Abstract
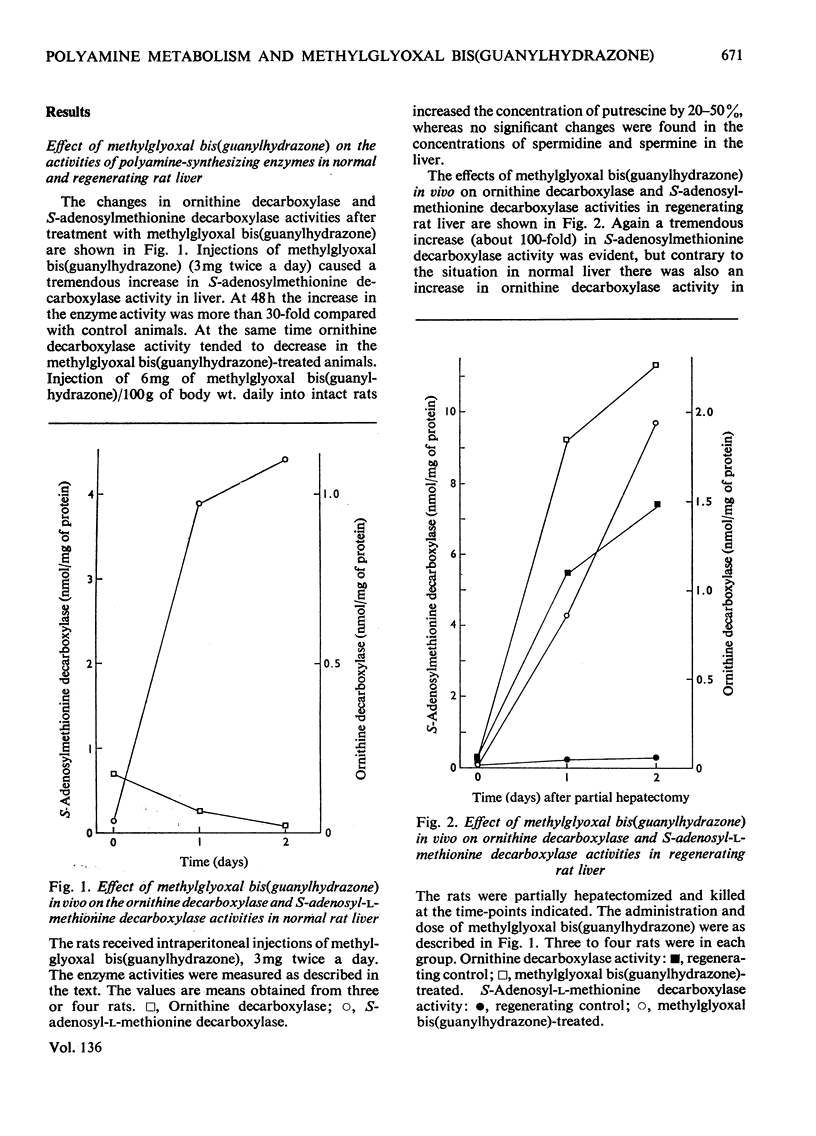
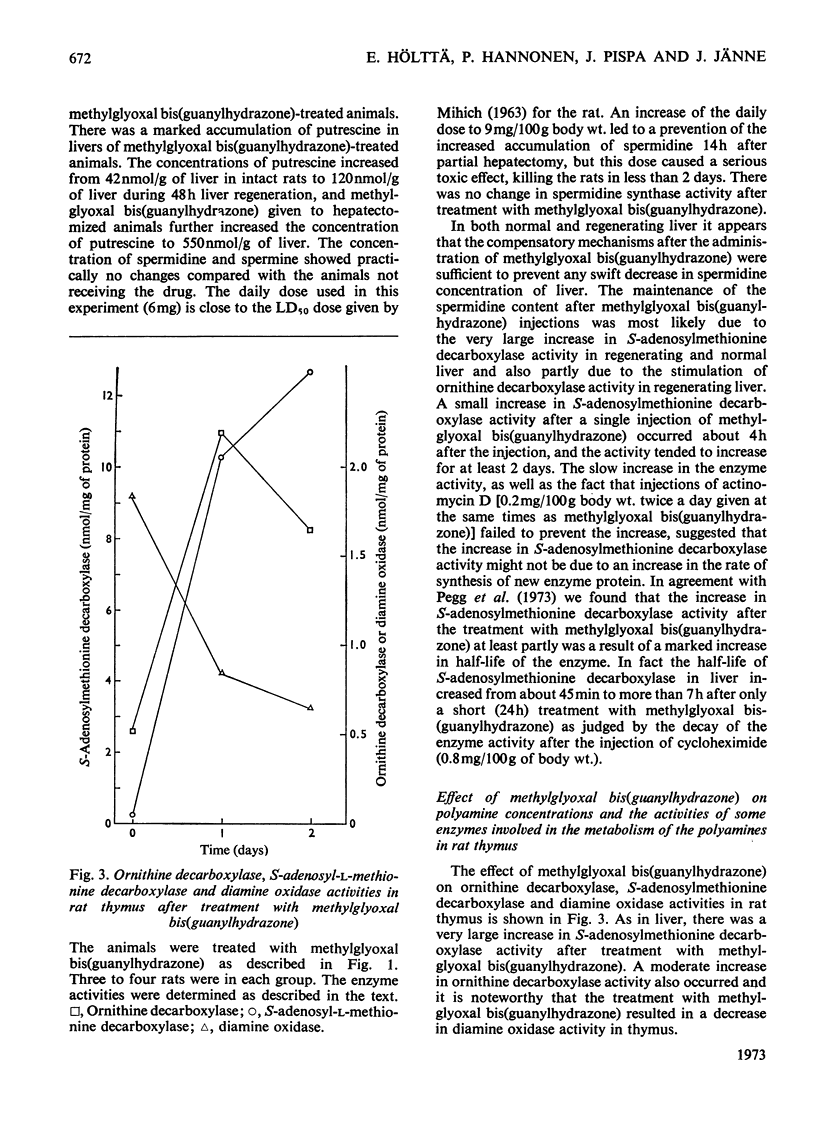
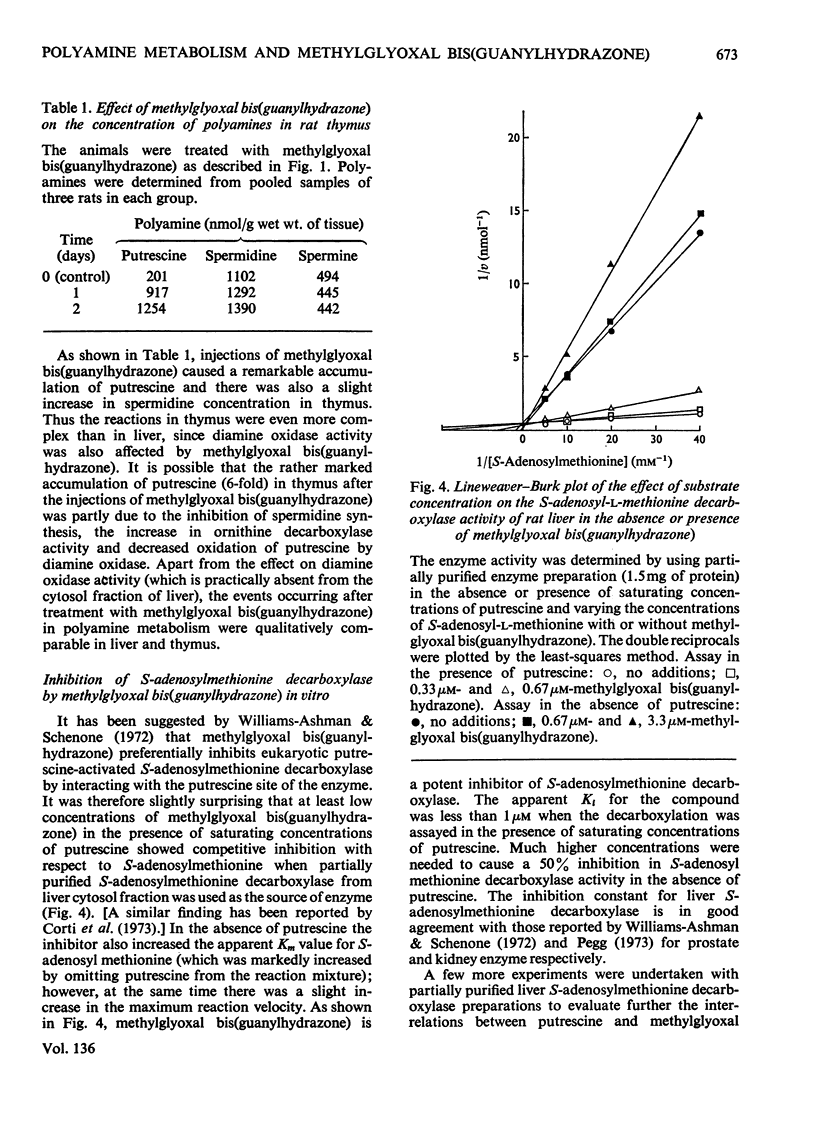
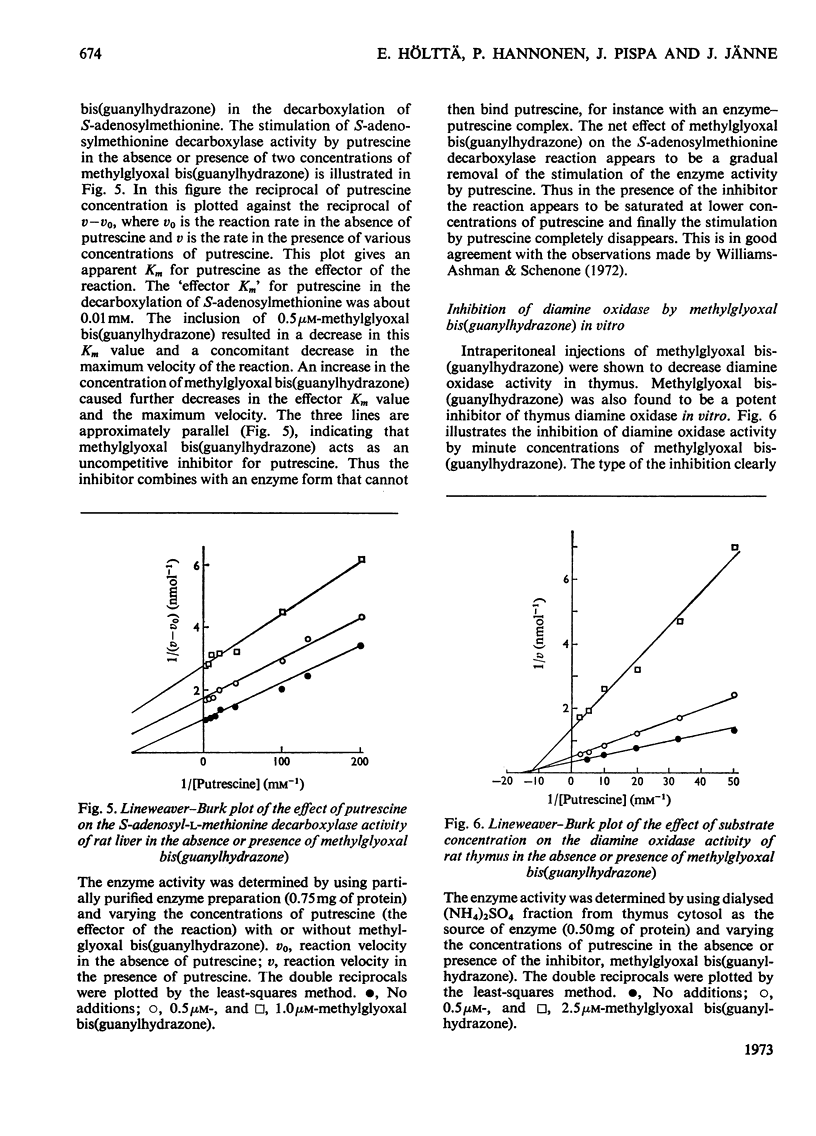
1. Injections of sublethal doses of methylglyoxal bis(guanylhydrazone), a potent inhibitor of putrescine-activated S-adenosylmethionine decarboxylase in vitro, resulted after a few days in an immense increase in the activity of S-adenosylmethionine decarboxylase in normal and regenerating rat liver and in rat thymus. The increase in the activity of S-adenosylmethionine decarboxylase was at least partly due to a marked lengthening of the half-life of the enzyme. 2. In regenerating liver and thymus there was also a moderate stimulation of the activity of ornithine decarboxylase (EC 4.1.1.17) and a marked accumulation of tissue putrescine. 3. Injection of methylglyoxal bis(guanylhydrazone) into the rat also markedly decreased the activity of diamine oxidase (EC 1.4.3.6) in thymus. 4. In no cases where doses of methylglyoxal bis(guanylhydrazone) close to the LD50 dose for the rat were used was it possible to lower tissue spermidine content to any significant extent. 