Abstract
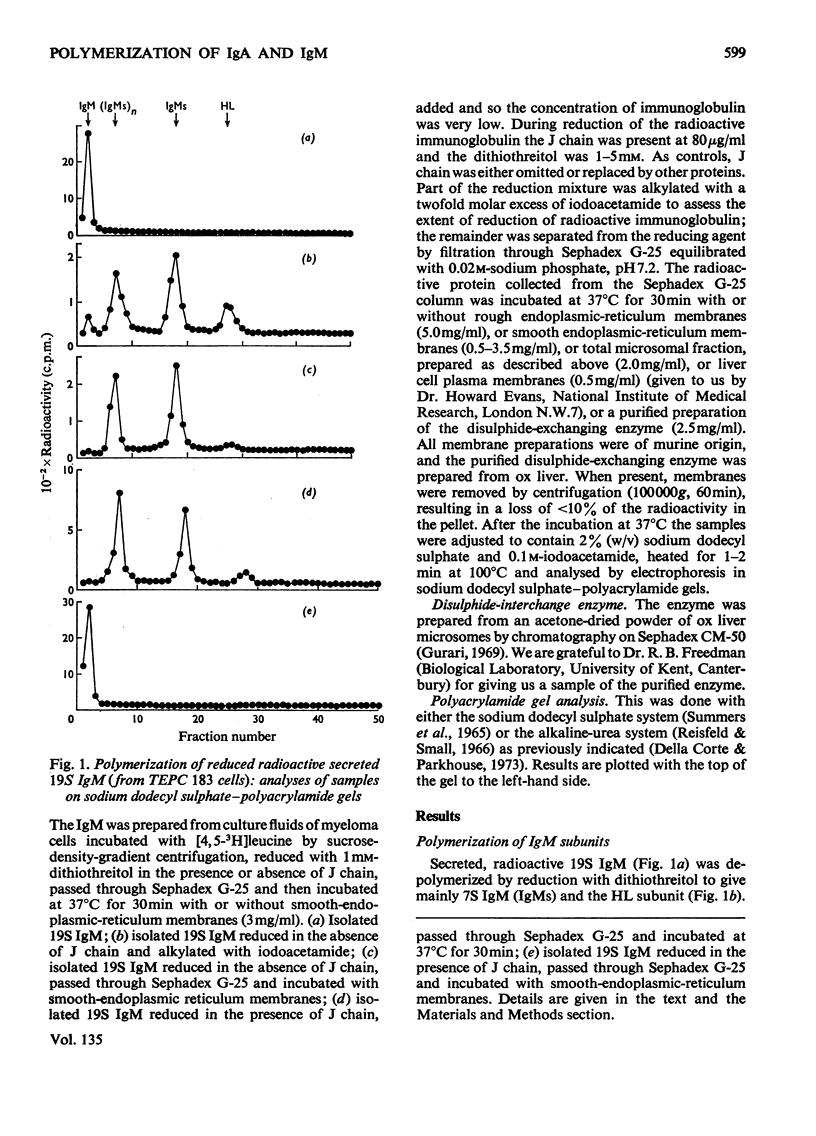
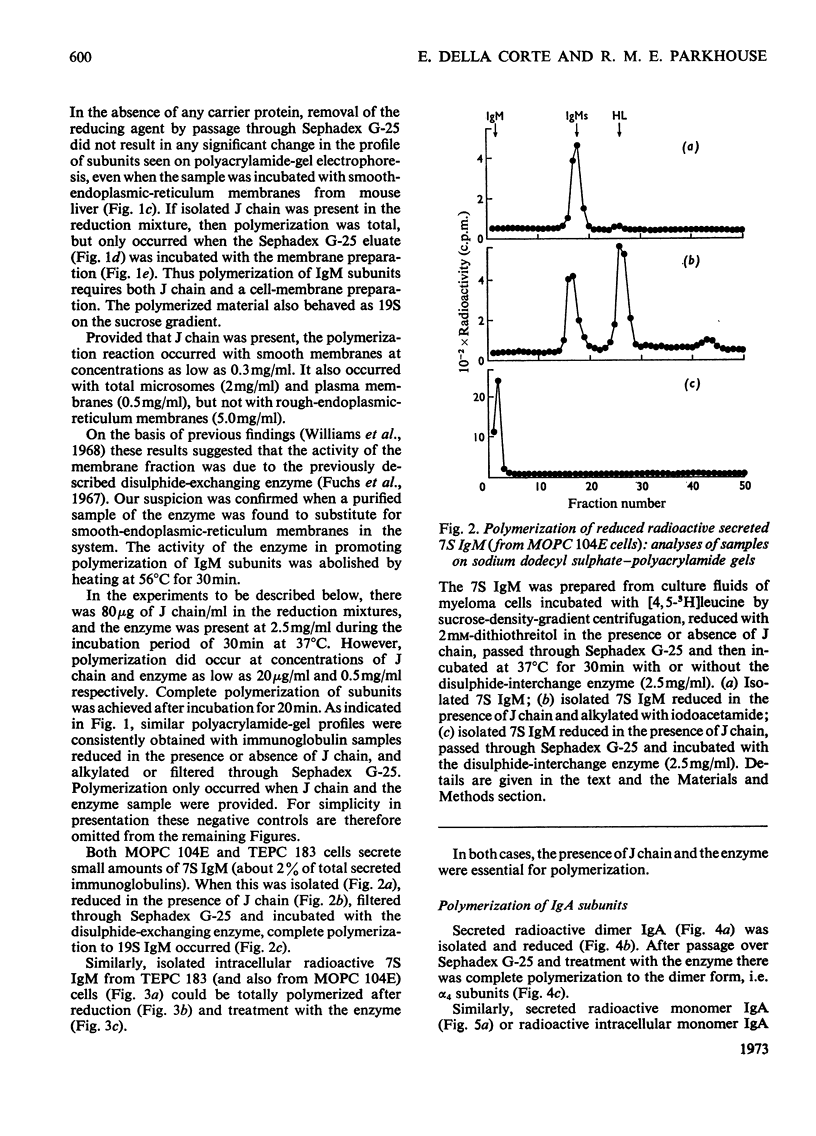
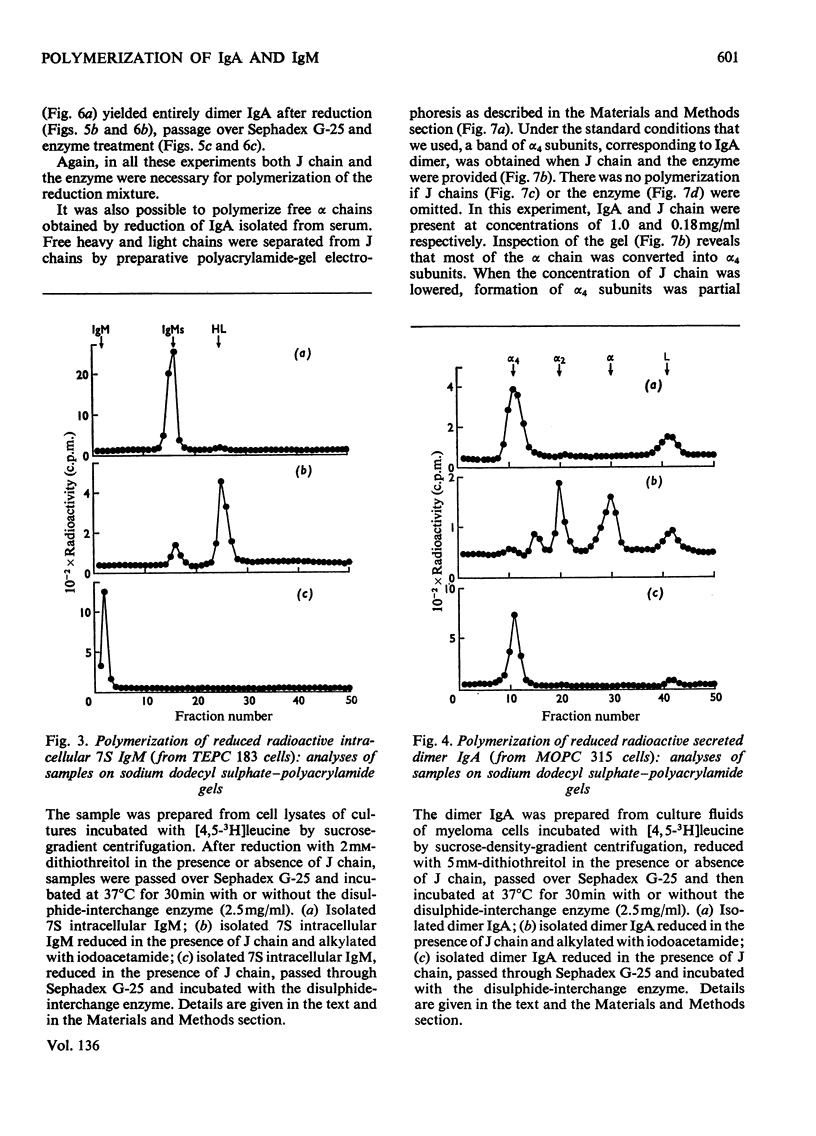
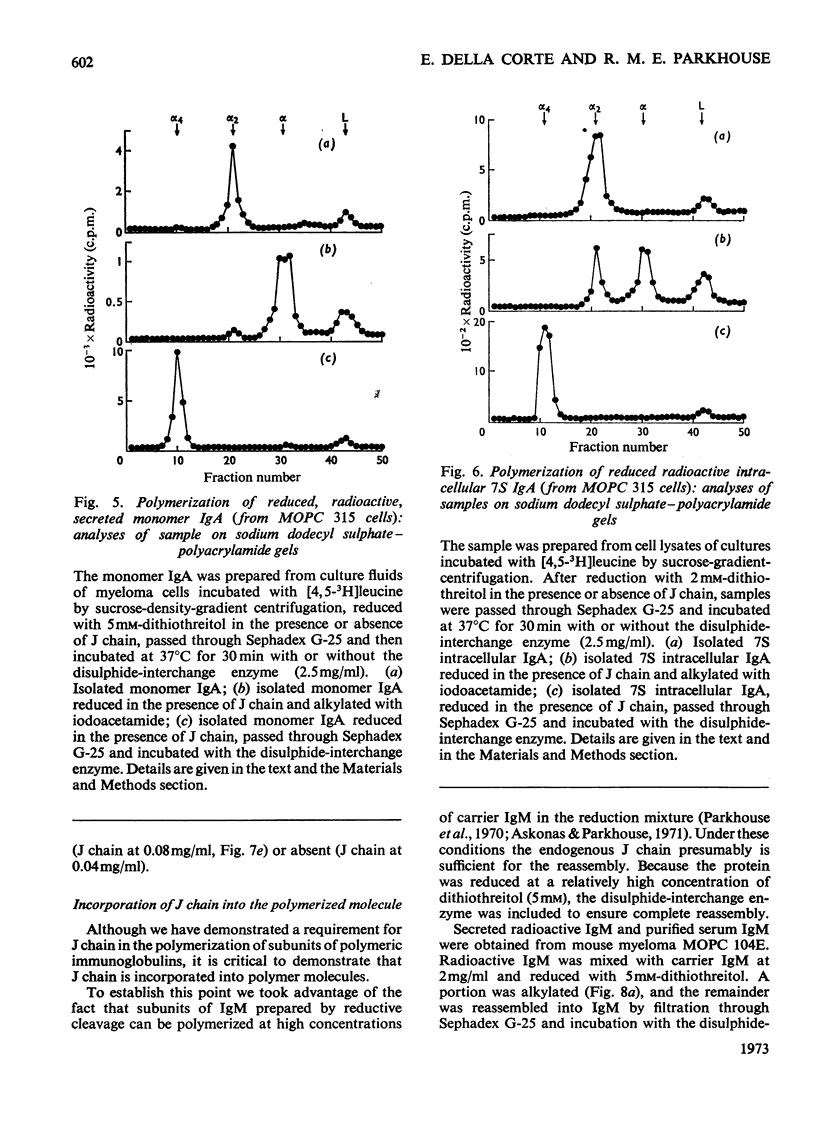
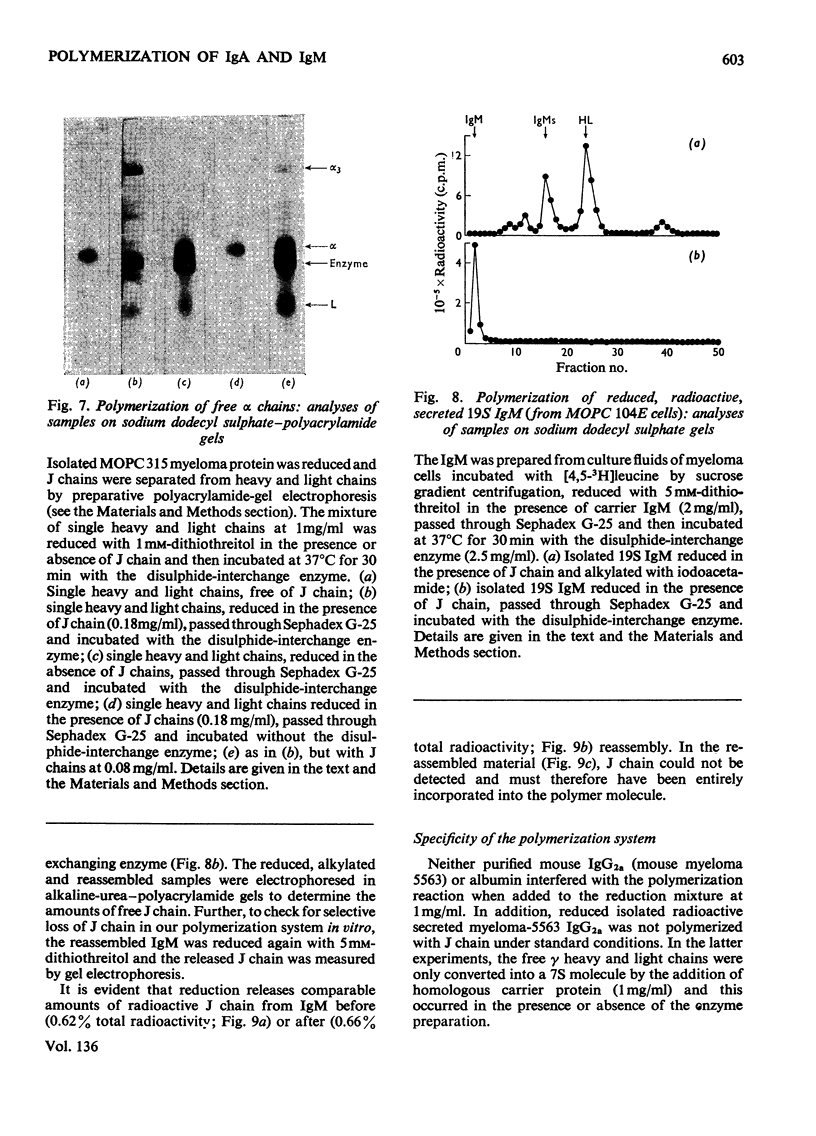
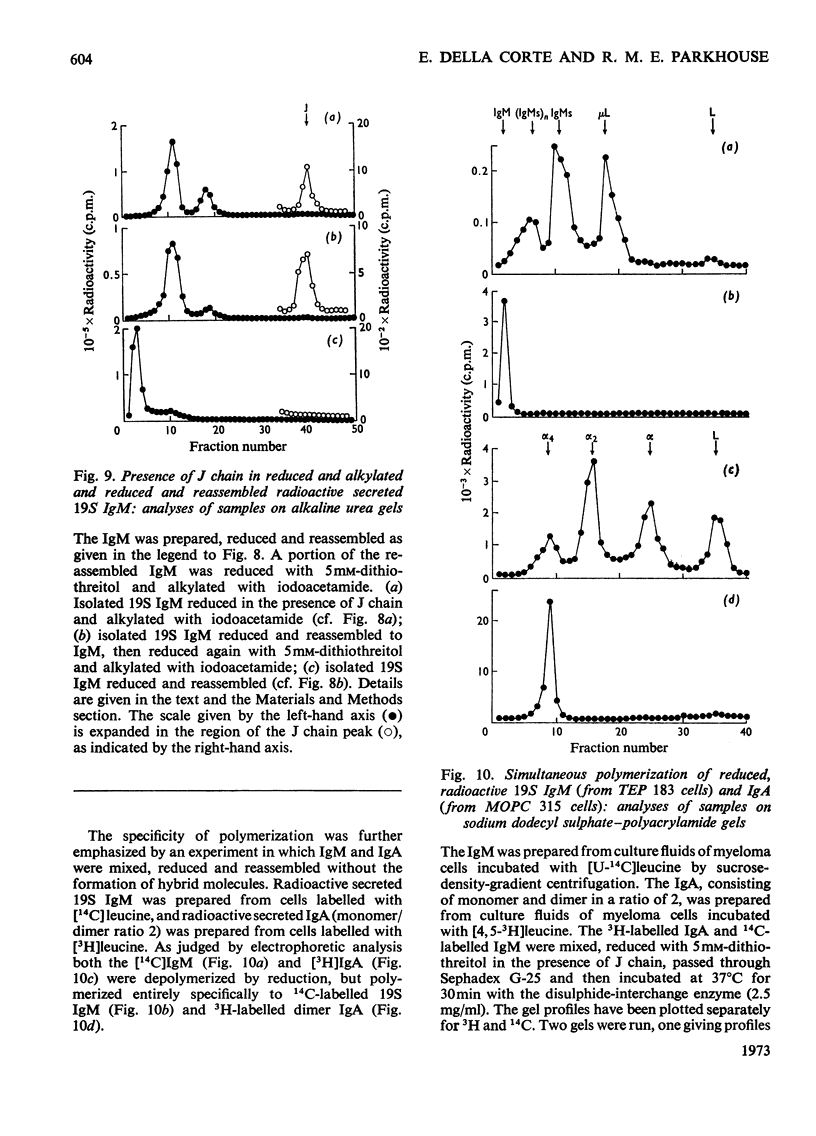
Mouse myeloma cells secreting 19S IgM (immunoglobulin M) (MOPC 104E and TEPC 183) or monomer and polymer IgA (immunoglobulin A) (MOPC 315) were incubated with radioactive leucine and the intracellular and secreted immunoglobulins and immunoglobulin subunits were prepared by preparative sucrose-density-gradient centrifugation. Samples were reduced in the presence or absence of isolated J chain, passed over Sephadex G-25 and then incubated at 37°C for 30min with or without a source of disulphide-interchange enzyme. The extent of reassembly of reduced subunits was then evaluated by electrophoresis in polyacrylamide gels. Provided that J chain and the disulphide-interchange enzyme were supplied, both IgM and IgA could be assembled from their respective subunits, obtained by reductive cleavage of polymeric forms. Under similar conditions, assembly of polymeric forms from intracellular or secreted 7S monomer subunits also occurred. Under these conditions polymerization was total, there being no residue of the monomeric