzonitrile and Its Related Compounds. J. Phys. Chem. 1992, 96, 8307–8309. 10.1021/j100200a019. [DOI] [Google Scholar]
- Rettig W.; Gilabert E.; Rullière C. Pressure Dependence of Bicimer Formation in 4-Dimethylamino-4’-cyanostilbene and Model Compounds. Chem. Phys. Lett. 1994, 229, 127–133. 10.1016/0009-2614(94)01005-6. [DOI] [Google Scholar]
- Asano T.; Furuta H.; Sumi H. “Two-Step” Mechanism in Single-Step Isomerizations. Kinetics in Highly Viscous Liquid Phase. J. Am. Chem. Soc. 1994, 116, 5545–5550. 10.1021/ja00092a004. [DOI] [Google Scholar]
- Hara K.; Kometani N.; Kajimoto O. High-Pressure Studies on the Excited-State Intramolecular Charge Transfer of 4-(N,N-Dimethylamino)triphenylphosphine in Alcohols. J. Phys. Chem. 1996, 100, 1488–1493. 10.1021/jp952270n. [DOI] [Google Scholar]
- Ruan K.; Tian S.; Lange R.; Balny C. Pressure Effects on Tryptophan and Its Derivatives. Biochem. Biophys. Res. Commun. 2000, 269, 681–686. 10.1006/bbrc.2000.2345. [DOI] [PubMed] [Google Scholar]
- Hablot D.; Ziessel R.; Alamiry M. A. H.; Bahraidah E.; Harriman A. Nanomechanical Properties of Molecular-Scale Bridges as Visualised by Intramolecular Electronic Energy Transfer. Chem. Sci. 2013, 4, 444–453. 10.1039/C2SC21505E. [DOI] [Google Scholar]
- Suhina T.; Weber B.; Carpentier C. E.; Lorincz K.; Schall P.; Bonn D.; Brouwer A. M. Fluorescence Microscopy Visualization of Contacts Between Objects. Angew. Chem., Int. Ed. 2015, 54, 3688–3691. 10.1002/anie.201410240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber G.; Tanaka F.; Okamoto B. Y.; Drickamer H. G. The Effect of Pressure on the Molecular Complex of Isoalloxazine and Adenine. Proc. Natl. Acad. Sci. U.S.A. 1974, 71, 1264–1266. 10.1073/pnas.71.4.1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letcher T. M.; Mercer-Chalmers J. D.; Kay R. L. Volume changes in complex formation between crown ethers of cryptand-222 and alkali metals in various solvents. Pure. Appl. Chem. 1994, 66, 419–427. 10.1351/pac199466030419. [DOI] [Google Scholar]
- Isaacs N. S.; Nichols P. J.; Raston C. L.; Sandova C. A.; Young D. J. Solution volume studies of a deep cavity inclusion complex of C60: [p-benzylcalix[5]arene ⊂ C60]. Chem. Commun. 1997, 1839–1997. [Google Scholar]
- Ariga K.; Terasaka Y.; Sakai D.; Tsuji H.; Kikuchi J. Piezoluminescence Based on Molecular Recognition by Dynamic Cavity Array of Steroid Cyclophanes at the Air-Water Interface. J. Am. Chem. Soc. 2000, 122, 7835–7836. 10.1021/ja000924m. [DOI] [Google Scholar]
- Saudan C.; Dunand F. A.; Abou-Hamdan A.; Bugnon P.; Lye P. G.; Lincoln S. F.; Merbach A. E. A Model for Sequential Threading of α-Cyclodextrin onto a Guest: A Complete Thermodynamic and Kinetic Study in Water. J. Am. Chem. Soc. 2001, 123, 10290–10298. 10.1021/ja010946o. [DOI] [PubMed] [Google Scholar]
- Ruloff R.; Seelbach U. P.; Merbach A. E.; Klärner F.-G. Molecular tweezers as synthetic receptors: the effect of pressure and temperature on the formation of host-guest complexes. J. Phys. Org. Chem. 2002, 15, 189–196. 10.1002/poc.477. [DOI] [Google Scholar]
- Yang C.; Nakamura A.; Fukuhara G.; Origane Y.; Mori T.; Wada T.; Inoue Y. Pressure and Temperature-Controlled Enantiodifferentiating [4 + 4] Photocyclodimerization of 2-Anthracenecarboxylate Mediated by Secondary Face- and Skeleton-Modified γ-Cyclodextrins. J. Org. Chem. 2006, 71, 3126–3136. 10.1021/jo0601718. [DOI] [PubMed] [Google Scholar]
- Yao J.; Mizuno H.; Xiao C.; Wu W.; Inoue Y.; Yang C.; Fukuhara G. Pressure-driven, solvation-directed planar chirality switching of cyclophano-pillar[5]arenes (molecular universal joints). Chem. Sci. 2021, 12, 4361–4366. 10.1039/D0SC06988D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita T.; Haketa Y.; Maeda H.; Fukuhara G. Ground- and excited-state dynamic control of an anion receptor by hydrostatic pressure. Chem. Sci. 2021, 12, 6691–6698. 10.1039/D1SC00664A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai H.; Chen Z.; Wang S.-H.; Quan M.; Yang L.-P.; Ke H.; Jiang W. Enantioselective Recognition of Neutral Molecules in Water by a Pair of Chiral Biomimetic Macrocyclic Receptors. CCS Chem. 2020, 2, 440–452. 10.31635/ccschem.020.202000160. [DOI] [Google Scholar]