5. Methylglyoxal bis(guanylhydrazone) seemed to act as a competitive inhibitor for the substrate S-adenosylmethionine and as an uncompetitive inhibitor for the activator putrescine in the decarboxylation of S-adenosylmethionine in vitro. 6. In the diamine oxidase reaction, with putrescine as the substrate, methylglyoxal bis(guanylhydrazone) was a non-competitive inhibitor for putrescine.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beaven M. A., de Jong W. Presence of histaminase and ornithine decarboxylase activities in rat thymus. Biochem Pharmacol. 1973 Jan 15;22(2):257–265. doi: 10.1016/0006-2952(73)90278-5. [DOI] [PubMed] [Google Scholar]
- Hannonen P., Raina A., Jänne J. Polyamine synthesis in the regenerating rat liver: stimulation of S-adenosyl methionine decarboxylase, and spermidine and spermine synthases after partial hepatectomy. Biochim Biophys Acta. 1972 Jun 26;273(1):84–90. doi: 10.1016/0304-4165(72)90194-8. [DOI] [PubMed] [Google Scholar]
- Hölttä E., Jänne J. Ornithine decarboxylase activity and the accumulation of putrescine at early stages of liver regeneration. FEBS Lett. 1972 Jun 1;23(1):117–121. doi: 10.1016/0014-5793(72)80298-9. [DOI] [PubMed] [Google Scholar]
- Janne J., Williams-Ashman H. G., Schenone A. Spermidine synthesizing enzymes in baker's yeast. Biochem Biophys Res Commun. 1971 Jun 18;43(6):1362–1368. doi: 10.1016/s0006-291x(71)80024-4. [DOI] [PubMed] [Google Scholar]
