Abstract
Case series
Patients: Female, 29-year-old • Female, 34-year-old • Female, 17-year-old • Female, 26-year-old
Final Diagnosis: Tirzepatide-induced hypoglycemic ketoacidosis for weight loss in four nondiabetic patients
Symptoms: Diarrhea • vomiting
Clinical Procedure: Fluid replacement
Specialty: Critical Care Medicine
Objective:
Unusual clinical course
Background:
Tirzepatide is a long-acting glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) receptor agonist administered via subcutaneous injection for weight reduction and treating type 2 diabetes.
Case Reports
We report case series of hypoglycemic ketoacidosis after the use of tirzepatide to treat nondiabetic patients with obesity from Kuwait. The first case was a 29-year-old woman with a body mass index (BMI) of 32 kg/m2 who developed abdominal pain and vomiting after increasing the dose to 5 mg subcutaneously in week 5 of treatment. The second case was a 34-year-old woman with a BMI of 31.3 kg/m2 who presented with abdominal pain, vomiting, and diarrhea after increasing the dose to 5 mg subcutaneously. The third case was a 17-year-old girl with a BMI of 30.4 kg/m2 who presented with abdominal pain, vomiting, and diarrhea in week 5 of treatment. The fourth case was a 26-year-old woman with a BMI of 30.8 kg/m2 who presented with abdominal pain, frequent loose motions, and vomiting. The median blood sugar level was <3.89 mmol/L and high anion gap metabolic acidosis with ketosis occurred. All the patients required inpatient treatment with intravenous fluid and the correction of hypoglycemia and ketosis.
Conclusions:
Tirzepatide can induce hypoglycemic ketoacidosis in nondiabetic patients with obesity when used for weight reduction. Measuring urine and serum ketone levels in patients with gastrointestinal symptoms who are taking dual GLP-1 and GIP receptor agonists is crucial. Medical supervision is recommended when this medication is prescribed.
Key words: Endocrine System Diseases, Hypoglycemia, Obesity
Introduction
Ketone compounds (acetoacetate and beta-hydroxybutyrate) and the occurrence of high anion gap metabolic acidosis are the main characteristics of ketoacidosis [1]. The main mechanism underlying ketoacid accumulation is the increased production of free fatty acids and their conversion to ketone acids in the liver, consumed mainly in the brain [2]. A decreased availability of excess insulin and glucagon is responsible for the increased mobilization of free fatty acids from adipose tissue and the metabolism of free fatty acids to keto acids in the liver [2].
Tirzepatide is a dual glucose-dependent insulinotropic poly-peptide (GIP) receptor and glucagon-like peptide 1 (GLP-1) receptor agonist [3]. It is administered once weekly via subcutaneous injection [3]. This medication is approved for type 2 diabetes management and as an adjunct for chronic weight management in adults with obesity or overweight, with or without diabetes [4]. A previous single-case report revealed tirzepatide-induced euglycemic ketoacidosis in nondiabetic patients [5]. Here, we present a case series of hypoglycemic ketoacidosis in nondiabetic patients with obesity treated with tirzepatide for weight reduction.
Case Reports
Four Kuwaiti patients were admitted to the Ahmadi Hospital Emergency Department (ED) and were then seen from January to June 2024. All patients were admitted to general medical wards for rehydration and a correction of their serum glucose levels. All of the patients returned for follow-up evaluations. The 4 patients received over-the-counter tirzepatide for weight reduction, and none were diabetic. A detailed history of dietary intake and drug use was obtained for each patient. Clinical evaluations were performed in the ED, followed by chemistry profiles, arterial blood gases, and lactic acid measurements. The blood levels of ketone bodies (serum p-hydroxybutyrate levels) were measured via capillary finger blood tests (free stylet test Optimum Neo; Abbott Diabetes Care), and urine acetone levels were checked.