- Yang L.-P.; Wang X.; Yao H.; Jiang W. Naphthotubes: Macrocyclic Hosts with a Biomimetic Cavity Feature. Acc. Chem. Res. 2020, 53, 198–208. 10.1021/acs.accounts.9b00415. [DOI] [PubMed] [Google Scholar]
- Rekharsky M. V.; Inoue Y. Complexation Thermodynamics of Cyclodextrins. Chem. Rev. 1998, 98, 1875–1917. 10.1021/cr970015o. [DOI] [PubMed] [Google Scholar]
- Crini G. Review: A History of Cyclodextrins. Chem. Rev. 2014, 114, 10940–10975. 10.1021/cr500081p. [DOI] [PubMed] [Google Scholar]
- Sansone F.; Barboso S.; Casnati A.; Sciotto D.; Ungaro R. A New Chiral Rigid Cone Water Soluble Peptidocalix[4]arene and Its Inclusion Complexes with α-Amino Acids and Aromatic Ammonium Cations. Tetrahedron Lett. 1999, 40, 4741–4744. 10.1016/S0040-4039(99)00838-2. [DOI] [Google Scholar]
- Dai L.; Wu W.; Liang W.; Chen W.; Yu X.; Ji J.; Xiao C.; Yang C. Enhanced chiral recognition by γ-cyclodextrin-cucurbit[6]uril-cowheeled [4]pseudorotaxanes. Chem. Commun. 2018, 54, 2643–2646. 10.1039/C8CC00840J. [DOI] [PubMed] [Google Scholar]
- Chen Y.; Fu L.; Sun B.; Qian C.; Pangannaya S.; Zhu H.; Ma J.; Jiang J.; Ni Z.; Wang R.; Lu X.; Wang L. Selection of Planar Chiral Conformations between Pillar[5,6]arenes Induced by Amino Acid Derivatives in Aqueous Media. Chem.—Eur. J. 2021, 27, 5890–5896. 10.1002/chem.202004003. [DOI] [PubMed] [Google Scholar]
- James T. D.; Sandanayake K. R. A. S.; Shinkai S. Chiral discrimination of monosaccharides using a fluorescent molecular sensor. Nature 1995, 374, 345–347. [Google Scholar]
- Singh H.; Warmuth R. Chiral recognition by hemicarcerand-like host in aqueous solution. Tetrahedron 2002, 58, 1257–1264. 10.1016/S0040-4020(02)00008-X. [DOI] [Google Scholar]
- Bouchet A.; Brotin T.; Linares M.; Ågren H.; Cavagnat D.; Buffeteau T. Enantioselective Complexation of Chiral Propylene Oxide by an Enantiopure Water-Soluble Cryptophane. J. Org. Chem. 2011, 76, 4178–4181. 10.1021/jo200519r. [DOI] [PubMed] [Google Scholar]
- Wang B.-Y.; Stojanović S.; Turner D. A.; Young T. L.; Hadad C. M.; Badjić J. D. The Entrapment of Chiral Guests with Gated Baskets: Can a Kinetic Discrimination of Enantiomers Be Governed through Gating?. Chem.—Eur. J. 2013, 19, 4767–4775. 10.1002/chem.201204344. [DOI] [PubMed] [Google Scholar]
- Ríos P.; Mooibroek T. J.; Carter T. S.; Williams C.; Wilson M. R.; Crump M. P.; Davis A. P. Enantioselective carbohydrate recognition by synthetic lectins in water. Chem. Sci. 2017, 8, 4056–4061. 10.1039/C6SC05399H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S.; Yao H.; Kameda T.; Jiang W.; Kitahara R. Volumetric Properties for the Binding of 1,4-Dioxane to Amide Naphthotubes in Water. J. Phys. Chem. B 2020, 124, 9175–9181. 10.1021/acs.jpcb.0c07690. [DOI] [PubMed] [Google Scholar]
- Mizuno H.; Kitamatsu M.; Imai Y.; Fukuhara G. Smart Fluorescence Materials that Are Controllable by Hydrostatic Pressure: Peptide-Pyrene Conjugates. ChemPhotoChem 2020, 4, 502–507. 10.1002/cptc.202000036. [DOI] [Google Scholar]
- Berova N.; Bari L. D.; Pescitelli G. Application of electronic circular dichroism in configurational and conformational analysis of organic compounds. Chem. Soc. Rev. 2007, 36, 914–931. 10.1039/b515476f. [DOI] [PubMed] [Google Scholar]
- Vuorimaa E.; Ikonen M.; Lemmetyinen H. Photophysics of rhodamine dimers in Langmuir-Blodgett films. Chem. Phys. 1994, 188, 289–302. 10.1016/0301-0104(94)00231-2. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


