Abstract
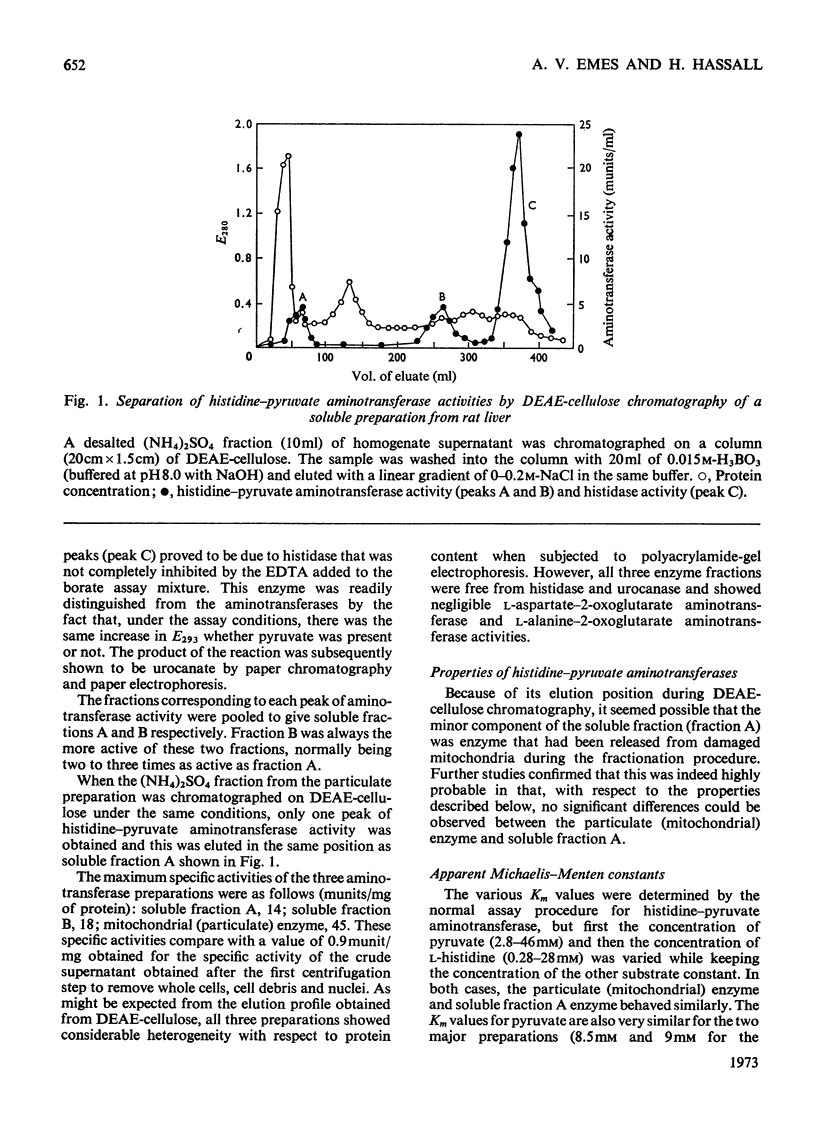
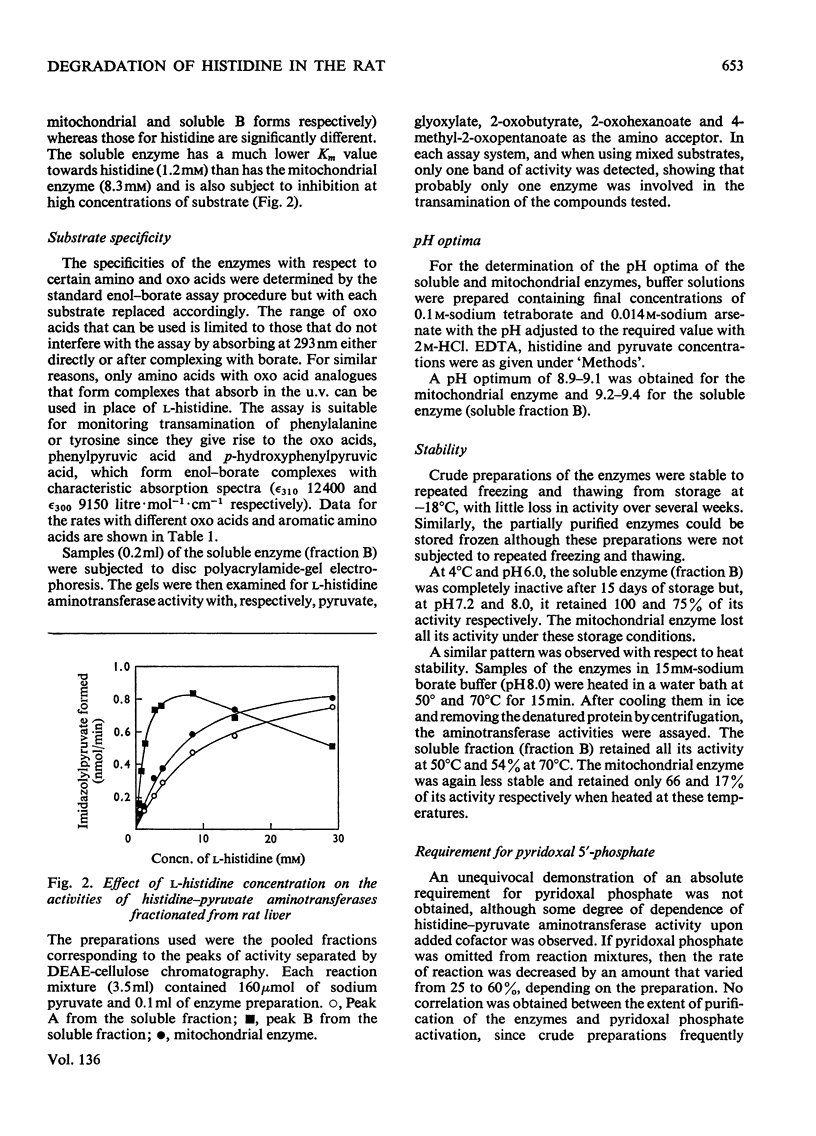
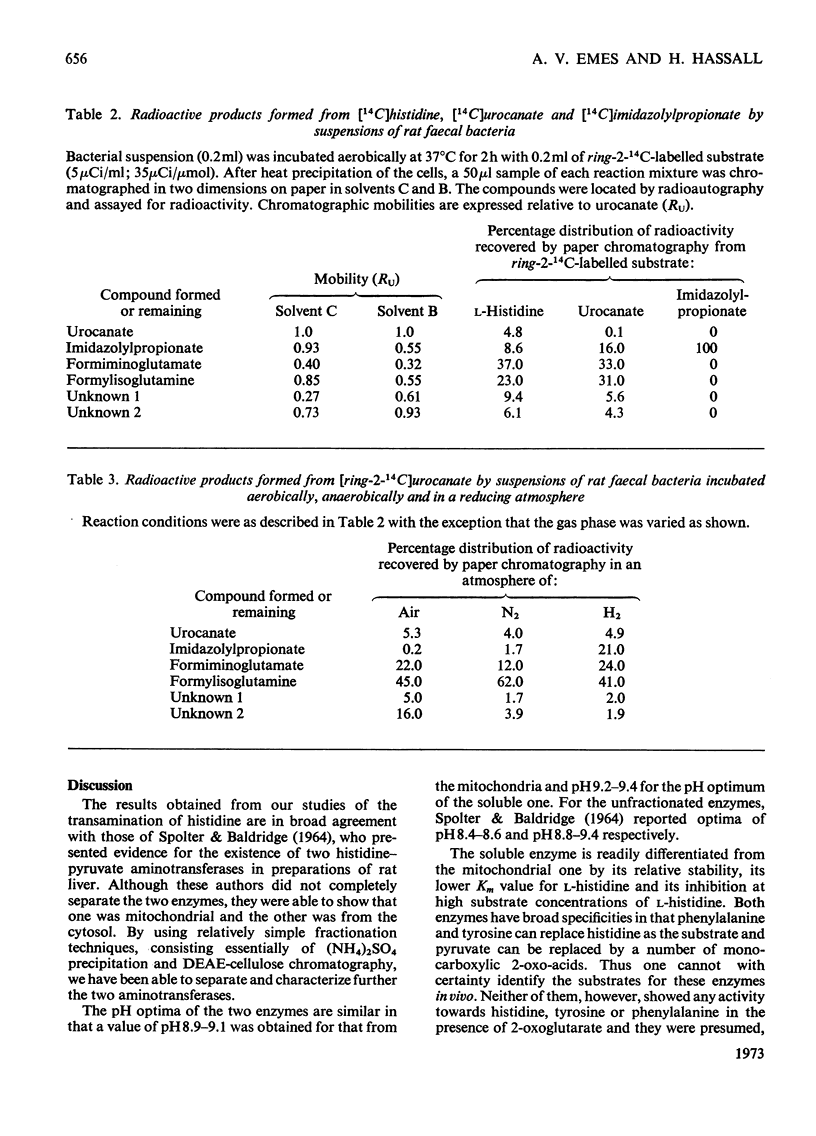
1. Soluble and mitochondrial forms of histidine–pyruvate aminotransferase were separated from rat liver preparations by chromatography on DEAE-cellulose. 2. These enzymes were characterized with respect to substrate specificity, substrate affinity, pH optimum, stability and molecular weight by chromatography on Sephadex G-200. 3. Each enzyme has a relatively broad specificity showing significant activity towards l-phenylalanine and l-tyrosine and catalysing transamination with a number of monocarboxylic 2-oxo acids. 2-Oxoglutarate is not a substrate for either enzyme. 4. The molecular weights of the two enzymes, by chromatography on Sephadex G-200, are in the range 130000–150000. 5. The formation in vitro of imidazolyl-lactate from imidazolylpyruvate and NADH was demonstrated by using liver preparations. 6. From a study of imidazolyl-lactate–NAD+ oxidoreductase activity after electrophoresis of liver preparations on polyacrylamide gel, and from an examination of the activity of l-lactate–NAD+ oxidoreductase (EC 1.1.1.27) towards imidazolylpyruvate, it is concluded that this latter enzyme is responsible for the formation of imidazolyl-lactate in the liver. 7. Preparations of bacteria obtained from rat faeces form imidazolylpropionate from l-histidine and urocanate without further subculture. The amount of imidazolylpropionate formed is increased under anaerobic conditions and more so in an atmosphere of H2. It is suggested that the gut flora of the rat contribute largely, if not exclusively, to the formation of imidazolylpropionate normally found in the urine.