Abstract
Background:
Capecitabine, a prodrug of 5-fluorouracil, is extensively utilized for the treatment of metastatic breast cancer, colorectal cancer, and gastric cancer. Nevertheless, there exist limitations in comprehending adverse reactions (AEs) in clinical practice. In this study, we investigated the distribution of AEs associated with capecitabine and explored potential rare adverse reactions by mining the Food and Drug Administration Adverse Event Reporting System (FAERS).
Objectives:
Our research aimed to explore the spectrum of AEs associated with capecitabine, including both documented and potential events, to provide a comprehensive understanding of the drug’s safety profile and guide clinical practice. At the same time, it provides a new direction for further research on AEs associated with capecitabine in the future.
Design:
We collected capecitabine-related adverse reactions from the FAERS and standardized the classification of AEs using the Medical Dictionary for Regulatory Activities 26.0. Four statistical schemes were used to analyze the obtained standardized signals.
Methods:
We collected AEs reported for capecitabine from the FAERS between 2004 and 2023. To ensure standardized data, the collected reports related to capecitabine-associated adverse events were categorized using the preferred terms (PTs) and system organ classes (SOCs) classifications provided by the Medical Dictionary for Regulatory Activities 26.0. Statistical analysis involved the utilization of reporting odds ratio, proportional reporting ratio, Bayesian confidence propagation neural network, and multi-item gamma Poisson shrinker. Four statistical schemes were employed to analyze the adverse reactions associated with capecitabine. A positive signal was considered when all four schemes indicated an association with the adverse event.
Results:
We collected a total of 45,011 AEs associated with the use of capecitabine from the database, covering 27 SOCs from 2004 to 2023. The nine SOC categories with the highest number of events were identified, which include gastrointestinal disorders; general disorders and administration site conditions; skin and subcutaneous tissue disorders; nervous system disorders; investigations, injury, poisoning, and procedural complications; blood and lymphatic system disorders; metabolism and nutrition disorders; infections and infestations; and neoplasms benign, malignant, and unspecified (including cysts and polyps). Among these 27 SOCs, we identified seven SOCs that met the signal value criteria. Notably, we discovered AEs not mentioned in the instructions, including intestinal obstruction in gastrointestinal disorders, penetrating aortic ulcer in cardiac disorders, and non-cirrhotic portal hypertension in hepatobiliary disorders, all of which exhibited signals. Furthermore, 40.1% of AEs associated with the use of capecitabine occurred within the first 30 days.
Conclusion:
Our study conducted a comprehensive analysis of capecitabine’s AEs using the FAERS database. We identified previously unreported AEs, mitigating the risk for patients and ensuring safe drug administration.
Keywords: adverse events, cancer, capecitabine, FAERS
Plain language summary
Adverse drug events of capecitabine
Introduction:
Capecitabine is a common chemotherapeutic drug; However, we do not have a comprehensive understanding of its adverse effects in clinical use. The FDA established the FDA Adverse Event Reporting System (FAERS) database to provide drug adverse event reports, medication error reports and product quality complaints.
Methods:
We analyzed the FAERS database to evaluate the common adverse events of capecitabine.
Results:
We collected a total of 45,011 AEs associated with capecitabine. The major adverse effects included leukopenia, colitis, radiation gastroenteritis, cardiotoxicity, mucosal toxicity, cryptitis, metabolic and nutritional disorders. In addition, we found unreported adverse events, such as intestinal obstruction, penetrating aortic ulcer, and non-cirrhotic portal hypertension. Our analysis also revealed that 40.1% of the AEs occurred within one month of initiating capecitabine treatment.
Conclusion:
Capecitabine can cause many adverse events during its use. A thorough understanding of these side effects is critical to providing personalized treatment and ensuring the safety of the medication.