Case 1
A 29-year-old woman with a body mass index (BMI) of 32 kg/m2 and no known diabetes or hypertension was included. She started tirzepatide over-the-counter for weight reduction. She was started on a dose of 2.5 mg weekly for 4 weeks and then increased to 5 mg for 3 weeks. In week 4, the patient developed severe generalized abdominal pain 5 days before admission, with a sensation of fullness but no diarrhea. This was accompanied by repeated vomiting 3 times per day for the same duration. There was severe anorexia and a sensation of fullness. She was admitted to the Medical Ward due to dehydration and tachycardia of 110 beats per min, blood pressure of 100/70 mm Hg, and oxygen saturation level of 99% on room air.
Tables 1 and 2 present the clinical characteristics and laboratory test results. The patient was admitted with ketotic high anion gap metabolic acidosis with hypoglycemia. She was started on dextrose 5% infusion and Ringer’s lactate infusion in another line until the anion gap was closed. Blood glucose levels were maintained between 4 and 6 mmol/L during treatment with dextrose infusion. A 4-week follow-up revealed the resolution of all symptoms, and the patient gained 1.5 kg. She declined to continue treatment with tirzepatide.
Table 1.
Clinical characteristics.
| Item | Case 1 | Case 2 | Case 3 | Case 4 |
|---|---|---|---|---|
| Age/sex | 29/F | 34/F | 17/F | 26/F |
| BMI (kg/m2) | 32 | 31.3 | 30.4 | 30.8 |
| Dehydration severity | Severe | Mild | Mild | Mild |
| Abdominal pain duration (duration in days) | 5 | 7 | 2 | 4 |
| Vomiting (frequency/day, duration in days) | 3, 5 | 4, 2 | 3, 2 | 3, 1 |
| Diarrhea (duration in days) | No | 3 | 3 | 3 |
| Duration of symptoms (days) | 5 | 2 | 2 | 4 |
| Dose of tirezaptide | 2.5 mg for 4 weeks, then 5 mg for 3 weeks | 2.5 mg for 4 weeks, then 5 mg for 2 weeks | 2.5 mg for 4 weeks, then 5 mg for 1 week | 2.5 mg for 4 weeks, then 5 mg for 2 weeks |
| Duration of tirzeptide until admission (weeks) | 7 | 6 | 5 | 6 |
| Weight loss (kg) | 5 | 7 | 8 | 7 |
| Calorie intake/day | 1400 | 1400 | 1500 | 1200 |
| Fluid resuscitation | Riger lactate | Dextrose 5%/saline infusion | Dextrose 10% for 1 h followed by dextrose 5% with saline | Dextrose 10% followed by ringer lactate |
| Ultrasound abdomen | Fatty liver grade 1 | Fatty liver grade 1 | Fatty liver grade 1 | Fatty liver grade 1 |
| Outcome | Admitted to ward | Ward admission | Ward admission | Ward admission |
Table 2.
Laboratory test results.
| Item (units) | Case 1 | Case 2 | Case 3 | Case 4 | Reference range |
|---|---|---|---|---|---|
| Glucose level on admission (mmol/L) | 3 | 2.8 | 2.9 | 3.1 | 4–6 |
| pH | 7.24 | 7.3 | 7.31 | 7.3 | 7.38–7.42 |
| HCO3 | 16 | 20 | 21.3 | 20.3 | 22–26 |
| Ketone level (mmol/L) | 4.5 | 5 | 1.2 | 3 | <0.2 |
| White blood count | 9.4 | 3.3 | 7.3 | 8.6 | 4–10×109/L |
| Hemoglobin (g/dL) | 14.8 | 13.0 | 12.5 | 16.5 | 12–15 |
| Platelet Count | 440×109/L | 140×109/L | 221×109/L | 322×109/L | 140–400 |
| Sodium (mmol/L) | 137 | 139 | 136 | 139 | 136–145 |
| Serum potassium (mmol/L) | 4.2 | 3.4 | 4 | 3.9 | 3.5–5.1 |
| Blood urea nitrogen, (mmol/L) | 2.6 | 1.8 | 3.5 | 2 | 2.14–7.14 |
| Corrected calcium (mmol/L) | 2.42 | 2.12 | 2.3 | 2.5 | 2.2–2.6 |
| Phosphorus (mmol/L) | 0.9 | 0.7 | 1.3 | 1.3 | 0.81–1.45 |
| Magnesium (mmol/L) | 0.7 | 0.85 | 1.2 | 0.8 | 0.66–1.07 |
| Total bilirubin (umol/L) | 10 | 12 | 30 | 21 | 2–17 |
