Abstract
Background.
The impact of post-diagnosis exercise on cause-specific mortality in cancer survivors and whether this differs based on cancer site is unclear.
Methods.
We performed an analysis of 11,480 cancer patients enrolled in the Prostate, Lung, Colorectal, and Ovarian (PLCO) cancer screening trial. Patients with a confirmed diagnosis of cancer completing a standardized survey quantifying exercise after diagnosis were included. The primary outcome was all-cause mortality; secondary endpoints were cancer mortality and mortality from other causes. Cox models were used to estimate the cause-specific hazard ratios (HRs) for all-cause (ACM), cancer, and non-cancer mortality as a function of meeting exercise guidelines versus not meeting guidelines with adjustment for important clinical covariates.
Results.
After a median follow-up of 16 years from diagnosis, 4,665 deaths were documented (1,940 due to cancer and 2,725 due to other causes). In multivariable analyses, exercise consistent with guidelines was associated with a 25% reduced risk of ACM compared with non-exercise (HR=0.75, 95% CI, 0.70, 0.80). Compared with non-exercise, exercise consistent with guidelines was associated with a significant reduction in cancer mortality (HR 0.79; 95% CI, 0.72, 0.88) and mortality from other causes (HR 0.72; 95% CI, 0.66, 0.78). The inverse relationship between exercise and cause-specific mortality varied by exercise dose. Exercise consistent with guidelines was associated with a reduced hazard of ACM for multiple cancer sites. Reduction in cancer mortality for exercisers was only observed in head and neck and renal cancer.
Conclusion.
In this pan-cancer sample of long-term cancer survivors, exercise consistent with guidelines was associated with substantial ACM benefit driven by both reductions in cancer and non-cancer mortality. The cause-specific impact of exercise differed as a function of cancer site.
Keywords: Physical activity, cause-specific mortality, malignancy, cancer survivorship, oncology
INTRODUCTION
Improvements in detection, risk stratification, and combination therapy have resulted in significant reductions in cancer mortality for patients diagnosed with early-stage disease.1,2 However, significant challenges remain. First, even patients living five years beyond early diagnosis remain at high-risk of distant recurrence and new primary malignanices.1,2 Second, as a consequence of improvements in cancer mortality, a large and rapidly growing number of cancer patients have sufficient longevity to be at elevated risk of non-cancer, competing causes of mortality.3 Certain adjuvant therapies can also lead to excess risk of comorbid conditions due to normal organ and tissue damage.4–7 Strategies that complement contemporary therapeutic approaches to further reduce cancer mortality while simultaneously lowering risk of death from other causes are therefore needed to improve all-cause mortality (ACM) among cancer survivors.8
In cancer survivors, “high” levels of post-diagnosis exercise is associated with a significant ACM benefit for several cancer types. However, most prior studies have focused on single cancer site, typically breast cancer, with fewer studies in colorectal, or prostate cancer.9–11 The few available pan-cancer analyses of post-diagnosis exercise and cause-specific mortality are mostly characterized by small overall sample sizes,12–15 resulting in a small number of patients in each cancer site, thereby limiting investigation of the clinically important question whether exercise benefit differs by cancer site. Finally, small sample sizes together with short duration of follow-up has resulted in a low number of ACM events; mortality from non-cancer causes is rarely reported.9 Thus, the impact of exercise on ACM and cause-specific mortality in cancer survivors is unclear. Such findings will facilitate recommendation and discussion of exercise in cancer survivor consultations.
We leveraged data from the Prostate, Lung, Colorectal, and Ovarian (PLCO) cancer screening trial to conduct a pan-cancer analysis of post-diagnosis exercise with ACM and mortality from cancer and other causes in long-term cancer survivors.
METHODS
PLCO Cohort, Patients and Setting
Full details are provided in the supplemental methods. Details of the PLCO screening trial design, methods, and cohort characteristics have been reported previously.16–18 In brief, between November 1993 and July 2001, 10 screening centers in the United States enrolled 76,678 men and 78,209 women between the ages of 55 to 74 years and with no history of prostate, lung, colorectal or ovarian cancer. The PLCO protocol was approved by the Institutional Review Board at each participating center and all participants provided written informed consent. Between 2006 and 2008, a one-time Supplemental Questionnaire (SQX) that contained patient-reported items, including questions on exercise, was sent to participants a median of 9 years following initial trial randomization. Of the 154,887 participants enrolled in the PLCO trial, a total of 40,126 (26%) had a confirmed cancer diagnosis. Of those, 12,277 (31%) completed the SQX after diagnosis. Patients were further excluded due to incomplete or missing exercise data (n=593) or completion of the SQX within 6 months of death (n=203), or with missing cause of death data (n=1), resulting in a final analytic cohort of 11,480 (Figure S1). Compared with excluded survivors (n=28,646), those included in this analysis were more likely to be diagnosed with breast cancer, prostate cancer, and localized disease, and less likely to be diagnosed with lung cancer or distant disease at diagnosis (Table S1).
