Abstract
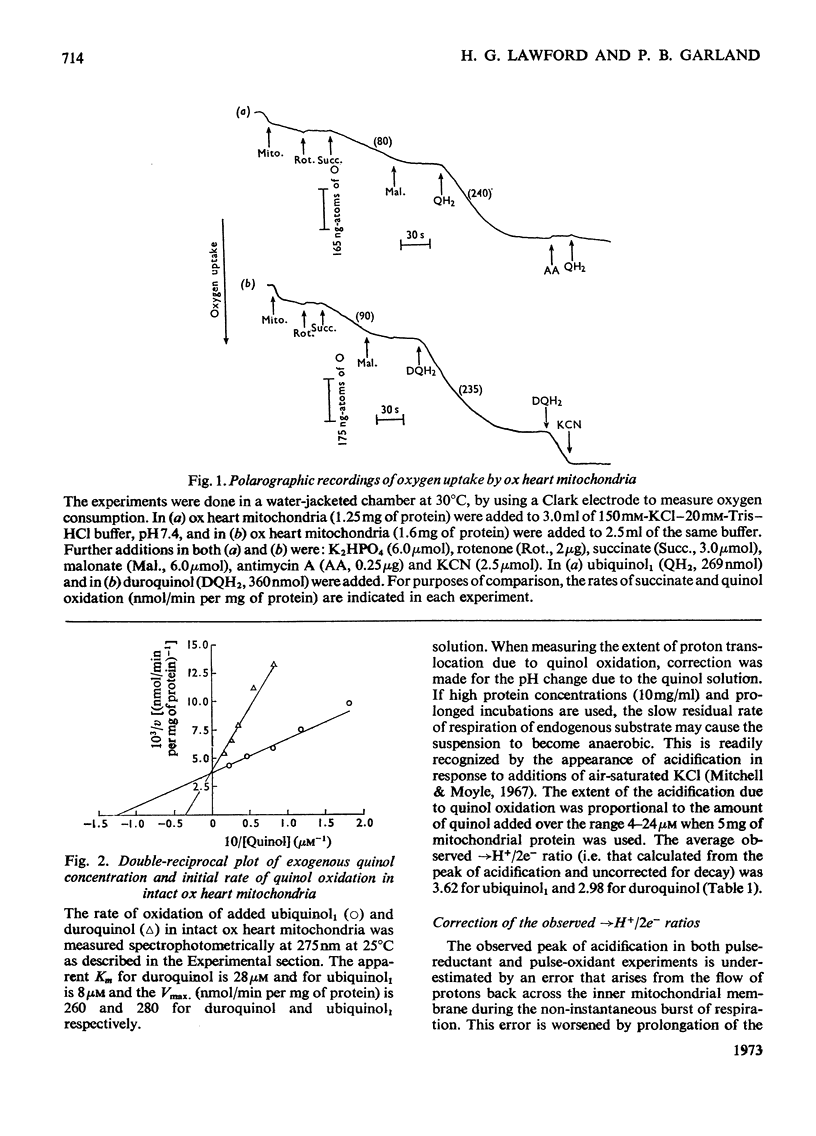
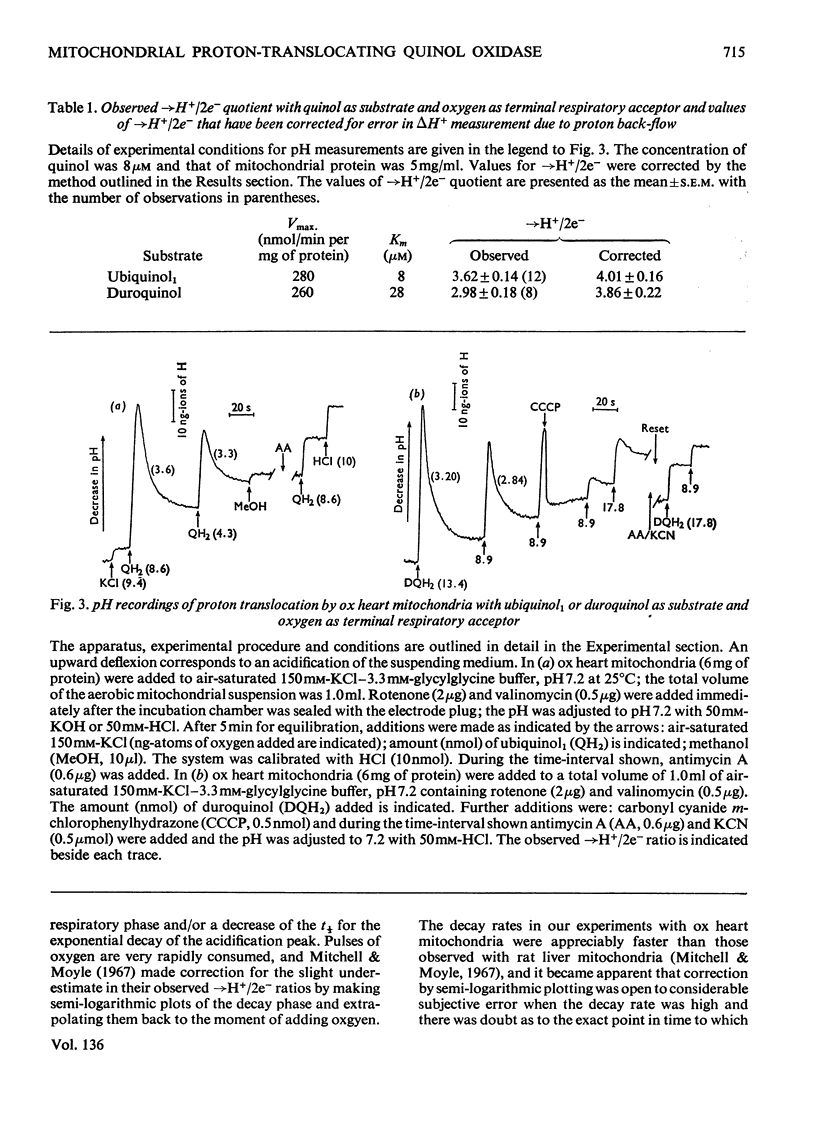
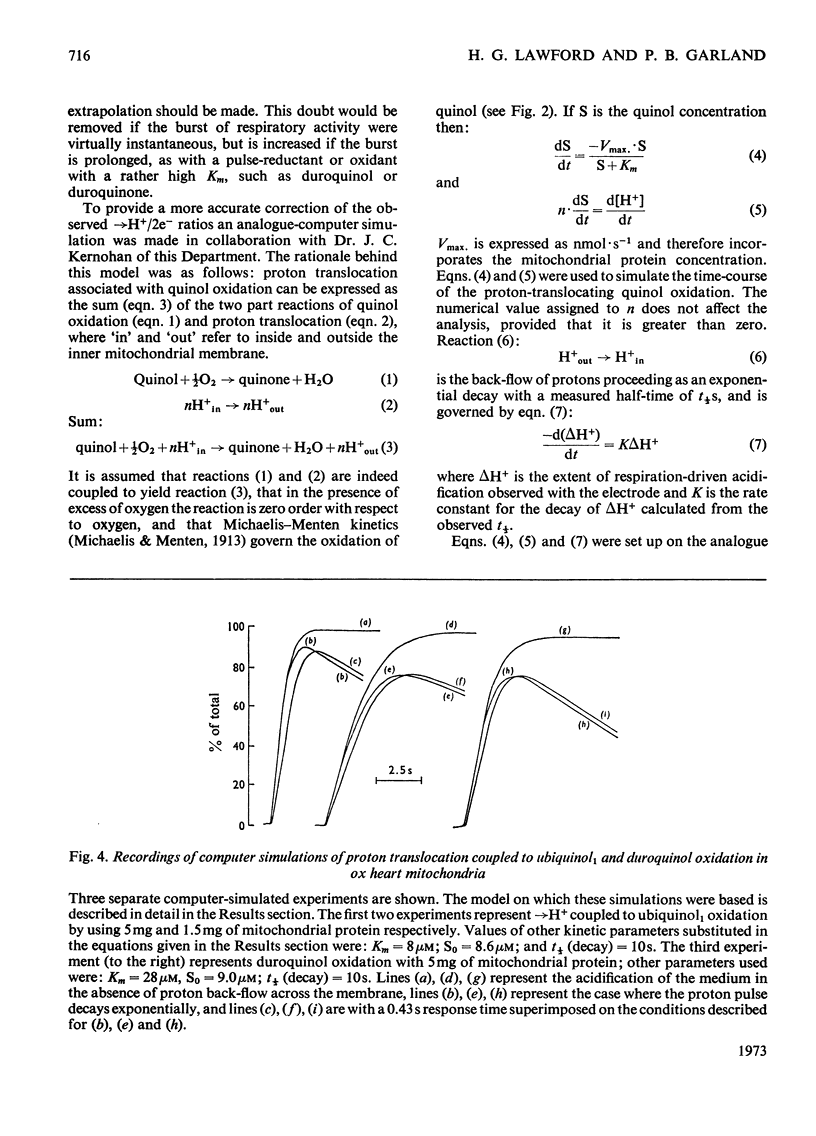
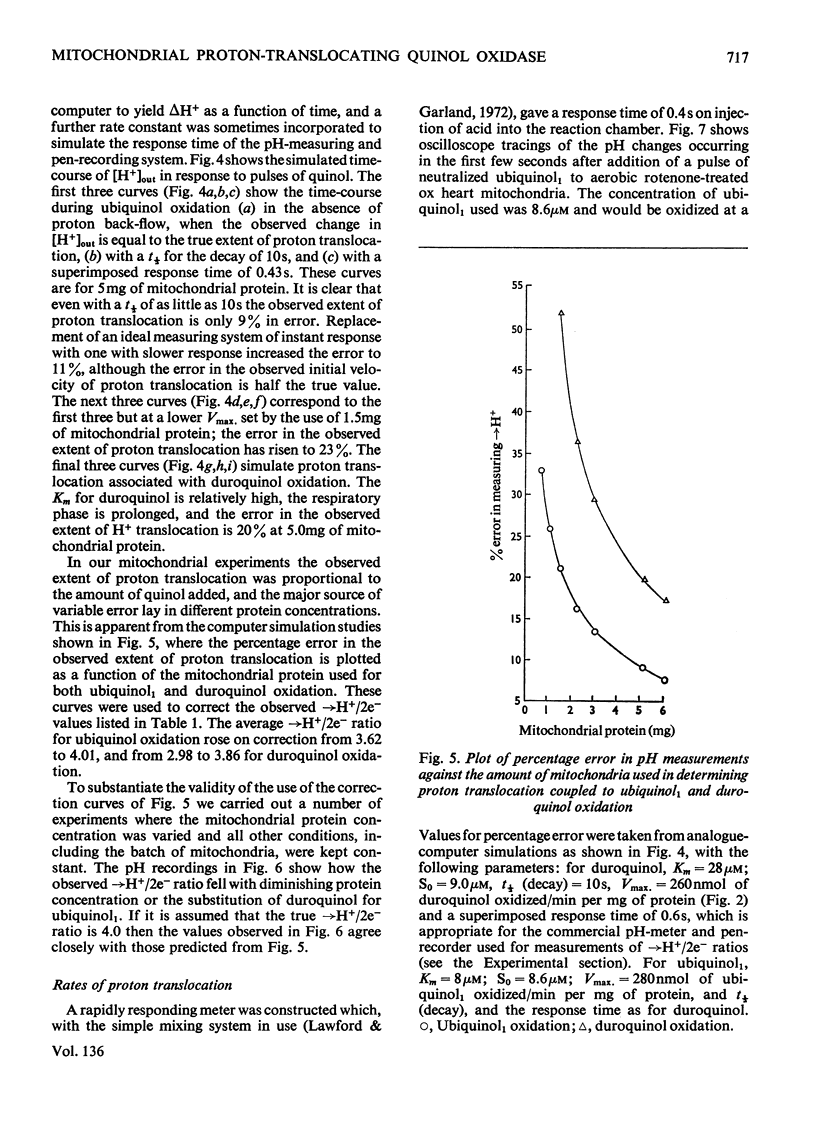
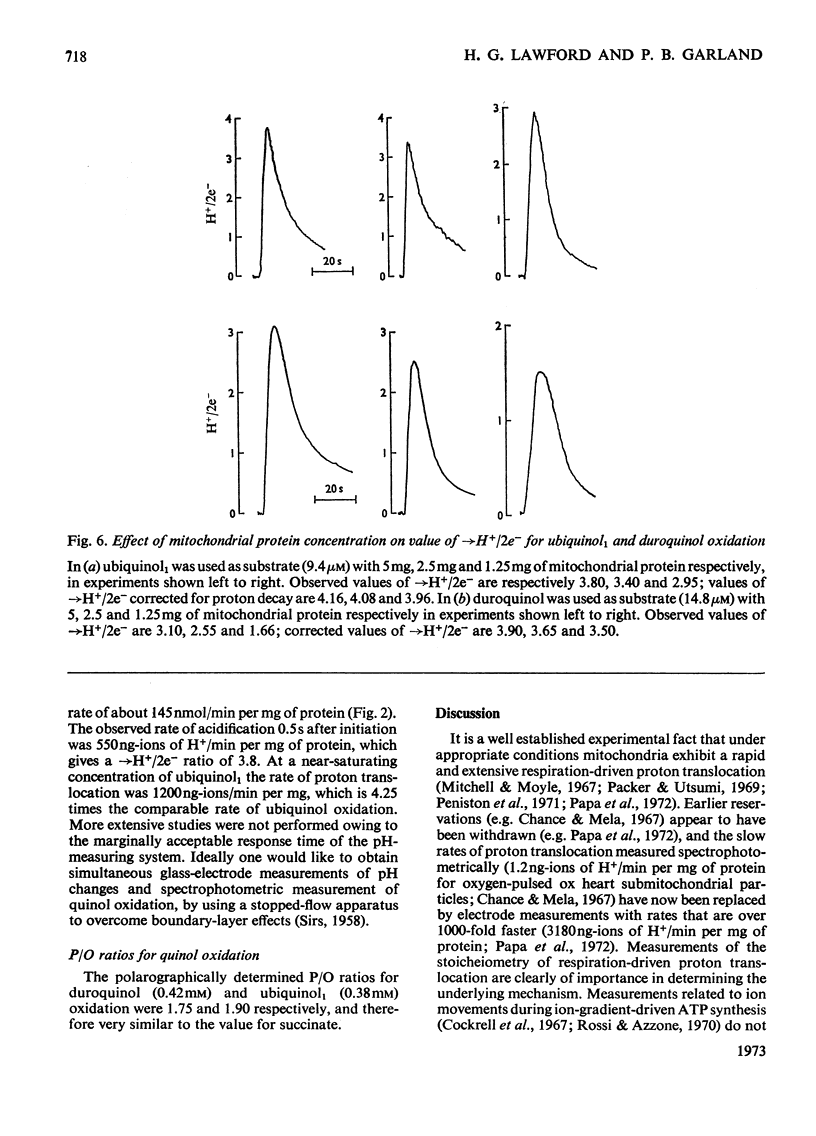
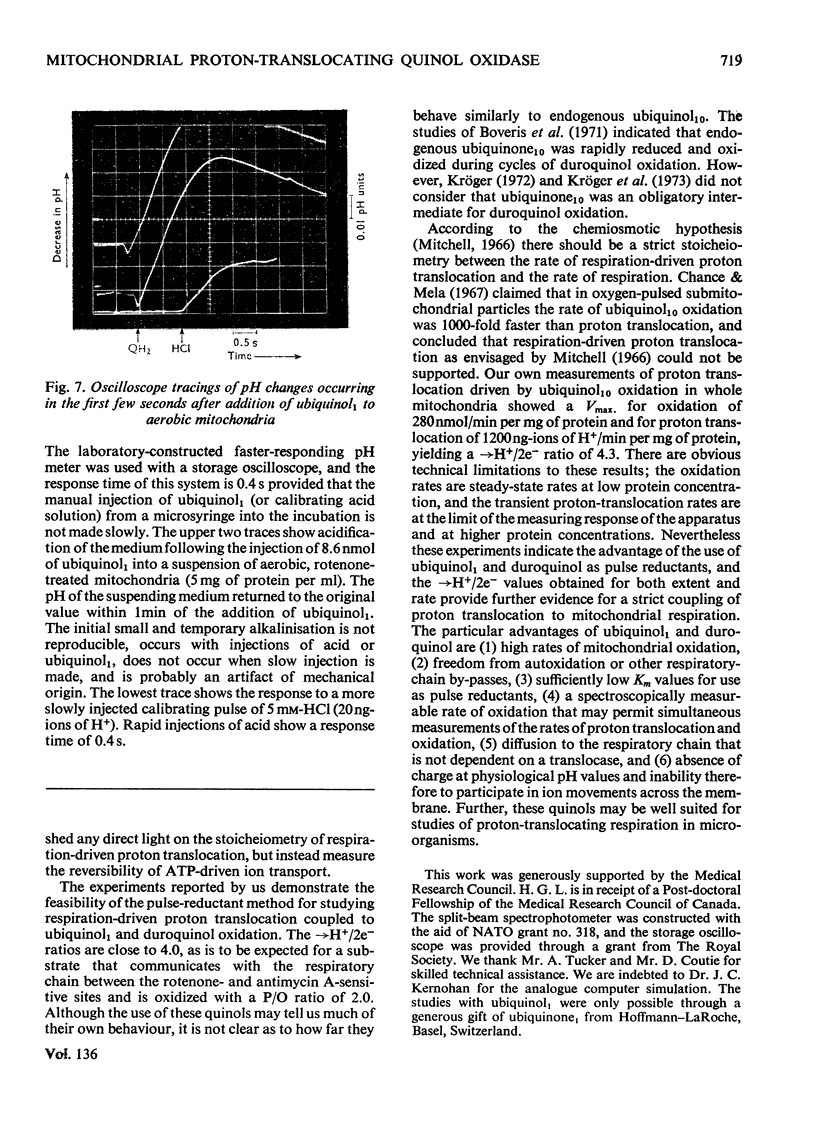
The suitability of ubiquinol1 and duroquinol as pulse reductants for initiating respirationdriven proton translocation by aerobic ox heart mitochondria was investigated. At 25°C the Vmax. for oxidation was close to 280nmol of quinol oxidized/min per mg of protein, and the Km values were 8μm for ubiquinol1 and 28μm for duroquinol. Pulses of ubiquinol1 and duroquinol were rapidly and completely oxidized by aerobic mitochondria with a simultaneous acidification of the suspending medium as detected with a glass electrode. The →H+/2e− ratios (Mitchell, 1966) calculated from the observed extent of acidification and the amount of quinol added were 3.62 for ubiquinol1 and 2.98 for duroquinol. These