| Gamma glutamyl transpeptidase (U/L) | 8 | 12 | 30 | 45 | 3–40 |
| Aspartate amino transf (AST/SGOT) (U/L) | 12 | 13 | 20 | 14 | 5–32 |
| Creatinine (umol/L) | 45 | 69 | 66 | 94 | 62–106 |
| HbA1c | 5 | 4.3 | 6.3 | 6.4 | <5.7 |
| Uric acid (umol/L) | 359 | 504 | 510 | 470 | 160–430 |
| Amylase (U/L) | 63 | 70 | 35 | 20 | 28–100 |
| Lipase (U/L) | 25 | 35 | 35 | (3–60) | |
| C-reactive protein (mg/L) | 7 | 3 | 9 | 23.7 | 0–5 |
| Lactate (mmol/L) | 1.0 | 0.9 | 1.5 | <1 | |
| Salicylates (mg/dL) | 3 | 1 | 0 | 0 | 50–300 |
Case 2
A 34-year-old woman with a BMI of 31.3 kg/m2 and not known to have diabetes mellitus or hypertension reported 7 days of abdominal pain after starting treatment, followed by vomiting 3 times/day for 2 days, with loose motion. She had started tirzepatide over-the-counter for weight reduction and was on her second week of 5 mg subcutaneous injection after completing 4 weeks of 2.5 mg/week. She was mildly dehydrated and had stable vital signs. Her blood sugar level at admission was 2.8 mmol (normal value is 4–6 mmol/L). Tables 1 and 2 present the clinical characteristics and laboratory test results. She was in ketotic high anion gap metabolic acidosis with hypoglycemia and normal lactate. She was started on dextrose 5% infusion and Ringer’s lactate infusion in another line until the anion gap was closed. Blood glucose levels were maintained between 5 and 7 mmol/L during treatment with dextrose infusion. A follow-up at 4 weeks showed the resolution of all symptoms, and the patient had gained 1 kg. She insisted on being on a lower dose and was advised against taking tirzepatide.
Case 3
A 17-year-old girl with a BMI of 30.4 kg/m2 was referred from the ED with consistent abdominal pain for 3 days, vomiting for 2 days, and diarrhea for 3 days. She had been taking tirzepatide for 5 weeks, starting with 2.5 mg/week for 4 weeks, after which the dose was increased to 5 mg in week 5. Her blood presssure was 104/56 mm Hg and pulse was 110 beats per min.
Tables 1 and 2 present the clinical characteristics and laboratory test results of the patient in the ED. The patient was treated for high anion gap metabolic acidosis with hypoglycemia and ketosis. She was started on dextrose 5% infusion and Ringer’s lactate infusion in another line until the anion gap was closed. Blood glucose levels were maintained above 4 mmol/L during her stay in the ED, and she was discharged in good condition and hydrated, with normal pH and blood bicarbonate levels. One month later, the patient was asymptomatic and had not gained weight.
Case 4
A 26-year-old woman with a BMI of 30.8 kg/m2 and no known diabetes or hypertension presented to the ED after experiencing abdominal pain for 4 days, followed by frequent loose motions and vomiting 3 times/day for a duration of 1 day. She was taking 2.5 mg tirzepatide for 4 weeks and 5 mg tirzepatide once weekly for 2 weeks before presenting to the ED. She was also mildly dehydrated. Tables 1 and 2 present the clinical characteristics and laboratory test results. Vital signs were normal, apart from tachycardia at 104 beats per min.
The patient’s blood sugar was 3.1 mmol/L (normal value is 4–6 mmol/L). Hypoglycemic ketotic high anion gap metabolic syndrome was diagnosed. A 10% dextrose infusion was started to correct the hypoglycemia, followed by adding Ringer’s lactate for rehydration. At the 4-week follow-up, the patient was asymptomatic and had gained 2 kg.