Exercise Assessment
The SQX contained a total of 12 items estimating occupational and non-occupational (i.e., exercise) physical activity. In this study, only the four items estimating strenuous or moderate exercise were analyzed. Items assessing mild exercise were not included in the SQX. Frequency was evaluated by the following two items: “Over the last 12 months, on average, how many days per week did you spend in” (1) “any physical activity strenuous enough to work up a sweat or to increase your breathing and heart rate to very high levels” and (2) “any moderate physical activity where you worked up a light sweat or increased your breathing and heart rate to moderately higher levels.” Four discrete response options were provided: 0 or less than 1 day per week; 2 to 3 days per week; 4 to 5 days per week; and 6 to 7 days per week. Average duration was evaluated by the following: “Over the last 12 months, on average, how long was each session of strenuous activity?” The same question assessed the duration of moderate activity. Five discrete response options were provided: 0 to less than 15 minutes; 16 to 19 minutes; 20 to 29 minutes; 30 to 39 minutes; and 40 minutes or more. These items are similar to those included in the International Physical Activity Questionnaire (IPAQ).23 Validity and reliability of the IPAQ has been established across multiple countries.23
For the primary analysis, exercise exposure was compared across two distinct categories: (1) meeting national guidelines: moderate-intensity exercise ≥4 days per week, with each session, on average, ≥30 minutes in duration and/or strenuous-intensity exercise ≥2 days per week, with each session, on average, ≥20 minutes in duration; and (2) not meeting national guidelines: any exercise below the criteria for meeting national guidelines, including 0 days of exercise per week. These exercise exposure classifications were selected given the close adherence with national and international exercise guidelines for cancer survivors.19–22 To examine dose-response, exercise was collapsed into four categories: (1) no exercise (n=3,111; 27%), (2) below exercise guidelines (n=3,995; 35%), (3) meeting exercise guidelines (n=2,515; 22%), and (4) exceeding exercise guidelines (n=1,859; 16%).
Follow-up, Ascertainment of Deaths, and End Points
PLCO trial participants were contacted annually to ascertain and confirm cancer diagnoses and deaths. This was supplemented by periodic linkage to the National Death Index to enhance completeness of end-point ascertainment. Death certificates were obtained to confirm the death. Cause of death was defined based on the National Center for Health Statistics guidance. The trial also used an end-point adjudication process to assign the cause of death in a uniform and unbiased manner.23,24 The last follow-up of end-point ascertainment in the PLCO was conducted in 2018. The primary endpoint was ACM, defined as death from any cause following a cancer diagnosis. Secondary end points were cancer mortality and death from other causes.
Statistical Analysis
ACM was analyzed using Kaplan-Meier methods and Cox models. Cumulative incidence curves for cause-specific mortality were estimated using the Aalen-Johansen method. Estimates of median survival (95% confidence intervals [CI]) are reported based on Kaplan-Meier methods. Cause-specific hazards were estimated using Cox regression models. For all time-to-event analyses, using methods for left-truncated data, cancer diagnosis was the origin time and patients entered the risk set six months after SQX completion. Delayed study entry is introduced by the requirement of the SQX post-diagnosis; any patients dying prior to completing the SQX are excluded by design. Additionally, the delayed entry is prolonged by the requirement that patients survive at least six months after the SQX. Therefore, patients can only have an event six months after completing the SQX, and consequently entered the risk set at that time.25
For Cox models of both ACM and cause-specific mortality, univariable analyses were performed considering relevant patient and cancer characteristics. Variables significant at a threshold of p ≤ 0.2 were included in a multivariable model. PLCO randomization group and time from diagnosis to SQX were included in all multivariable models. Hazard ratios (HR) and 95% confidence intervals from Cox models are presented. The proportional hazards assumption was assessed based on tests of weighted residuals.26 The dose-response relationship between exercise and the hazard of mortality was assessed using a linear model of the hazard ratios from the resulting models as a function of exercise dose, weighted by the number of participants at risk.27 Additionally, to reduce potential bias associated with reverse causation we conducted a sensitivity analysis excluding all patients dying within two years following completion of the SQX; the results were consistent with the primary analysis (results not presented). Analyses were performed in R version 4.1.2.28
RESULTS
Of the 11,480 patients providing complete exercise data, 4,374 (38%) patients were defined as exercisers and 7,106 (62%) were defined as non-exercisers. Across both groups, the estimated median time spent on moderate and strenuous exercise per week was 44 minutes (IQR 8, 100) and 19 minutes (IQR 8, 86), respectively. Exercisers were more likely to be male, nonsmokers, and had a lower prevalence of CVD history (coronary heart disease or history of heart attack) compared to non-exercisers (Table 1). Among the types of cancer diagnoses observed during follow-up, prostate cancer (n=4,261; 37%) was the most common diagnosis followed by breast cancer (n=2,276; 20%). The median interval between cancer diagnosis and completion of the SQX was 4.5 years (interquartile range, [IQR] 2.1, 7.0 years). The median time between landmark study entry (six months after completion of the SQX) and last follow-up was 11.6 years (IQR 11.4, 12.2 years), among the 6,815 patients alive at the end of the study. During this period, 4,665 deaths were documented (1,940 due to cancer and 2,725 due to other causes; the number of deaths in each cancer site is presented in Table 3).
Table 1.
