Abstract
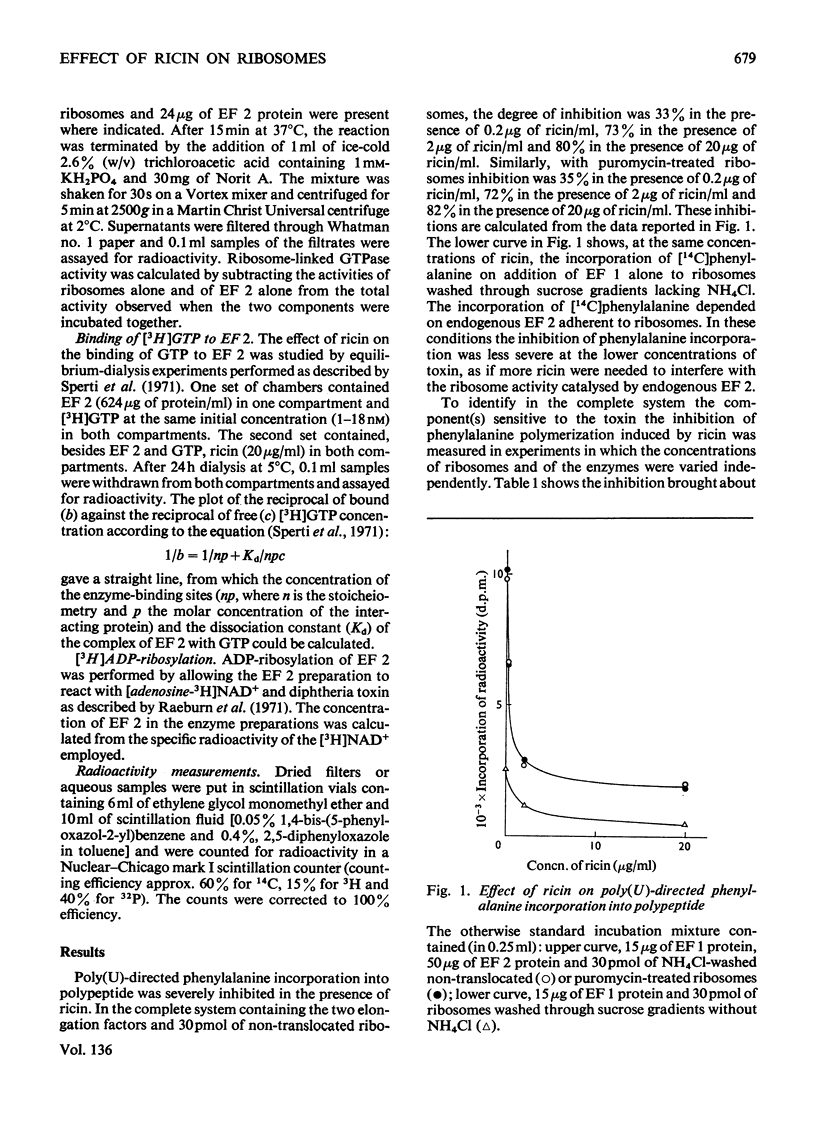
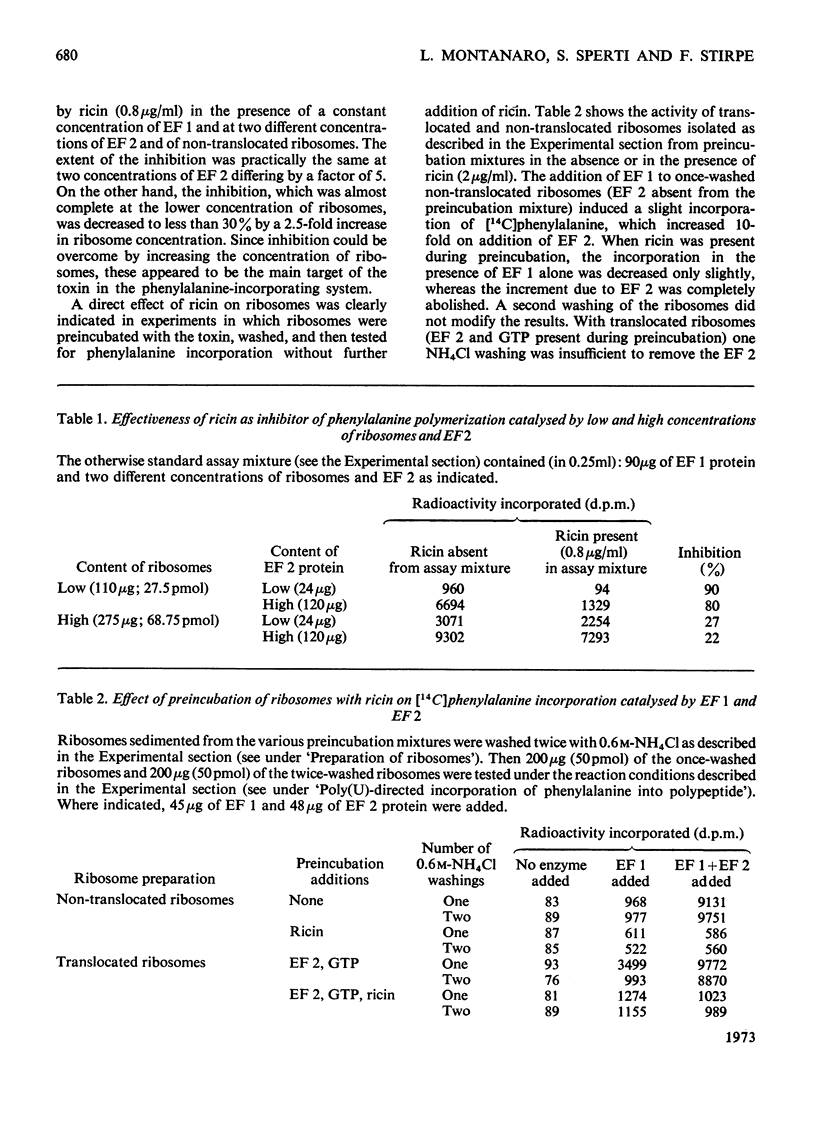
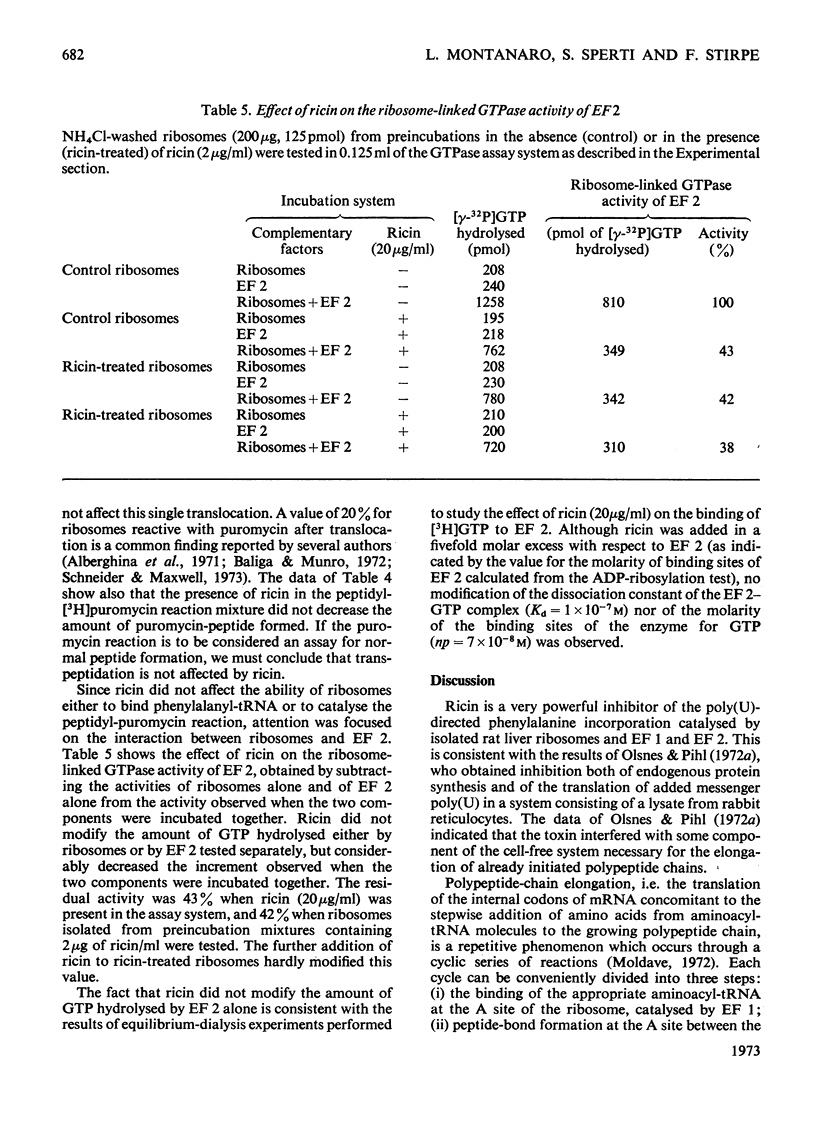
1. Ricin (a toxic protein from the seeds of Ricinus communis) is a powerful inhibitor of the poly(U)-directed incorporation of phenylalanine into polypeptides catalysed by isolated rat liver ribosomes and elongation factors 1 and 2 (EF 1 and EF 2). The inhibition can be largely overcome by increasing the concentration of ribosomes. 2. The toxin does not affect the binding of phenylalanyl-tRNA to ribosomes catalysed by EF 1, nor does it inhibit the puromycin reaction used as a test for peptide-bond formation catalysed by ribosomes. 3. Ricin inhibits the ribosome-linked GTP hydrolysis catalysed by EF 2. 4. Ribosomes treated with ricin and washed through sucrose gradients containing 0.6m-NH4Cl are functionally inactive in those assay systems that are sensitive to the presence of added toxin. 5. It is suggested that ricin brings about an irreversible modification of ribosomes which impairs their ability to interact with EF 2. Since ricin inhibits at a molar concentration much lower than that of ribosomes it probably acts catalytically. No added cofactor is necessary for the inhibitory action of the toxin.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alberghina F. A., Sturani E., Schiaffonati L. The peptidyl-puromycin reaction with Neurospora crassa ribosomes. The number of active ribosomes in different conditions of growth. Arch Mikrobiol. 1971;80(2):166–175. doi: 10.1007/BF00411881. [DOI] [PubMed] [Google Scholar]
- Baliga B. S., Munro H. N. Evidence of formation of a complex between GTP and elongation factor two and the binding of the complex to a specific site on mammalian ribosomes. Biochim Biophys Acta. 1972 Aug 25;277(2):368–383. doi: 10.1016/0005-2787(72)90418-2. [DOI] [PubMed] [Google Scholar]