Discussion
Tirzepatide is a dual-acting therapy comprising GLP-1 and GIP receptor agonists that control serum glucose by increasing glucose-dependent insulin secretion, modifying gastric emptying, and decreasing postprandial glucagon and food in-take [6]. Tirzepatide has been approved for weight management alongside diet and exercise in patients with a BMI ≥30 kg/m2 and in patients with a BMI ≥27 kg/m2 and 1 weight-associated comorbidity (eg, cardiac disease, chronic hypertension, obstructive sleep apnea, type 2 diabetes mellitus) [4]. The drug is also approved for treating type 2 diabetes mellitus [4]. The major adverse effects are gastrointestinal effects, mainly nausea, vomiting, and diarrhea, which occur in 10% of individuals on therapy [7]. There are reported cases of pancreatitis, hepatobiliary cholelithiasis, and cholecystitis [8]. Tirzepatide does not usually cause hypoglycemia in the absence of other diabetic therapies that might induce hypoglycemia. Even with overdoses of GLP1 agonists, hypoglycemia has not been reported [9,10]. The proposed mechanism for the hypoglycemia observed in the present case series could be multifactorial. The most important factor is the depletion of available glycogen stores in the liver after a period of starvation for days or even hours. Additionally, GLP-1 therapy can augment insulin release and inhibit the effect of glucagon, thereby decreasing hepatic gluconeogenesis [11]. Insulin and glucagon work in a counteractive mode to regulate glucose metabolism. Low insulin and high glucagon levels occur during fasting. These hormones increase hepatic gluconeogenesis and glycogenolysis (glycogen breakdown) while decreasing glycogen synthesis, to prevent blood sugar from dropping too low [11]. GLP-1 therapy antagonizes all these mechanisms.
Hypoglycemia with ketoacidosis occurs in certain situations, such as alcoholism, starvation, and cases where birth defects result in faulty fatty acid metabolism. This condition can also occur when insulin release is blocked by alpha receptor blockers and diazoxide, salicylate intoxication, and renal sodium-glucose transporter 2 inhibitor therapy [12]. A previous single-case report revealed tirzepatide-induced euglycemic ketoacidosis in nondiabetic patients [5]. The main mechanism of euglycemic ketoacidosis is starvation due to vomiting, diarrhea, and poor caloric intake induced by dual-acting GLP-1 agonists. In addition, dual-acting GLP-1 agonists can suppress appetite, prolong gastric emptying, and decrease calorie intake [5]. The present case series featured hypoglycemia with ketoacidosis.
In contrast, the Food and Drug Administration and the Medicines and Healthcare Products Regulatory Agency of the United Kingdom reported a link between GLP-1 receptor agonists (GLP-1RAs) and diabetic ketoacidosis in people with diabetes who were taking medications targeting GLP-1. The FDA Adverse Event Reporting System (FAERS) database was used to assess the association between GLP-1RAs and diabetic ketoacidosis/ketosis. A total of 1382 diabetic ketoacidosis cases from the first quarter of 2004 to the fourth quarter of 2019 were associated with GLP-1RAs in the FAERS database. After the effects of SGLT2i and insulin were removed, a slightly higher rate of diabetic ketoacidosis was reported when GLP-1RAs were used (proportional reporting ratio 1.49, 95% CI 1.24–1.79, P<0.001). After GLP-1RAs were combined with insulin for comparison, this disproportionality disappeared. Disproportionate reporting of diabetic ketoacidosis associated with GLP-1RAs occurred when they were not used in combination with insulin [13].