Characteristics of the Patients
| Characteristic | Exercise Classification1 |
||
|---|---|---|---|
| Overall | Non-Exercisers | Exercisers | |
| No. of patients – (row %) | 11,480 (100) | 7,106 (62) | 4,374 (38) |
| Estimated minutes of moderate exercise per week – median (IQR) | 44 (8, 100) | 19 (8, 61) | 100 (61, 180) |
| Unknown | 38 | 0 | 38 |
| Estimated minutes of strenuous exercise per week – median (IQR) | 19 (8, 86) | 8 (8, 19) | 100 (61, 155) |
| Unknown | 17 | 0 | 17 |
| Age at exercise survey completion – median (IQR) | 73 (68, 77) | 73 (69, 78) | 72 (68, 76) |
| Age at diagnosis – median (IQR) | 68 (64, 72) | 68 (64, 73) | 67 (63, 71) |
| Interval between diagnosis and survey completion (years) – median (IQR) | 4.50 (2.09, 6.98) | 4.36 (2.02, 6.92) | 4.69 (2.25, 7.07) |
| PLCO Intervention Arm | 6,030 (53) | 3,691 (52) | 2,339 (53) |
| Female – no. (%) | 4,567 (40) | 2,992 (42) | 1,575 (36) |
| Race/ethnicity – no. (%) | |||
| Non-Hispanic white | 10,461 (93) | 6,467 (93) | 3,994 (93) |
| Other group | 807 (7.2) | 495 (7.1) | 312 (7.2) |
| Missing | 212 | 144 | 68 |
| Body mass index – kg/m2 | |||
| 0–18.5 | 55 (0.5) | 41 (0.6) | 14 (0.3) |
| >18.5–24.9 | 3,716 (33) | 2,100 (31) | 1,616 (38) |
| >25–29.9 | 4,998 (45) | 3,105 (45) | 1,893 (45) |
| >30 | 2,350 (21) | 1,623 (24) | 727 (17) |
| Unknown | 361 | 237 | 124 |
| Smoking, pack-years – median (IQR) | 5 (0, 34) | 7 (0, 37) | 3 (0, 26) |
| Unknown | 327 | 224 | 103 |
| Primary diagnosis – no. (%) | |||
| Prostate | 4,261 (37) | 2,452 (35) | 1,809 (41) |
| Breast (female) | 2,276 (20) | 1,435 (20) | 841 (19) |
| Colon | 872 (7.6) | 598 (8.4) | 274 (6.3) |
| Hematopoietic | 855 (7.4) | 565 (8.0) | 290 (6.6) |
| Melanoma | 773 (6.7) | 423 (6.0) | 350 (8.0) |
| Bladder | 535 (4.7) | 348 (4.9) | 187 (4.3) |
| Lung | 391 (3.4) | 278 (3.9) | 113 (2.6) |
| Endometrial | 374 (3.3) | 244 (3.4) | 130 (3.0) |
| Renal | 240 (2.1) | 165 (2.3) | 75 (1.7) |
| Head and neck | 204 (1.8) | 133 (1.9) | 71 (1.6) |
| Ovarian | 112 (1.0) | 74 (1.0) | 38 (0.9) |
| Thyroid | 106 (0.9) | 64 (0.9) | 42 (1.0) |
| Upper Gastrointestinal | 94 (0.8) | 62 (0.9) | 32 (0.7) |
| Pancreas | 36 (0.3) | 30 (0.4) | 6 (0.1) |
| Male Breast | 14 (0.1) | 9 (0.1) | 5 (0.1) |
| Biliary | 12 (0.1) | 10 (0.1) | 2 (<0.1) |
| Glioma | 12 (0.1) | 8 (0.1) | 4 (<0.1) |
| Liver | 9 (<0.1) | 7 (<0.1) | 2 (<0.1) |
| Other2 | 304 (2.6) | 201 (2.8) | 103 (2.4) |
| Cancer stage at diagnosis – no. (%) | |||
| In situ | 926 (8.1) | 522 (7.3) | 404 (9.2) |
| Localized | 6,738 (59) | 4,076 (57) | 2,662 (61) |
| Regional | 1,463 (13) | 953 (13) | 510 (12) |
| Distant | 464 (4.0) | 306 (4.3) | 158 (3.6) |
| Unknown | 1,889 (16) | 1,249 (18) | 640 (15) |
| History of chronic conditions– no. (%) | |||
| Arthritis | 3,976 (36) | 2,614 (38) | 1,362 (32) |
| Unknown | 289 | 187 | 102 |
| Chronic bronchitis | 449 (4.0) | 317 (4.6) | 132 (3.1) |
| Unknown | 287 | 187 | 100 |
| Colon-related comorbidity (ulcerative colitis, Crohn’s disease, Gardner’s syndrome, or familial polyposis) | 148 (1.3) | 98 (1.4) | 50 (1.2) |
| Unknown | 313 | 202 | 111 |
| Diabetes | 640 (5.7) | 460 (6.6) | 180 (4.2) |
| Unknown | 282 | 183 | 99 |
| Diverticulitis/diverticulosis | 830 (7.4) | 530 (7.7) | 300 (7.0) |
| Unknown | 286 | 188 | 98 |
| Emphysema | 249 (2.2) | 188 (2.7) | 61 (1.4) |
| Unknown | 283 | 185 | 98 |
| Gallbladder stones or inflammation | 1,203 (11) | 819 (12) | 384 (9.0) |
| Unknown | 289 | 190 | 99 |
| Coronary heart disease or history of heart attack | 916 (8.2) | 617 (8.9) | 299 (7.0) |
| Unknown | 288 | 186 | 102 |
| Hypertension | 3,636 (32) | 2,409 (35) | 1,227 (29) |
| Unknown | 279 | 181 | 98 |
| Liver-related co-morbidity (hepatitis or cirrhosis) | 416 (3.7) | 267 (3.9) | 149 (3.5) |
| Unknown | 294 | 189 | 105 |
| Osteoporosis | 422 (3.8) | 308 (4.5) | 114 (2.7) |
| Unknown | 302 | 194 | 108 |
| Stroke | 205 (1.8) | 144 (2.1) | 61 (1.4) |
| Unknown | 279 | 182 | 97 |
Definitions.
Exercisers: moderate-intensity exercise >=4 days per week, with each session, on average, >=30 minutes in duration and/or strenuous-intensity exercise >=2 days per week, with each session, on average, >=20 minutes in duration; and (2) Non-exercisers: any exercise below the criteria for meeting national guidelines, including patients reporting 0 days of exercise per week
Other cancers include any other cancer site not listed.
Table 3.