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AUERBACH V. H., DIGEORGE A. M., BALDRIDGE R. C., TOURTELLOTTE C. D., BRIGHAM M. P. Histidinemia. A deficiency in histidase resulting in the urinary excretion of histidine and of imidazolepyruvic acid. J Pediatr. 1962 Apr;60:487–497. doi: 10.1016/s0022-3476(62)80109-7. [DOI] [PubMed] [Google Scholar]
- Andrews P. The gel-filtration behaviour of proteins related to their molecular weights over a wide range. Biochem J. 1965 Sep;96(3):595–606. doi: 10.1042/bj0960595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BALDRIDGE R. C., TOURTELLOTTE C. D. The metabolism of histidine. III. Urinary metabolites. J Biol Chem. 1958 Jul;233(1):125–127. [PubMed] [Google Scholar]
- BROWN D. D., KIES M. W. The mammalian metabolism of L-histidine. I. The enzymatic formation of L-hydantion-5-propionic acid. J Biol Chem. 1959 Dec;234:3182–3187. [PubMed] [Google Scholar]
- Coote J. G., Hassall H. The control of the enzymes degrading histidine and related imidazolyl derivates in Pseudomonas testosteroni. Biochem J. 1973 Mar;132(3):423–433. doi: 10.1042/bj1320423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coote J. G., Hassall H. The degradation of L-histidine, imidazolyl-L-lactate and imidazolylpropionate by Pseudomonas testosteroni. Biochem J. 1973 Mar;132(3):409–422. doi: 10.1042/bj1320409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- GHADIMI H., PARTINGTON M. W., HUNTER A. A familial disturbance of histidine metabolism. N Engl J Med. 1961 Aug 3;265:221–224. doi: 10.1056/NEJM196108032650504. [DOI] [PubMed] [Google Scholar]