The present study is an important case series in which hypoglycemic ketoacidosis in nondiabetic patients with obesity receiving the dual-acting GLP-1 agonist therapy, tirzepatide, for weight reduction was studied. Starvation ketoacidosis with depletion of liver glycogen is the most likely mechanism. Starvation ketoacidosis is caused by vomiting, diarrhea, and poor caloric intake induced by dual-acting GLP-1 agonists. In addition, dual-acting GLP-1 agonists reduce appetite, delay gastric emptying, and lower caloric intake (Figure 1). Patients with obesity with a limited caloric intake, sometimes less than 500 calories a day, are at risk of ketosis [14]. Another possible mechanism is the association between metabolism-related unhealthy obesity and insulin resistance [15]. Our patients met the criteria for metabolically unhealthy obesity in the form of visceral fat depositions (fatty liver), impaired lipid profiles, high uric acid levels, and high BMIs [15]. The excessive accumulation of free fatty acids in insulin-sensitive non-adipose tissues causes increased insulin resistance [16]. In patients with obesity and diabetes, insulin resistance can result in relative insulin deficiency. Insulin withdrawal or dose reduction has been identified as a critical factor in the development of diabetic ketoacidosis among patients treated with GLP-1 receptor agonists [13].
Figure 1.
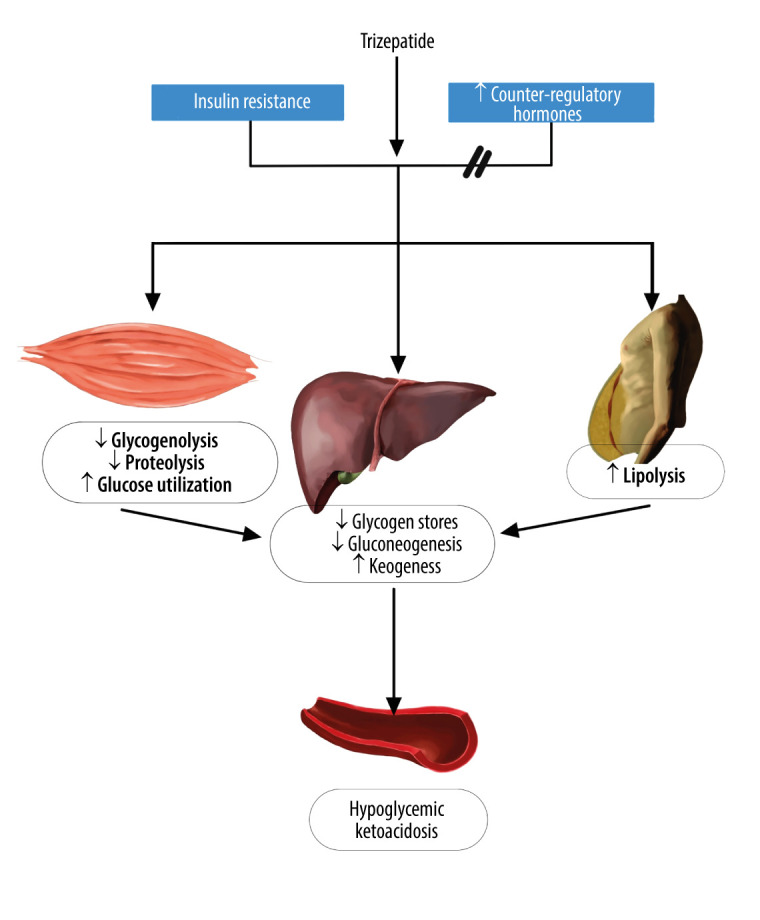
Mechanism of hypoglycemic ketoacidosis. Depleting available glycogen stores in the liver and tirzepatide can increase insulin release and inhibit the effect of counterregulatory hormones, thereby decreasing hepatic gluconeogenesis in a state of insulin resistance in the peripheral tissue. This figure was created using digital art on an iPad, an Apple pencil, and an application called “Notebook”.
Conclusions
We reported a case series of hypoglycemic ketoacidosis caused by the dual-acting GLP-1 and GIP receptor agonist tirzepatide in nondiabetic patients with obesity. The most likely causes are starvation ketosis and impaired hepatic gluconeogenesis in patients with insulin resistance. Measuring urine and serum ketone levels in patients with gastrointestinal symptoms who are taking dual GLP-1 and GIP receptor agonists is necessary, and stopping the medication is necessary when needed. Tirzepatide should be prescribed only under medical supervision, not for over-the-counter use.
Acknowledgments
The authors thank the Ahmadi Hospital administration for their unlimited support.