Multivariable Hazard Ratios for Exercise and Cause-Specific Mortality
| All-Cause Mortality |
Cancer-Specific Mortality |
Non-Cancer Mortality |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Site | N | N Events | Multivariable HR (95% CI) | N | N Events | Multivariable HR (95% CI) | N | N Events | Multivariable HR (95% CI) |
| All cancers | 10,852 | 4,397 | 0.75 (0.70, 0.80) | 10,896 | 1,851 | 0.79 (0.72, 0.88) | 10,852 | 2,552 | 0.72 (0.66, 0.78) |
| Bladder | 520 | 241 | 0.79 (0.59, 1.04) | 524 | 104 | 0.85 (0.56, 1.30) | 519 | 137 | 0.73 (0.49, 1.07) |
| Breast | 2,159 | 607 | 0.76 (0.63, 0.91) | 2,199 | 250 | 0.80 (0.61, 1.06) | 2,159 | 365 | 0.75 (0.59, 0.96) |
| Colon | 809 | 351 | 0.81 (0.63, 1.04) | 827 | 146 | 1.04 (0.72, 1.50) | 817 | 210 | 0.68 (0.48, 0.96) |
| Endometrial | 361 | 95 | 0.41 (0.24, 0.72) | 365 | 31 | 0.55 (0.23, 1.34) | 361 | 65 | 0.41 (0.21, 0.81) |
| Head & Neck | 196 | 117 | 0.62 (0.40, 0.96) | 196 | 51 | 0.49 (0.25, 0.96) | 196 | 66 | 0.83 (0.47, 1.48) |
| Hematopoietic | 818 | 458 | 0.72 (0.59, 0.89) | 818 | 241 | 0.81 (0.61, 1.07) | 826 | 217 | 0.62 (0.45, 0.85) |
| Lung | 361 | 245 | 1.01 (0.74, 1.38) | 362 | 147 | 1.00 (0.68, 1.47) | 369 | 101 | 0.83 (0.50, 1.38) |
| Melanoma | 744 | 241 | 0.78 (0.59, 1.02) | 745 | 90 | 0.74 (0.47, 1.15) | 741 | 151 | 0.81 (0.57, 1.16) |
| Ovarian* | 109 | 56 | 1.04 (0.52, 2.05) | 111 | 47 | 1.12 (0.55, 2.28) | - | - | - |
| Prostate | 3,980 | 1,590 | 0.78 (0.70, 0.86) | 4,055 | 556 | 0.85 (0.71, 1.02) | 4,035 | 1,052 | 0.72 (0.63, 0.82) |
| Renal | 234 | 125 | 0.50 (0.31, 0.81) | 234 | 56 | 0.34 (0.15, 0.75) | 234 | 69 | 0.84 (0.45, 1.58) |
| Upper GI | 92 | 59 | 0.87 (0.47, 1.61) | 92 | 28 | 0.63 (0.26, 1.55) | 92 | 31 | 1.27 (0.49, 3.28) |
HR = Hazard Ratio; CI = Confidence Interval
There were only 9 non-cancer deaths among patients with ovarian cancer; the non-cancer mortality model could not be fit for this outcome.
N and N Events shown are based on the multivariable models.
Covariates included in each multivariable model are shown in Supplemental Table 3.
Pan-Cancer
During follow-up, 1,459 (33%) total deaths had occurred among the 4,374 patients classified as exercisers and 3,206 (45%) among the 7,106 classified as non-exercisers. Median overall survival from diagnosis was 19 years (95% CI, 19, 20) for exercisers and 14 years (95% CI, 13, 15) for non-exercisers. In multivariable analysis, exercisers had a 25% reduced risk of ACM compared with non-exercisers (HR=0.75, 95% CI, 0.70, 0.80; Figure 1A). The reduction in ACM in exercisers was apparent within five years, persisting for at least 20 years following diagnosis (Table 2). Exercise consistent with national guidelines was associated with a statistically significant reduction in cancer mortality (HR 0.79; 95% CI, 0.72, 0.88) and mortality from other causes (HR 0.72; 95% CI, 0.66, 0.78). The five year cumulative incidence of cancer mortality was 12% (95% CI, 10%, 16%) for exercisers compared with 16% (95% CI 14%, 18%) for non-exercisers (HR 0.79, 95% CI, 0.72, 0.88; Figure 1B). The five-year cumulative incidence for death from other causes was 2.4% (95% CI 1.5%, 3.8%) for exercisers and 6.4% (95% CI, 5.3%, 7.7%) for non-exercisers (HR 0.72, 95% CI, 0.66, 0.78; Figure 1C). Exercise consistent with national guidelines was associated with a reduction in cancer mortality and mortality from other causes over the entire follow-up period (Table 2).
Figure 1.

Cumulative incidence for all-cause mortality (A), cancer mortality (B) and mortality from other causes (C) by meeting exercise guidelines versus not meeting guidelines. The x-axis indicates years from diagnosis and begins at 0.5 years to reflect the landmark time.
Table 2.
Pan-Cancer Cumulative Incidence of All-Cause Mortality and Cause-Specific Mortality by Exercise Classification
| Cumulative Incidence (95% Confidence Interval) |
||||
|---|---|---|---|---|
| Outcome by Exercise Status1 | 5 Years | 10 Years | 15 Years | 20 Years |
| All-Cause Mortality | ||||
| Non-exercisers | 22% (20%, 25%) | 38% (36%, 40%) | 53% (51%, 55%) | 68% (66%, 69%) |
| Exercisers | 15% (12%, 18%) | 25% (22%, 28%) | 38% (35%, 40%) | 52% (50%, 55%) |
| Cancer Mortality | ||||
| Non-exercisers | 16% (14%, 18%) | 23% (21%, 26%) | 29% (27%, 31%) | 33% (31%, 35%) |
| Exercisers | 12% (9.6%, 16%) | 18% (15%, 21%) | 23% (20%, 26%) | 27% (24%, 30%) |
| Other Mortality | ||||
| Non-exercisers | 6.4% (5.3%, 7.7%) | 14% (13%, 16%) | 25% (23%, 26%) | 35% (33%, 37%) |
| Exercisers | 2.4% (1.5%, 3.8%) | 7.1% (5.9%, 8.5%) | 15% (13%, 16%) | 25% (23%, 27%) |
Definitions.