- Collier R. J. Effect of diphtheria toxin on protein synthesis: inactivation of one of the transfer factors. J Mol Biol. 1967 Apr 14;25(1):83–98. doi: 10.1016/0022-2836(67)90280-x. [DOI] [PubMed] [Google Scholar]
- Felicetti L., Lipmann F. Comparison of amino acid polymerization factors isolated from rat liver and rabbit reticulocytes. Arch Biochem Biophys. 1968 May;125(2):548–557. doi: 10.1016/0003-9861(68)90613-9. [DOI] [PubMed] [Google Scholar]
- Honjo T., Nishizuka Y., Hayaishi O. Diphtheria toxin-dependent adenosine diphosphate ribosylation of aminoacyl transferase II and inhibition of protein synthesis. J Biol Chem. 1968 Jun 25;243(12):3553–3555. [PubMed] [Google Scholar]
- Ibuki F., Moldave K. Evidence for the enzymatic binding of aminoacyl transfer ribonucleic acid to rat liver ribosomes. J Biol Chem. 1968 Feb 25;243(4):791–798. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lin J. Y., Liu K., Chen C. C., Tung T. C. Effect of crystalline ricin on the biosynthesis of protein, RNA, and DNA in experimental tumor cells. Cancer Res. 1971 Jul;31(7):921–924. [PubMed] [Google Scholar]
- Lin J. Y., Tserng K. Y., Chen C. C., Lin L. T., Tung T. C. Abrin and ricin: new anti-tumour substances. Nature. 1970 Jul 18;227(5255):292–293. doi: 10.1038/227292a0. [DOI] [PubMed] [Google Scholar]
- MOULE Y. Préparation et toxicité de la ricine pure. Bull Soc Chim Biol (Paris) 1951;33(10):1461–1466. [PubMed] [Google Scholar]
- Olsnes S., Pihl A. Ricin - a potent inhibitor of protein synthesis. FEBS Lett. 1972 Feb 15;20(3):327–329. doi: 10.1016/0014-5793(72)80098-x. [DOI] [PubMed] [Google Scholar]