Introduction
Capecitabine is an oral prodrug of 5-fluorouracil (FU) that was developed to reduce the potential risk of infection and the burden of reduced quality of life associated with intravenous administration of FU. 1 Capecitabine was approved by the Food and Drug Administration (FDA) for the first time for the treatment of metastatic breast cancer, and later expanded to include other cancers such as colorectal cancer and gastric cancer. 2 Once absorbed in the gastrointestinal mucosa, this oral prodrug of FU undergoes a series of cascade reactions, producing 5′-deoxy-5-fluorocytidine and 5′-deoxy-5-fluorouridine. The resulting FU interferes with DNA replication in cancer cells and inhibits tumor growth. 3
The FDA Adverse Event Reporting System (FAERS) is a crucial database employed by the FDA for the reporting of adverse events (AEs). 4 It aggregates reports of AEs linked to FDA-approved drugs, encompassing submissions from manufacturers, healthcare professionals, and users alike. 5 With the development of data mining technology, numerous studies have been conducted utilizing FAERS databases to monitor signals of adverse drug events (ADEs), offering valuable insights for clinical practice. For instance, Yang et al. suggested an association between the combined use of Glucagon-like peptide-1 (GLP-1) receptor agonists and dipeptidyl peptidase IV inhibitors and the occurrence of thyroid tumors (both benign and malignant), pancreatic tumors, and other related cases. The researchers utilized the FAERS database to investigate potential ADEs, presenting novel real-world evidence regarding the risk of tumors associated with GLP-1 receptor agonists. 6 Furthermore, studies have emerged that integrate FAERS data with pharmacovigilance analysis and bioinformatics technology to investigate ADEs and underlying biological mechanisms. These studies offer novel insights and suggest innovative approaches for utilizing the FAERS database. 7
Currently, researchers commonly employ two algorithms, namely, reporting odds ratio (ROR) 8 and proportional reporting ratio (PRR), 9 to assess adverse reaction signals. Moreover, several other algorithms, such as Bayesian confidence propagation neural network (BCPNN), 10 multi-item gamma Poisson shrinker (MGPS), 11 and empirical Bayesian geometric mean, 12 have been utilized in combination in certain studies to enhance the reliability of signals for AEs. Our study aims to analyze adverse reaction reports of capecitabine in a large real-world population sample, providing epidemiological data evidence to support these findings.
Methods
Source of data
We conducted a search in the FAERS database for AEs reported between 2004 and 2023 using “capecitabine” as the keyword. In this study, we established the following inclusion criteria: (1) The screening period encompassed 2004–2023, and the data originated from the FAERS database. (2) We collected reports of AEs associated with the use of capecitabine, including both individual and combination medications. Exclusion criteria were as follows: (1) Incomplete or ambiguous reporting of essential information or adverse reaction event records. (2) Reports containing inconsistent information or erroneous data input. (3) Duplicate reports were excluded (reports with identical values in fields such as gender, age, country, date of event, and adverse reactions were considered duplicates).
Data processing
We categorized the reports gathered from the FAERS database based on “CASEID,” “FDA_DT,” and “PRIMARYID,” while preserving reports with the highest values for “FDA-DT” and “PRIMARYID” to ensure access to the most current and comprehensive data. Subsequently, we standardized the collected AE reports related to capecitabine using Preferred Terms (PTs) and System Organ Classes (SOCs) from the Medical Dictionary for Regulatory Activities (MedDRA®), version 20.0. MedDRA® is owned and maintained by the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) and is managed by the MedDRA Maintenance and Support Services Organization (MSSO), which operates under Qualys. The most recent version used in this study was version 20.0, and it is regularly updated to reflect new medical knowledge and regulatory requirements. 13 Adverse events were coded using MedDRA PTs, and this study employed standardized MedDRA for querying. The reporting of this study conforms to the STROBE statement. 14
Statistical analysis
We employ the ROR as the primary method for signal mining in the disproportionality analysis. After obtaining ADE data reports for capecitabine and other drugs using the ratio imbalance measurement method in the four-grid table (Table 1), we utilize the ROR, PRR, BCPNN, and MGPS. Four statistical approaches are applied to simultaneously screen for positive signals that meet all four thresholds (Table 2). In the statistical analysis process, the software involved included SPSS Statistics 26.0 (IBM, Chicago, IL, USA), Excel 2021 (Microsoft, Redmond, WA, USA), and R 4.2.2 (R Foundation for Statistical Computing, USA), with a significance level set at p < 0.05. During the R data processing, the packages used mainly included ggplot2 (version 3.4.4), ggrepel (version 0.9.4), dplyr (version 1.1.4), and DescTools (version 0.99.52).
Table 1.