- HASSALL H., GREENBERG D. M. Studies on the enzymic decomposition of urocanic acid. V. The formation of 4-oxoglutaramic acid, a nonenzymic oxidation product of 4(5)-imidazolone-5(4)-propionic acid. J Biol Chem. 1963 Apr;238:1423–1431. [PubMed] [Google Scholar]
- Hassall H., Lunn P. J., Ryall-Wilson J. Detection of histidase and urocanase after disc electrophoresis on polyacrylamide gels. Anal Biochem. 1970 Jun;35(2):326–334. doi: 10.1016/0003-2697(70)90192-2. [DOI] [PubMed] [Google Scholar]
- KRAML M., BOUTHILLIER L. P. The conversion of urocanic acid to glutamic acid in the intact rat. Can J Biochem Physiol. 1955 Jul;33(4):590–598. [PubMed] [Google Scholar]
- LIN E. C., PITT B. M., CIVEN M., KNOX W. E. The assay of aromatic amino acid transaminations and keto acid oxidation by the enol borate-tautomerase method. J Biol Chem. 1958 Sep;233(3):668–673. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MAKOFF R., BALDRIDGE R. C. THE METABOLISM OF HISTIDINE. LIVER-ENZYME CHANGES DURING DEVELOPMENT. Biochim Biophys Acta. 1964 Aug 19;90:282–286. doi: 10.1016/0304-4165(64)90190-4. [DOI] [PubMed] [Google Scholar]
- PECK H. D., Jr, SMITH O. H., GEST H. Comparative biochemistry of the biological reduction of fumaric acid. Biochim Biophys Acta. 1957 Jul;25(1):142–147. doi: 10.1016/0006-3002(57)90431-6. [DOI] [PubMed] [Google Scholar]