- Olsnes S., Pihl A. Treatment of abrin and ricin with -mercaptoethanol opposite effects on their toxicity in mice and their ability to inhibit protein synthesis in a cell-free system. FEBS Lett. 1972 Nov 15;28(1):48–50. doi: 10.1016/0014-5793(72)80674-4. [DOI] [PubMed] [Google Scholar]
- POPE C. G., STEVENS M. F. The purification of diphtheria toxin and the isolation of crystalline toxin-protein. Br J Exp Pathol. 1958 Apr;39(2):139–149. [PMC free article] [PubMed] [Google Scholar]
- Raeburn S., Collins J. F., Moon H. M., Maxwell E. S. Aminoacyltransferase II from rat liver. I. Purification and enzymatic properties. J Biol Chem. 1971 Feb 25;246(4):1041–1048. [PubMed] [Google Scholar]
- Schneider J. A., Maxwell E. S. Peptidylpuromycin formation on mammalian polysomes. Studies on transpeptidation and translocation. Biochemistry. 1973 Jan 30;12(3):475–481. doi: 10.1021/bi00727a018. [DOI] [PubMed] [Google Scholar]
- Skogerson L., Moldave K. Evidence for aminoacyl-tRNA binding, peptide bond synthesis, and translocase activities in the aminoacyl transfer reaction. Arch Biochem Biophys. 1968 May;125(2):497–505. doi: 10.1016/0003-9861(68)90607-3. [DOI] [PubMed] [Google Scholar]
- Sperti S., Montanaro L., Mattioli A. Studies on diphtheria toxin. The effect of GTP on the toxin-dependent adenosine diphosphate ribosylation of rat liver aminoacyl transferase. II. Chem Biol Interact. 1971 Apr;3(2):141–148. doi: 10.1016/0009-2797(71)90094-9. [DOI] [PubMed] [Google Scholar]
- Waller G. R., Ebner K. E., Scroggs R. A., Das Gupta B. R., Corcoran J. B. Studies on the toxic action of ricin. Proc Soc Exp Biol Med. 1966 Mar;121(3):685–691. doi: 10.3181/00379727-121-30861. [DOI] [PubMed] [Google Scholar]