- Jänne J., Schenone A., Williams-Ashman H. G. Separation of two proteins required for synthesis of spermidine from S-adenosyl-L-methionine and putrescine in rat prostate. Biochem Biophys Res Commun. 1971 Feb 19;42(4):758–764. doi: 10.1016/0006-291x(71)90552-3. [DOI] [PubMed] [Google Scholar]
- Jänne J., Williams-Ashman H. G. Dissociation of putrescine-activated decarboxylation of S-adenosyl-L-methionine from the enzymic synthesis of spermidine and spermine by purified prostatic enzyme preparations. Biochem Biophys Res Commun. 1971 Jan 22;42(2):222–229. [PubMed] [Google Scholar]
- Jänne J., Williams-Ashman H. G. On the purification of L-ornithine decarboxylase from rat prostate and effects of thiol compounds on the enzyme. J Biol Chem. 1971 Mar 25;246(6):1725–1732. [PubMed] [Google Scholar]
- Kay J. E., Pegg A. E. Effect of inhibition of spermidine formation on protein and nucleic acid synthesis during lymphocyte activation. FEBS Lett. 1973 Feb 1;29(3):301–304. doi: 10.1016/0014-5793(73)80044-4. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MIHICH E. CURRENT STUDIES WITH METHYLGLYOXAL-BIS(GUANYLHYDRAZONE). Cancer Res. 1963 Sep;23:1375–1389. [PubMed] [Google Scholar]