values are underestimates of the true value owing to proton back-flow across the membrane. An analogue computer model was used to correct the observed extent of respirationdriven acidification for proton back-flow. The corrected →H+/2e− values were 4.01 for ubiquinol and 3.86 for duroquinol oxidation. Attempts to measure the rate of proton translocation with a pH-measuring system with a response time of 0.4s were not entirely satisfactory, owing to the relative slowness of the electrode response. Nevertheless the maximal rate of proton generation during ubiquinol1 oxidation was about 1200ng-ions of H+/min per mg of mitochondrial protein. It is concluded, contrarily to Chance & Mela (1967), that mitochondria exhibit a proton-translocating ubiquinol oxidase activity with a →H+/2e− ratio of 4.0.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boveris A., Oshino R., Erecińska M., Chance B. Reduction of mitochondrial components by durohydroquinone. Biochim Biophys Acta. 1971 Aug 6;245(1):1–16. doi: 10.1016/0005-2728(71)90002-8. [DOI] [PubMed] [Google Scholar]
- CHANCE B., WILLIAMS G. R. The respiratory chain and oxidative phosphorylation. Adv Enzymol Relat Subj Biochem. 1956;17:65–134. doi: 10.1002/9780470122624.ch2. [DOI] [PubMed] [Google Scholar]
- Chance B., Mela L. Energy-linked changes of hydrogen ion concentration in submitochondrial particles. J Biol Chem. 1967 Mar 10;242(5):830–844. [PubMed] [Google Scholar]
- Chappell J. B. The oxidation of citrate, isocitrate and cis-aconitate by isolated mitochondria. Biochem J. 1964 Feb;90(2):225–237. doi: 10.1042/bj0900225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockrell R. S., Harris E. J., Pressman B. C. Synthesis of ATP driven by a potassium gradient in mitochondria. Nature. 1967 Sep 30;215(5109):1487–1488. doi: 10.1038/2151487a0. [DOI] [PubMed] [Google Scholar]