form. Reassembly did not occur in the absence of either J chain or the enzyme. All of the J chain released from IgM by reductive cleavage was incorporated back into the reassembled polymer. The J chain is therefore likely to be an essential structural requirement for polymeric immunoglobulins. A variety of controls ruled out non-specific interactions, and further suggested that the amino acid sequence of polypeptide chains determines the specificity of polymerization. The fact that intracellular IgA and IgM monomer subunits known to be deficient in galactose and fucose can be completely polymerized suggests that the addition of carbohydrate does not control polymerization.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Askonas B. A., Parkhouse R. M. Assembly of immunoglobulin M. Blocked thiol groups of intracellular 7S subunits. Biochem J. 1971 Jul;123(4):629–634. doi: 10.1042/bj1230629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bargellesi A., Periman P., Scharff M. D. Synthesis, assembly, and secretion of globulin by mouse myeloma cells. IV. Assembly of IgA. J Immunol. 1972 Jan;108(1):126–134. [PubMed] [Google Scholar]
- Bevan M. J. Interchain disulfide bond formation studied in two mouse myelomas which secrete immunoglobulin A. Eur J Immunol. 1971 Apr;1(2):133–138. doi: 10.1002/eji.1830010212. [DOI] [PubMed] [Google Scholar]
- Brownstone A. D. A versatile system for preparative electrophoresis in acrylamide gel. Anal Biochem. 1969 Jan;27(1):25–46. doi: 10.1016/0003-2697(69)90216-4. [DOI] [PubMed] [Google Scholar]
- Cowan N. J., Robinson G. B. The sequence of addition of terminal sugars to an immunoglobulin A myeloma protein. Biochem J. 1972 Feb;126(3):751–754. doi: 10.1042/bj1260751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Della Corte E., Parkhouse R. M. Biosynthesis of immunoglobulin A (IgA). Secretion and addition of carbohydrate to monomer and polymer forms of a mouse myeloma protein. Biochem J. 1973 Nov;136(3):589–596. doi: 10.1042/bj1360589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAHEY J. L. Physiocochemical characterization of mouse myeloma proteins: demonstration of heterogeneity for each myeloma globulin. J Exp Med. 1961 Sep 1;114:399–413. doi: 10.1084/jem.114.3.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahey J. L. HETEROGENEITY OF MYELOMA PROTEINS. J Clin Invest. 1963 Jan;42(1):111–123. doi: 10.1172/JCI104688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs S., De Lorenzo F., Anfinsen C. B. Studies on the mechanism of the enzymic catalysis of disulfide interchange in proteins. J Biol Chem. 1967 Feb 10;242(3):398–402. [PubMed] [Google Scholar]