Exercisers: moderate-intensity exercise >=4 days per week, with each session, on average, >=30 minutes in duration and/or strenuous-intensity exercise >=2 days per week, with each session, on average, >=20 minutes in duration; and (2) Non-exercisers: any exercise below the criteria for meeting national guidelines, including patients reporting 0 days of exercise per week
Pan-Cancer Dose-Response
The inverse relationship between exercise and cause-specific mortality varied by dose (Table S2). For ACM, compared with no exercise, exercise below guidelines was associated with a 25% reduction (HR 0.75, 95% CI, 0.70 to 0.80), meeting guidelines a 35% reduction (HR 0.65, 95% CI, 0.59 to 0.71), and a 36% reduction for exceeding exercise guidelines (HR 0.64, 95% CI, 0.58 to 0.71) (p<0.001; average change in HR for each increasing exercise dose: –0.12, 95% CI, –0.31 to 0.06, p=0.10; Figure 2A). For cancer mortality, exercise below guidelines was associated with a 19% reduction (HR 0.81, 95% CI, 0.72 to 0.90), meeting guidelines a 25% reduction (HR 0.75, 95% CI, 0.66 to 0.86), and a 33% reduction for exceeding exercise guidelines (HR 0.67, 95% CI, 0.58 to 0.78) compared with no exercise (p<0.001; average change in HR for each increasing exercise dose: –0.11, 95% CI, –0.21 to 0.00, p=0.052; Figure 2B). For death from other causes, compared with no exercise, exercise below guidelines was associated with a 29% reduction (HR 0.71, 95% CI, 0.65 to 0.78), meeting guidelines a 42% reduction (HR 0.58, 95% CI, 0.52 to 0.65), and a 37% reduction for exceeding exercise guidelines (HR 0.63, 95% CI, 0.55 to 0.72; p<0.001; average change in HR for each increasing exercise dose: –0.13, 95% CI, –0.38 to 0.11, p=0.14; Figure 2C).
Figure 2.

Cumulative incidence for all-cause mortality (A), cancer mortality (B) and mortality from other causes (C) by exercise dose. The x-axis indicates years from diagnosis and begins at 0.5 years to reflect the landmark time.
Mortality by Cancer Site
Exercise consistent with national guidelines was associated with a reduction in the hazard for ACM for patients with breast, endometrial, head and neck, hematopoetic, prostate, and renal cancer (Figure 3A; Table 3). The reduction in hazard of ACM ranged from 22% (HR 0.78; 95% CI, 0.70, 0.86) for prostate cancer to 59% (HR 0.41; 95% CI, 0.24, 0.72) for endometrial cancer for patients meeting exercise guidelines compared with patients not meeting exercise guidelines. Exercise consistent with national guidelines was associated with a reduction in cancer mortality for two sites: head and neck, and renal cancer (Figure 3B; Table 3). Compared with non-exercisers, exercisers had a significant reduction in death from other causes for breast, colon, endometrial, hematopoietic, and prostate cancer (Figure 3C; Table 3).
Figure 3.
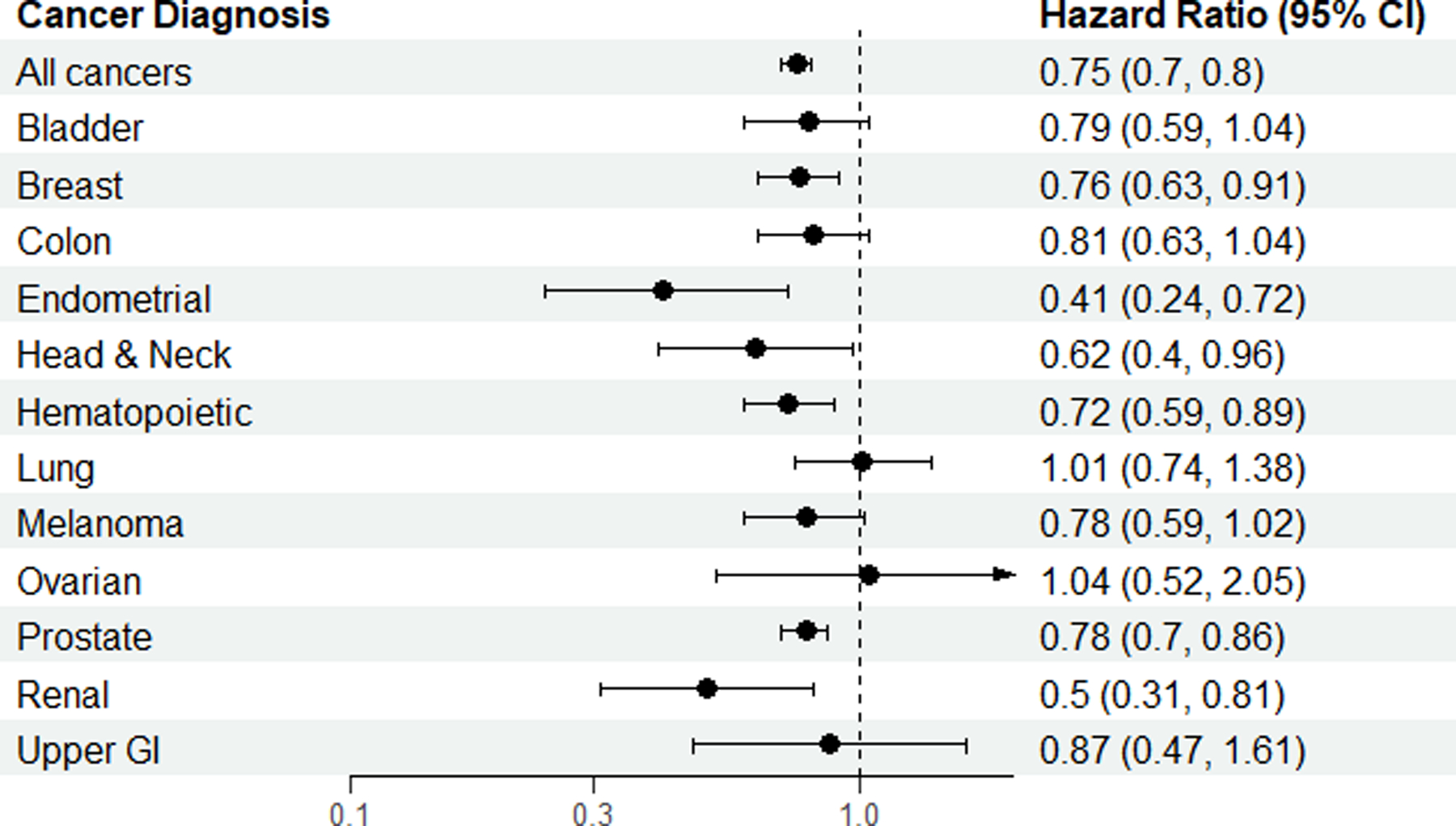


Hazard ratios for all-cause mortality (A), cancer mortality (B) and mortality from other causes (C) for all cancers and by cancer site.
DISCUSSION
Findings of the present study corroborate prior work investigating the relationship of post-diagnosis exercise and cause-specific mortality in cancer survivors. For example, a pooled analysis of six prospective pancancer studies (representing 22,511 survivors) found the highest level of exercise associated with a significant reduction in ACM compared with low exercise.9 In the same review, pooled analysis of four pan-cancer prospective studies found high exercise also associated with a significant reduction in cancer mortality.9 Dose-reponse analysis was not performed nor was analysis of non-cancer deaths due to the small number of studies reporting such events. However, evidence from pooled analyses has important limitations. We leveraged the PLCO screening trial data to overcome these challenges: in addition to the large sample size, long follow-up and resulting high event rate, uniform assessment and classification of exercise exposure together with rigorous ascertainment and adjudication of mortality attribution permits rigorous examination of the post-diagnosis exercise – mortality relationship in cancer survivors thereby significantly extending the current evidence base.