- Garland P. B., Chance B., Ernster L., Lee C. P., Wong D. Flavoproteins of mitochondrial fatty acid oxidation. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1696–1702. doi: 10.1073/pnas.58.4.1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEYTLER P. G. uncoupling of oxidative phosphorylation by carbonyl cyanide phenylhydrazones. I. Some characteristics of m-Cl-CCP action on mitochondria and chloroplasts. Biochemistry. 1963 Mar-Apr;2:357–361. doi: 10.1021/bi00902a031. [DOI] [PubMed] [Google Scholar]
- Haddock B. A., Garland P. B. Effect of sulphate-limited growth on mitochondrial electron transfer and energy conservation between reduced nicotinamide-adenine dinucleotide and the cytochromes in Torulopsis utilis. Biochem J. 1971 Aug;124(1):155–170. doi: 10.1042/bj1240155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kröger A., Klingenberg M. The kinetics of the redox reactions of ubiquinone related to the electron-transport activity in the respiratory chain. Eur J Biochem. 1973 Apr;34(2):358–368. doi: 10.1111/j.1432-1033.1973.tb02767.x. [DOI] [PubMed] [Google Scholar]
- LESTER R. L., HATEFI Y., WIDMER C., CRANE F. L. Studies on the electron transport system. XX. Chemical and physical properties of the coenzyme Q family of compounds. Biochim Biophys Acta. 1959 May;33(1):169–185. doi: 10.1016/0006-3002(59)90511-6. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lawford H. G., Garland P. B. Proton translocation coupled to quinone reduction by reduced nicotinamide--adenine dinucleotide in rat liver and ox heart mitochondria. Biochem J. 1972 Dec;130(4):1029–1044. doi: 10.1042/bj1301029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massari S., Azzone G. F. The mechanism of ion translocation in mitochondria. 1. Coupling of K+ and H+ fluxes. Eur J Biochem. 1970 Feb;12(2):301–309. doi: 10.1111/j.1432-1033.1970.tb00851.x. [DOI] [PubMed] [Google Scholar]
- Mitchell P., Moyle J. Respiration-driven proton translocation in rat liver mitochondria. Biochem J. 1967 Dec;105(3):1147–1162. doi: 10.1042/bj1051147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packer L., Utsumi K. The relation of respiration-dependent proton transfer to mitochondrial structure. Arch Biochem Biophys. 1969 May;131(2):386–403. doi: 10.1016/0003-9861(69)90411-1. [DOI] [PubMed] [Google Scholar]
- Papa S., Scarpa A., Lee C. P., Chance B. Effect of ion conductance changes in the mitochondrial membrane on the kinetics of respiratory carriers. Biochemistry. 1972 Aug 1;11(16):3091–3098. doi: 10.1021/bi00766a024. [DOI] [PubMed] [Google Scholar]
- Penniston J. T., Southard J. H., Green D. E., Luzzana M. Speed of respiration-dependent proton ejection by mitochondria. Application of a pH-measuring system with 10-msec resolution. Arch Biochem Biophys. 1971 Feb;142(2):638–644. doi: 10.1016/0003-9861(71)90529-7. [DOI] [PubMed] [Google Scholar]