Four-grid table of disproportionality analysis method.
| Item | Target adverse events | All other adverse events | Total |
|---|---|---|---|
| Target drugs | a | b | a + b |
| All other drugs | c | d | c + d |
| Total | a + c | b + d | a + b + c + d |
Table 2.
Principle of disproportionality measure and standard of signal detection.
| Methods | Calculation formula | Inclusion standard of positive signal |
|---|---|---|
| ROR | a ⩾ 3 and 95% CI > 1 | |
| PRR | a ⩾ 3 and 95% CI > 1 | |
| BCPNN | (1) No signal (−): E(IC) ⩽ 0 |
|
| (2) Low signal (+): 0 < E(IC) ⩽ 1.5 |
||
| (3) Medium signal (++): 1.5 < E(IC) ⩽ 3 |
||
| (4) High signal (+++): E(IC) > 3 | ||
| Where α1 = β1 = 1; α = β = 2; | ||
| MGPS | EBGM05 > 2 and a > 0 | |
BCPNN, Bayesian confidence propagation neural network; CI, confidence interval; EBGM, empirical Bayesian geometric mean; IC, information component; MGPS, multi-item gamma Poisson shrinker; PRR, proportional reported ratio; ROR, reporting odds ratio.
Results
Patients characteristics
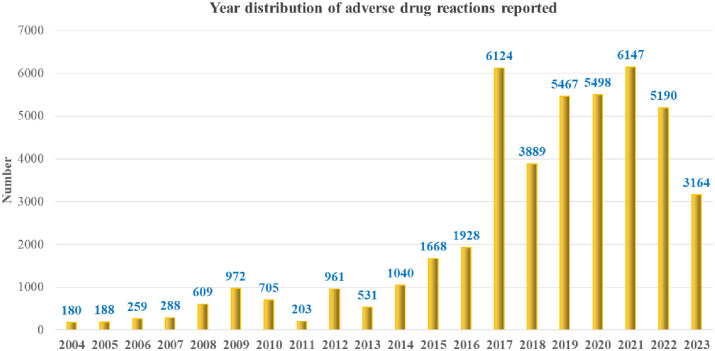
We collected a total of 45,011 reports on AEs following the use of capecitabine between 2004 and 2023. On average, there were 2250 reported cases of AEs per year. Notably, since 2017, there has been a significant increase in the number of reports, with an average annual increase of 5068 cases (Figure 1). Among the 45,011 reports, females accounted for 58.9% and males accounted for 33.1% of the cases, indicating a higher proportion among females. The age distribution of the reporters was mainly between 18–64.9 years (33.6%) and 65–85 years (28.5%). In terms of reporter identities, consumers, physicians, pharmacists, and other health professionals accounted for a relatively high proportion, representing 76.3% of the total. The three most common categories of severe adverse reactions were Other Serious (Important Medical Event) (25.3%), Hospitalization Initial Prolonged (22.5%), and Death (14.0%). The majority of reports came from the United States, with a total of 31,141 reports, accounting for 69.2% of the total. In addition, China, the United Kingdom, and Japan accounted for 9.3% of the reports (Table 3).
Figure 1.
Annual distribution of reported adverse drug reactions.
Table 3.
Clinical characteristics of reports with capecitabine from the FAERS database (2004–2023).
| Variables | Overall (N = 45,011) |
|---|---|
| Sex | |
| Female | 26,498 (58.9%) |
| Male | 14,908 (33.1%) |
| Missing | 3605 (8.0%) |
| Weight (kg) | |
| <50 | 928 (2.1%) |
| >100 | 861 (1.9%) |
| 50–100 | 8802 (19.6%) |
| Missing | 34,420 (76.5%) |
| Age (years old) | |
| <18 | 71 (0.2%) |
| 18~64.9 | 15,111 (33.6%) |
| 65–85 | 12,829 (28.5%) |
| >85 | 820 (1.8%) |
| Missing | 16,180 (35.9%) |
| Reporter | |
| Consumer | 12,203 (27.1%) |
| Physician | 10,319 (22.9%) |
| Pharmacist | 6988 (15.5%) |
| Other health professional | 4875 (10.8%) |
| Lawyer | 29 (0.1%) |
| Missing | 9586 (23.5%) |
| Outcome | |
| Other serious (important medical events) | 12,630 (25.3%) |
| Hospitalization—initial or prolonged | 11,228 (22.5%) |
| Death | 6972 (14.0%) |
| Life-threatening | 1217 (2.4%) |
| Disability | 359 (0.7%) |
| Required intervention to prevent permanent impairment/damage | 110 (0.2%) |
| Congenital anomaly | 16 (0.0%) |
| Missing | 17,334 (34.8%) |
| Reporter country | |
| United States of America | 31,141 (69.2%) |
| China | 1743 (3.9%) |
| United Kingdom | 1601 (3.5%) |
| Japan | 1157 (2.6%) |
| Canada | 838 (1.9%) |
| France | 855 (1.9%) |
| Germany | 800 (1.8%) |
| Italy | 772 (1.7%) |
| Spain | 517 (1.1%) |
| Netherlands | 498 (1.1%) |
| Others | 4456 (9.9%) |
FAERS, Food and Drug Administration Adverse Event Reporting System.