Footnotes
Publisher’s note: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher
Declaration of Figures’ Authenticity
All figures submitted have been created by the authors who confirm that the images are original with no duplication and have not been previously published in whole or in part.
References:
- 1.Halperin ML, Hammeke M, Josse RG, Jungas RL. Metabolic acidosis in the alcoholic: A pathophysiologic approach. Metabolism. 1983;32(3):308–15. doi: 10.1016/0026-0495(83)90197-x. [DOI] [PubMed] [Google Scholar]
- 2.Davids MR, Segal AS, Brunengraber H, Halperin ML. An unusual cause for ketoacidosis. QJM. 2004;97(6):365–76. doi: 10.1093/qjmed/hch064. [DOI] [PubMed] [Google Scholar]
- 3.Willard FS, Douros JD, Gabe MB, et al. Tirzepatide is an imbalanced and biased dual GIP and GLP-1 receptor agonist. JCI Insight. 2020;5:e140532. doi: 10.1172/jci.insight.140532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jastreboff AM, Aronne LJ, Ahmad NN, et al. SURMOUNT-1 Investigators Tirzepatide once weekly for the treatment of obesity. N Engl J Med. 2022;387:205–16. doi: 10.1056/NEJMoa2206038. [DOI] [PubMed] [Google Scholar]
- 5.Iqbal PMR, Maadarani OS, Bitar ZI. Tirzepatide-induced ketoacidosis in non-diabetic patients. Eur J Case Rep Intern Med. 2024;11(4):004357. doi: 10.12890/2024_004357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Calanna S, Christensen M, Holst JJ. Secretion of glucagon-like peptide-1 in patients with type 2 diabetes mellitus: Systematic review and meta-analyses of clinical studies. Diabetologia. 2013;56(5):965–72. doi: 10.1007/s00125-013-2841-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tirzepatide (Mounjaro) for type 2 diabetes. Med Lett Drugs Ther. 2022;64(1654):105–7. [PubMed] [Google Scholar]
- 8.Farzam K, Patel P. StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024. Tirzepatide. [Updated 2024 Feb 20]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK585056/ [Google Scholar]
- 9.Krishnan L, Dhatariya K, Gerontitis D. No clinical harm from a massive exenatide overdose: A short report. Clin Toxicol (Phila) 2013;51(1):61. doi: 10.3109/15563650.2012.752495. [DOI] [PubMed] [Google Scholar]
- 10.Elmehdawi RR, Elbarsha AM. An accidental liraglutide overdose: Case report. Libyan J Med. 2014;9:23055. doi: 10.3402/ljm.v9.23055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koliaki C, Doupis J. Incretin-based therapy: A powerful and promising weapon in the treatment of type 2 diabetes mellitus. Diabetes Ther. 2011;2(2):101–21. doi: 10.1007/s13300-011-0002-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meoli M, Lava SAG, Bronz G, et al. Eu- or hypoglycemic ketosis and ketoacidosis in children: A review. Pediatr Nephrol. 2024;39(4):1033–40. doi: 10.1007/s00467-023-06115-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang Z, Yu M, Mei M, et al. The association between GLP-1 receptor agonist and diabetic ketoacidosis in the FDA adverse event reporting system. Nutr Metab Cardiovasc Dis. 2022;32:504–10. doi: 10.1016/j.numecd.2021.10.003. [DOI] [PubMed] [Google Scholar]
- 14.Cahill GF., Jr Fuel metabolism in starvation. Annu Rev Nutr. 2006;26:1–22. doi: 10.1146/annurev.nutr.26.061505.111258. [DOI] [PubMed] [Google Scholar]
- 15.Samocha-Bonet D, Dixit VD, Kahn CR, et al. Metabolically healthy and unhealthy obese – the 2013 Stock Conference report. Obes Rev. 2014;15:697–708. doi: 10.1111/obr.12199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tong Y, Xu S, Huang L, Chen C. Obesity and insulin resistance: Pathophysiology and treatment. Drug Discov Today. 2022;27:822–30. doi: 10.1016/j.drudis.2021.11.001. [DOI] [PubMed] [Google Scholar]