- OKUYAMA T., KOBAYASHI Y. Determination of diamine oxidase activity by liquid scintillation counting. Arch Biochem Biophys. 1961 Nov;95:242–250. doi: 10.1016/0003-9861(61)90141-2. [DOI] [PubMed] [Google Scholar]
- Pegg A. E., Corti A., Williams-Ashman H. G. Paradoxical enhancement of S-adenosylmethionine decarboxylase in rat tissues following administration of the specific inhibitor methyl glyoxal bis(guanylhydrazone). Biochem Biophys Res Commun. 1973 May 15;52(2):696–701. doi: 10.1016/0006-291x(73)90768-7. [DOI] [PubMed] [Google Scholar]
- Pegg A. E. Inhibition of spermidine formation in rat liver and kidney by methylglyoxal bis(guanylhydrazone). Biochem J. 1973 Mar;132(3):537–540. doi: 10.1042/bj1320537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegg A. E., Williams-Ashman H. G. On the role of S-adenosyl-L-methionine in the biosynthesis of spermidine by rat prostate. J Biol Chem. 1969 Feb 25;244(4):682–693. [PubMed] [Google Scholar]
- Raina A., Cohen S. S. Polyamines and RNA synthesis in a polyauxotrophic strain of E. coli. Proc Natl Acad Sci U S A. 1966 Jun;55(6):1587–1593. doi: 10.1073/pnas.55.6.