Signal detects at SOC Level
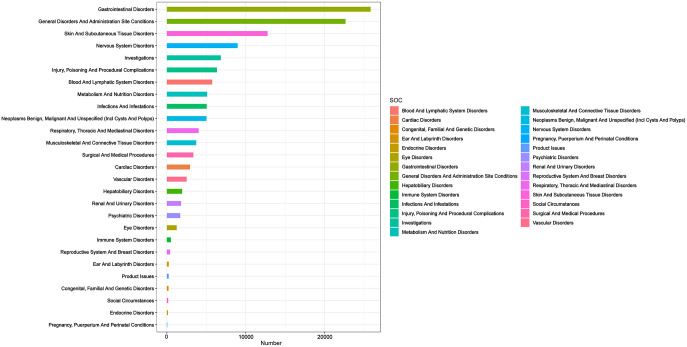
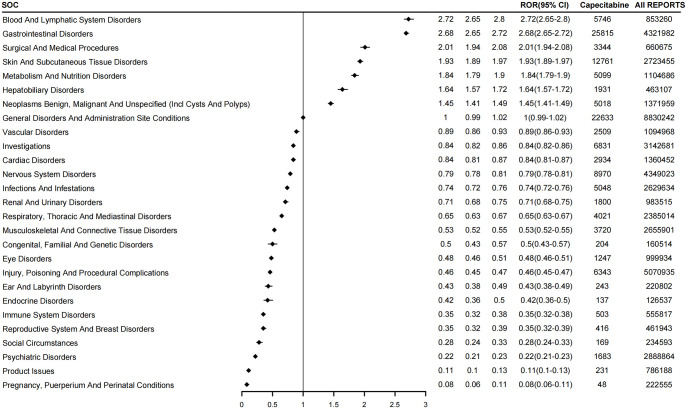
We distributed the identified positive signals across 27 SOCs and ranked them in terms of ADE report count and signal strength, respectively (Figure 2). In terms of the number of reports, gastrointestinal disorders, general disorders, and administration site conditions are the two most common types of SOCs with the highest number of events (greater than 20,000). In addition, skin and subcutaneous tissue disorders; nervous system disorders; investigations, injury, poisoning, and procedural complications; blood and lymphatic system disorders; metabolism and nutrition disorders; infections and infestations; and neoplasm benign, malignant, and unspecified (including cysts and polyps). These eight types of occurrences are greater than 10,000. On the mining signal, we found that there are seven types of SOC with signal strength greater than 1 (Figure 3): blood and lymphatic system disorders (ROR: 2.72, 95% CI: 2.65–2.80); gastrointestinal disorders (ROR: 2.68, 95% CI: 2.65–2.72); surgical and medical procedures (ROR: 2.01, 95% CI: 1.94–2.08); skin and subcultural tissues disorders (ROR: 1.93, 95% CI: 1.89–1.97); metabolism and nutrition disorders (ROR: 1.84, 95% CI: 1.79–1.90); hepatobiliary disorders (ROR: 1.64, 95% CI: 1.57–1.72); and neoplasm benign, malignant, and unspecified (including cysts and polyps; ROR: 1.45, 95% CI: 1.41–1.49).
Figure 2.
Number of 27 SOC-related PT reports.
PT, preferred term; SOC, system organ class.
Figure 3.
Signal strength of capecitabine reports at the SOC level.