A strength of the PLCO screening trial dataset was adequate representation of patients across different cancer sites, permitting investigation of the clinically important question of whether exercise benefit on mortality differs as a function of cancer site. The present findings indicate that exercise consistent with national guidelines associates with near universal ACM benefit for most cancers included, although the cause-specific mortality events contributing to this benefit appeared cancer site-specific. For instance, in renal cancer, ACM benefit was driven by reductions in cancer mortality, whereas in bladder, colon, endometrial, and hematopoietic cancers, the potential reduction appeared driven by reduction in death from other causes. In breast, prostate, and melanoma cancers ACM benefit was derived from reductions in both cancer mortality and other causes. It is important to interpret these findings within the context of the selected PLCO cohort, which was restricted to patients alive for a median time of 4.5 years after initial diagnosis. Thus, the generalizability of our findings is likely restricted to patients diagnosed with less aggressive tumors at lower risk of disease recurrence and/or cancer mortality and, consequently, with sufficient longevity to be at higher risk of other causes of mortality (e.g., CVD).
The observed variability in the post-diagnosis exercise – cancer mortality relationship across cancer sites is worth considering in this context. Most studies investigating this question have been conducted in breast cancer,29–31 with fewer in prostate32,33 and colorectal cancer.34,35 Systematic reviews indicate post-diagnosis exercise (highest versus lowest exercise) associates with significant reductions in cancer mortality risk, even after adjustment for important clinical covariates9–11 – findings not replicated in our analysis, at least for these 3 cancers. Differences in sample attributable mortality risk might contribute to the discrepant findings given our sample consisted of long-term survivors at high risk of non-cancer mortality, thereby reducing the number cancer-specific events. Interestingly, we observed a significant univariable inverse relationship between exercise at recommended levels and cancer mortality for breast and prostate cancer, as well as for melanoma – all became non-significant in adjusted models suggesting that the multivariable models accounted for observed confounding. Nonetheless, our study is the first to show exercise at recommended levels lowers the risk of cancer death in head and neck and renal cancer (when unadjusted for treatment), and the first to examine, and reveal no association, between exercise and cancer mortality in lung, upper GI, melanoma, ovarian, bladder, endometrial, and hematopoietic cancers. Overall, characterizing and understanding the variability in tumor response to exercise both across and within cancer sites will be a critical and exciting area of future work in exercise-oncology.36
Limitations of our study require consideration. Self-reported assessment of exercise has well-known limitations, and therefore some misclassification of exercise exposure is expected. Generalizability of our findings are limited since our analyses were restricted to a selective sample of primarily non-Hispanic white, long-term survivors with a distribution of cancer sites not representative of general US survivor population,37 which introduces selection bias. Further, our sample consisted of survivors who were alive and willing to complete the SQX after initial cancer diagnosis and therefore perhaps more motivated to engage in heathy lifestyle behaviors.