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raina A., Jänne J. Biosynthesis of putrescine: characterization of ornithine decarboxylase from regenerating rat liver. Acta Chem Scand. 1968;22(7):2375–2378. doi: 10.3891/acta.chem.scand.22-2375. [DOI] [PubMed] [Google Scholar]
- Raina Aarne, Hannonen Pekka. Separation of enzyme activities catalysing spermidine and spermine synthesis in rat brain. FEBS Lett. 1971 Jul 15;16(1):1–4. doi: 10.1016/0014-5793(71)80669-5. [DOI] [PubMed] [Google Scholar]
- Tryding N., Willert B. Determination of plasma diamine oxidase (histaminase) in clinical practice. A comparison between a biological method and a radiochemical micromethod. Scand J Clin Lab Invest. 1968;22(1):29–32. doi: 10.3109/00365516809160732. [DOI] [PubMed] [Google Scholar]
- Williams-Ashman H. G., Jänne J., Coppoc G. L., Geroch M. E., Schenone A. New aspects of polyamine biosynthesis in eukaryotic organisms. Adv Enzyme Regul. 1972;10:225–245. doi: 10.1016/0065-2571(72)90016-7. [DOI] [PubMed] [Google Scholar]
- Williams-Ashman H. G., Schenone A. Methyl glyoxal bis(guanylhydrazone) as a potent inhibitor of mammalian and yeast S-adenosylmethionine decarboxylases. Biochem Biophys Res Commun. 1972 Jan 14;46(1):288–295. doi: 10.1016/0006-291x(72)90661-4. [DOI] [PubMed] [Google Scholar]