- POLAN C. E., MCNEILL J. J., TOVE S. B. BIOHYDROGENATION OF UNSATURATED FATTY ACIDS BY RUMEN BACTERIA. J Bacteriol. 1964 Oct;88:1056–1064. doi: 10.1128/jb.88.4.1056-1064.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEN N. P., McGEER P. L., PAUL R. M. Imidazolepropionic acid as a urinary metabolite of L-histidine. Biochem Biophys Res Commun. 1962 Oct 17;9:257–261. doi: 10.1016/0006-291x(62)90069-4. [DOI] [PubMed] [Google Scholar]
- SPOLTER H., BALDRIDGE R. C. MULTIPLE FORMS OF HISTIDINE-PYRUVATE TRANSAMINASE IN RAT LIVER. Biochim Biophys Acta. 1964 Aug 19;90:287–290. doi: 10.1016/0304-4165(64)90191-6. [DOI] [PubMed] [Google Scholar]
- SPOLTER P. D., BALDRIDGE R. C. The metabolism of histidine. V. On the assay of enzymes in rat liver. J Biol Chem. 1963 Jun;238:2071–2074. [PubMed] [Google Scholar]
- WOLIN M. J., WOLIN E. A., JACOBS N. J. Cytochrome-producing anaerobic Vibrio succinogenes, sp. n. J Bacteriol. 1961 Jun;81:911–917. doi: 10.1128/jb.81.6.911-917.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilde P. F., Dawson R. M. The biohydrogenation of alpha-linolenic acid and oleic acid by rumen micro-organisms. Biochem J. 1966 Feb;98(2):469–475. doi: 10.1042/bj0980469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Heiden C., Wadman S. K., de Bree P. K., Wauters E. A. Increased urinary imidazolepropionic acid, N-acetylhistamine and other imidazole compounds in patients with intestinal disorders. Clin Chim Acta. 1972 Jun;39(1):201–214. doi: 10.1016/0009-8981(72)90317-8. [DOI] [PubMed] [Google Scholar]