Relatedly, it is not possible to delineate whether exercise simply reflects lower disease and/or treatment-related toxicities, as opposed to direct exercise-induced effects or better adherence to a healthier lifestyle (i.e., residual confounding). We adjusted all analyses for available important clinical covariates and conducted a sensitivity analysis excluding all patients dying within two years of SQX completion; however, the contribution of unmeasured confounding, including diet and alcohol habits, cannot be disregarded, and only data from randomized controlled trials can definitively prove causality. Given the survey instrument used to evaluate exercise exposure and cross-sectional design, it is not known how long an individual had been exercising (or not) at the time of survey completion or whether exercise was continued after survey completion. Longitudinal studies, preferrably using wearable devices to objectively assess exercise and physical activity, are required to address this important question. Due to the definitions and scope of exercise exposure used in the present study, total physical activity (i.e., occupational plus non-occupational) or specific components of exercise such as intensity or duration were not investigated. These will be important analyses for future studies. Finally, information on primary cancer treatment was only available for PLCO cancers.
In summary, our findings show exercise is a holistic strategy that may complement contemporary management approaches to further reduce cancer mortality (in select sites) while simultaneously lowering risk of death from other competing causes, which combine to improve ACM. This benefit was observed within a few years after diagnosis and sustained for at least 20 years but was not dose-dependent. The cancer mortality impact of exercise differed by cancer site and requires further investigation.
Supplementary Material
CONTEXT SUMMARY.
Key objective.
Does exercise impact cause-specific mortality in long-term cancer survivors?
Knowledge generated.
In pan-cancer analysis, exercise consistent with guidelines was associated with a significant reduction in the hazard of all-cause mortality and the hazards for cancer mortality and mortality from other causes. The inverse relationship between exercise and cause-specific mortality varied by dose. For individual cancer sites, the all-cause mortality benefit of exercise was driven primarily by a reduction in death from other causes; the impact on cancer mortality differed as a function of cancer site.
Relevance:
In this analysis of more than 11,000 patients participating in a cancer screening trial, exercise was associated with reduced all-cause mortality across cancer sites, whereas cancer-specific mortality differed by cancer site. Further prospective studies are necessary to investigate variability in tumor response to exercise across cancer sites.
Relevance section written by JCO Associate Editor Camilla Zimmermann, MD, PhD, FRCPC
Acknowledgement.
The authors thank the National Cancer Institute for access to NCI’s data collected by the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial. Cancer incidence data have been provided by the Colorado Central Cancer Registry, District of Columbia Cancer Registry, Georgia Cancer Registry, Hawaii Cancer Registry, Cancer Data Registry of Idaho, Minnesota Cancer Surveillance System, Missouri Cancer Registry, Nevada Central Cancer Registry, Pennsylvania Cancer Registry, Texas Cancer Registry, Virginia Cancer Registry, and Wisconsin Cancer Reporting System. All are supported in part by funds from the Center for Disease Control and Prevention, National Program for Central Registries, local states or by the National Cancer Institute, Surveillance, Epidemiology, and End Results program. The results reported here and the conclusions derived are the sole responsibility of the authors.
Sources of Funding.
This study was supported by AKTIV Against Cancer (awarded to LWJ). JAL, JMS, TT, CSM, and LWJ are supported by the Memorial Sloan Kettering Cancer Center Support Grant/Core Grant (P30 CA008748). PCB is supported by the UCLA Cancer Center Support Grant (P30 CA016042).
Footnotes
Disclosures.
LWJ – stock ownership in Pacylex, Inc. and Illumisonics, Inc.
JAL – salary support from AACR Project GENIE BPC.
PCB – scientific advisory boards for Sage Bionetworks, BioSymetrics Inc. and Intersect Diagnostics Inc.
TT – scientific advisory board for Lime Therapeutics.
REFERENCES
- 1.Siegel RL, Miller KD, Fuchs HE, et al. : Cancer statistics, 2022. CA Cancer J Clin 72:7–33, 2022 [DOI] [PubMed] [Google Scholar]
- 2.Kratzer TB, Siegel RL, Miller KD, et al. : Progress Against Cancer Mortality 50 Years After Passage of the National Cancer Act. JAMA Oncol 8:156–159, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moslehi J: The cardiovascular perils of cancer survivorship. N Engl J Med 368:1055–6, 2013 [DOI] [PubMed] [Google Scholar]
- 4.Darby SC, Ewertz M, McGale P, et al. : Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med 368:987–98, 2013 [DOI] [PubMed] [Google Scholar]
- 5.Oeffinger KC, Mertens AC, Sklar CA, et al. : Chronic health conditions in adult survivors of childhood cancer. N Engl J Med 355:1572–82, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Mackey JR, Martin M, Pienkowski T, et al. : Adjuvant docetaxel, doxorubicin, and cyclophosphamide in node-positive breast cancer: 10-year follow-up of the phase 3 randomised BCIRG 001 trial. Lancet Oncol 14:72–80, 2013 [DOI] [PubMed] [Google Scholar]
- 7.Jones LW, Courneya KS, Mackey JR, et al. : Cardiopulmonary function and age-related decline across the breast cancer survivorship continuum. J Clin Oncol 30:2530–7, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DeGregori J, Pharoah P, Sasieni P, et al. : Cancer Screening, Surrogates of Survival, and the Soma. Cancer Cell 38:433–437, 2020 [DOI] [PubMed] [Google Scholar]