SOC, system organ class.
Signal detects at PTs level
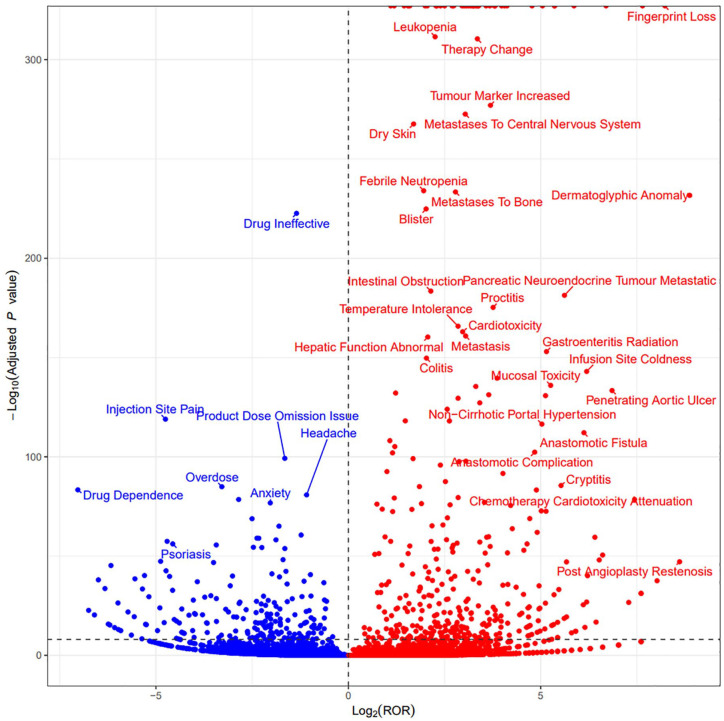
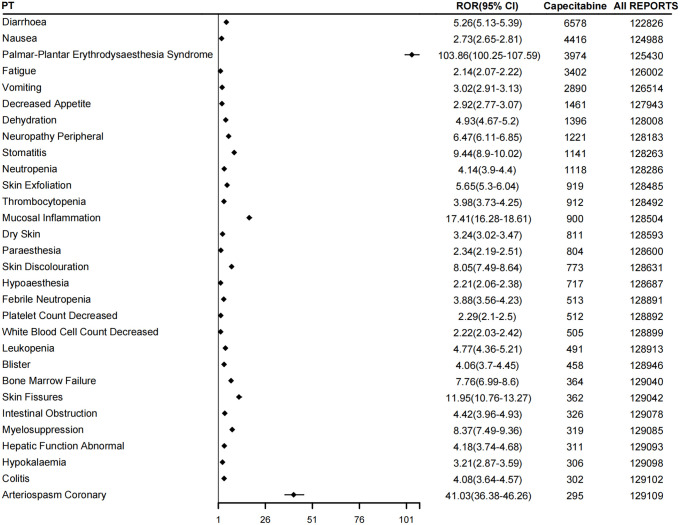
After administering capecitabine, we identified several AEs (Figure 4). Blood and lymphatic system disorders included leukopenia and febrile neutropenia. Gastrointestinal disorders encompass intestinal obstruction, proctitis, colitis, gastroenteritis radiation, mucosal toxicity, and cryptitis, which are the two most significant types of SOC. Furthermore, we observed five positive signals of SOC and related PTs: surgical and medical procedures, such as anastomotic fistula, anastomotic complication, and post-angioplasty restenosis. Skin and subcutaneous tissue disorders consisted of fingerprint loss, dermatoglyphic anomaly, blister, and dry skin, metabolism and nutrition disorders, hepatological disorders comprised of non-cirrhotic portal hypertension and abnormal liver function. Neoplasms benign, malignant, and unspecified (including cysts and polyps) included tumor marker increased and metastasis, metastatic pancreatic neuroendocrine tumor, metastases to bone, and metastases to the central nervous system. In addition, we identified cardiac disorders such as cardiotoxicity, chemotherapy cardiotoxicity attenuation, as well as penetrating aortic ulcer. Furthermore, general disorders and administration site conditions were observed, including treatment changes and infusion site coldness. We counted the distribution of signal values of the first 30 PT (Supplemental Table 1). The five PTs with the highest distribution in terms of the number of events are: diarrhea (ROR: 5.26, 95% CI: 5.13–5.39), nausea (ROR: 2.73,95% CI: 2.65–2.81), palmar-plantar erythrodysaesthesia syndrome (ROR: 103.86, 95%CI: 100.25–107.59), fatigue (ROR: 2.14, 95% CI: 2.07–2.22), and vomiting (ROR: 3.02, 95% CI: 2.91–3.13; Table 4). Interestingly, we found that most of these PTs had ROR less than 20, except for palmar-plantar erythrodysaesthesia syndrome (ROR: 103.86, 95% CI: 100.25–107.59) and arteriospasm coronary (ROR: 41.03, 95% CI: 36.38–46.26; Figure 5).