- Halpern M. S., Koshland M. E. Noval subunit in secretory IgA. Nature. 1970 Dec 26;228(5278):1276–1278. doi: 10.1038/2281276a0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mestecky J., Zikan J., Butler W. T. Immunoglobulin M and secretory immunoglobulin A: presence of a common polypeptide chain different from light chains. Science. 1971 Mar 19;171(3976):1163–1165. doi: 10.1126/science.171.3976.1163. [DOI] [PubMed] [Google Scholar]
- Metzger H. Structure and function of gamma M macroglobulins. Adv Immunol. 1970;12:57–116. doi: 10.1016/s0065-2776(08)60168-6. [DOI] [PubMed] [Google Scholar]
- Morrison S. L., Koshland M. E. Characterization of the J chain from polymeric immunoglobulins (IgA-IgM-immunological specificity-primary structure). Proc Natl Acad Sci U S A. 1972 Jan;69(1):124–128. doi: 10.1073/pnas.69.1.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkhouse R. M., Askonas B. A., Dourmashkin R. R. Electron microscopic studies of mouse immunoglobulin M; structure and reconstitution following reduction. Immunology. 1970 Apr;18(4):575–584. [PMC free article] [PubMed] [Google Scholar]
- Parkhouse R. M., Askonas B. A. Immunoglobulin M biosynthesis. Intracellular accumulation of 7S subunits. Biochem J. 1969 Nov;115(2):163–169. doi: 10.1042/bj1150163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkhouse R. M. Assembly and secretion of immunoglobulin M (IgM) by plasma cells and lymphocytes. Transplant Rev. 1973;14:131–144. doi: 10.1111/j.1600-065x.1973.tb00104.x. [DOI] [PubMed] [Google Scholar]
- Parkhouse R. M. Biosynthesis of J-chain in mouse IgA and IgM. Nat New Biol. 1972 Mar 1;236(61):9–11. doi: 10.1038/newbio236009a0. [DOI] [PubMed] [Google Scholar]
- Parkhouse R. M.E. Immunoglobulin a biosynthesis. Intracellular accumulation of 7 S subunits. FEBS Lett. 1971 Jul 15;16(1):71–73. doi: 10.1016/0014-5793(71)80689-0. [DOI] [PubMed] [Google Scholar]
- Parkhouse R. M. Immunoglobulin M biosynthesis. Production of intermediates and excess of light-chain in mouse myeloma MOPC 104E. Biochem J. 1971 Jul;123(4):635–641. doi: 10.1042/bj1230635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkhouse R. M., Melchers F. Biosynthesis of the carbohydrate portions of immunoglobulin M. Biochem J. 1971 Nov;125(1):235–240. doi: 10.1042/bj1250235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkhouse R. M., Virella G., Dourmashkin R. R. Structural characterization of a human monoclonal IgA protein. Clin Exp Immunol. 1971 Apr;8(4):581–591. [PMC free article] [PubMed] [Google Scholar]