- 9.Friedenreich CM, Stone CR, Cheung WY, et al. : Physical Activity and Mortality in Cancer Survivors: A Systematic Review and Meta-Analysis. JNCI Cancer Spectr 4:pkz080, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patel AV, Friedenreich CM, Moore SC, et al. : American College of Sports Medicine Roundtable Report on Physical Activity, Sedentary Behavior, and Cancer Prevention and Control. Med Sci Sports Exerc 51:2391–2402, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rock CL, Thomson CA, Sullivan KR, et al. : American Cancer Society nutrition and physical activity guideline for cancer survivors. CA Cancer J Clin 72:230–262, 2022 [DOI] [PubMed] [Google Scholar]
- 12.Cao C, Friedenreich CM, Yang L: Association of Daily Sitting Time and Leisure-Time Physical Activity With Survival Among US Cancer Survivors. JAMA Oncol 8:395–403, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hamer M, Stamatakis E, Saxton JM: The impact of physical activity on all-cause mortality in men and women after a cancer diagnosis. Cancer Causes Control 20:225–31, 2009 [DOI] [PubMed] [Google Scholar]
- 14.Inoue-Choi M, Robien K, Lazovich D: Adherence to the WCRF/AICR guidelines for cancer prevention is associated with lower mortality among older female cancer survivors. Cancer Epidemiol Biomarkers Prev 22:792–802, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gunnell AS, Knuiman MW, Divitini ML, et al. : Leisure time physical activity and long-term cardiovascular and cancer outcomes: the Busselton Health Study. Eur J Epidemiol 29:851–7, 2014 [DOI] [PubMed] [Google Scholar]
- 16.Gohagan JK, Prorok PC, Hayes RB, et al. : The Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial of the National Cancer Institute: history, organization, and status. Control Clin Trials 21:251S–272S, 2000 [DOI] [PubMed] [Google Scholar]
- 17.Prorok PC, Andriole GL, Bresalier RS, et al. : Design of the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial. Control Clin Trials 21:273S–309S, 2000 [DOI] [PubMed] [Google Scholar]
- 18.Gohagan JK, Prorok PC, Greenwald P, et al. : The PLCO Cancer Screening Trial: Background, Goals, Organization, Operations, Results. Rev Recent Clin Trials 10:173–80, 2015 [DOI] [PubMed] [Google Scholar]
- 19.Campbell KL, Winters-Stone KM, Wiskemann J, et al. : Exercise Guidelines for Cancer Survivors: Consensus Statement from International Multidisciplinary Roundtable. Med Sci Sports Exerc 51:2375–2390, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ligibel JA, Bohlke K, May AM, et al. : Exercise, Diet, and Weight Management During Cancer Treatment: ASCO Guideline. J Clin Oncol:JCO2200687, 2022 [DOI] [PubMed] [Google Scholar]
- 21.Denlinger CS, Ligibel JA, Are M, et al. : NCCN Guidelines Insights: Survivorship, Version 1.2016. J Natl Compr Canc Netw 14:715–24, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cormie P, Atkinson M, Bucci L, et al. : Clinical Oncology Society of Australia position statement on exercise in cancer care. Med J Aust 209:184–187, 2018 [DOI] [PubMed] [Google Scholar]
- 23.Miller AB, Yurgalevitch S, Weissfeld JL, et al. : Death review process in the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial. Control Clin Trials 21:400S–406S, 2000 [DOI] [PubMed] [Google Scholar]
- 24.Miller AB, Feld R, Fontana R, et al. : Changes in and Impact of the Death Review Process in the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial. Rev Recent Clin Trials 10:206–11, 2015 [DOI] [PubMed] [Google Scholar]
- 25.Brown S, Lavery JA, Shen R, et al. : Implications of Selection Bias Due to Delayed Study Entry in Clinical Genomic Studies. JAMA Oncol 8:287–291, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grambsch PT T: Proportional hazards tests and diagnostics based on weighted residuals. Biometrika 81:515–526, 1994 [Google Scholar]
- 27.Brownstein NC, Cai J: Tests of trend between disease outcomes and ordinal covariates discretized from underlying continuous variables: simulation studies and applications to NHANES 2007–2008. BMC Med Res Methodol 19:2, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.R Core Team: R: A Language and Environment for Statistical Computing. Vienna, Austria, R Foundation for Statistical Computing, 2021 [Google Scholar]
- 29.Holmes MD, Chen WY, Feskanich D, et al. : Physical activity and survival after breast cancer diagnosis. JAMA 293:2479–86, 2005 [DOI] [PubMed] [Google Scholar]
- 30.Jones LW, Kwan ML, Weltzien E, et al. : Exercise and Prognosis on the Basis of Clinicopathologic and Molecular Features in Early-Stage Breast Cancer: The LACE and Pathways Studies. Cancer Res 76:5415–22, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Irwin ML, Smith AW, McTiernan A, et al. : Influence of pre- and postdiagnosis physical activity on mortality in breast cancer survivors: the health, eating, activity, and lifestyle study. J Clin Oncol 26:3958–64, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kenfield SA, Stampfer MJ, Giovannucci E, et al. : Physical activity and survival after prostate cancer diagnosis in the health professionals follow-up study. J Clin Oncol 29:726–32, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Friedenreich CM, Wang Q, Neilson HK, et al. : Physical Activity and Survival After Prostate Cancer. Eur Urol 70:576–585, 2016 [DOI] [PubMed] [Google Scholar]
- 34.Lee S, Meyerhardt JA: Impact of Diet and Exercise on Colorectal Cancer. Hematol Oncol Clin North Am 36:471–489, 2022 [DOI] [PubMed] [Google Scholar]
- 35.Brown JC, Ma C, Shi Q, et al. : Physical Activity in Stage III Colon Cancer: CALGB/SWOG 80702 (Alliance). J Clin Oncol:JCO2200171, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jones LW: Precision Oncology Framework for Investigation of Exercise As Treatment for Cancer. J Clin Oncol 33:4134–7, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miller KD, Siegel RL, Lin CC, et al. : Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin 66:271–89, 2016 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