- Reisfeld R. A., Small P. A., Jr Electrophoretic heterogeneity of polypeptide chains of specific antibodies. Science. 1966 May 27;152(3726):1253–1255. doi: 10.1126/science.152.3726.1253. [DOI] [PubMed] [Google Scholar]
- Summers D. F., Maizel J. V., Jr, Darnell J. E., Jr Evidence for virus-specific noncapsid proteins in poliovirus-infected HeLa cells. Proc Natl Acad Sci U S A. 1965 Aug;54(2):505–513. doi: 10.1073/pnas.54.2.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Underdown B. J., Simms E. S., Eisen H. N. Subunit structure and number of combining sites of the immunoglobulin A myeloma protein produced by mouse plasmacytoma MOPC-315. Biochemistry. 1971 Nov 23;10(24):4359–4368. doi: 10.1021/bi00800a002. [DOI] [PubMed] [Google Scholar]
- VOGT M., DULBECCO R. Steps in the neoplastic transformation of hamster embryo cells by polyoma virus. Proc Natl Acad Sci U S A. 1963 Feb 15;49:171–179. doi: 10.1073/pnas.49.2.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams D. J., Gurari D., Rabin B. R. The effects of ribosomes on the activity of a membrane bound enzyme catalysing thiol-disulphide interchange. FEBS Lett. 1968 Dec;2(2):133–135. doi: 10.1016/0014-5793(68)80123-1. [DOI] [PubMed] [Google Scholar]