Figure 4.
Signal detects at PTs level.
PT, preferred term.
Table 4.
Distribution of signal values of the first 30 PTs.
| PT | ROR (95% CI) | PRR (X2) | MGPS (95% CI lower) | BCPNN (95% CI lower) | Capecitabine | All reports | p Value |
|---|---|---|---|---|---|---|---|
| Diarrhea | 5.26 (5.13–5.39) | 5.04 (21,241.3) | 4.99 (4.88) | 2.32 (0.65) | 6578 | 122,826 | <0.001 |
| Nausea | 2.73 (2.65–2.81) | 2.67 (4636.55) | 2.66 (2.59) | 1.41 (−0.26) | 4416 | 124,988 | <0.001 |
| Palmar-plantar erythrodysaesthesia syndrome | 103.86 (100.25–107.59) | 100.7 (311,968.28) | 80.26 (77.92) | 6.33 (4.66) | 3974 | 125,430 | <0.001 |
| Fatigue | 2.14 (2.07–2.22) | 2.11 (2006.7) | 2.11 (2.05) | 1.07 (−0.59) | 3402 | 126,002 | <0.001 |
| Vomiting | 3.02 (2.91–3.13) | 2.97 (3787.86) | 2.96 (2.87) | 1.57 (−0.1) | 2890 | 126,514 | <0.001 |
| Decreased appetite | 2.92 (2.77–3.07) | 2.9 (1810.03) | 2.88 (2.76) | 1.53 (−0.14) | 1461 | 127,943 | <0.001 |
| Dehydration | 4.93 (4.67–5.2) | 4.89 (4271.01) | 4.84 (4.63) | 2.27 (0.61) | 1396 | 128,008 | <0.001 |
| Neuropathy peripheral | 6.47 (6.11–6.85) | 6.42 (5505.09) | 6.33 (6.04) | 2.66 (1) | 1221 | 128,183 | <0.001 |
| Stomatitis | 9.44 (8.9–10.02) | 9.37 (8335.97) | 9.17 (8.73) | 3.2 (1.53) | 1141 | 128,263 | <0.001 |
| Neutropenia | 4.14 (3.9–4.4) | 4.12 (2614.76) | 4.08 (3.89) | 2.03 (0.36) | 1118 | 128,286 | <0.001 |
| Skin exfoliation | 5.65 (5.3–6.04) | 5.62 (3445.4) | 5.55 (5.26) | 2.47 (0.81) | 919 | 128,485 | <0.001 |
| Thrombocytopenia | 3.98 (3.73–4.25) | 3.96 (1999.86) | 3.93 (3.72) | 1.97 (0.31) | 912 | 128,492 | <0.001 |
| Mucosal inflammation | 17.41 (16.28–18.61) | 17.29 (13,235.6) | 16.6 (15.7) | 4.05 (2.39) | 900 | 128,504 | <0.001 |
| Dry skin | 3.24 (3.02–3.47) | 3.22 (1236.21) | 3.21 (3.02) | 1.68 (0.01) | 811 | 128,593 | <0.001 |
| Paraesthesia | 2.34 (2.19–2.51) | 2.33 (611.32) | 2.33 (2.2) | 1.22 (−0.45) | 804 | 128,600 | <0.001 |
| Skin discoloration | 8.05 (7.49–8.64) | 8 (4645.64) | 7.86 (7.41) | 2.98 (1.31) | 773 | 128,631 | <0.001 |
| Hypoesthesia | 2.21 (2.06–2.38) | 2.21 (472.26) | 2.2 (2.07) | 1.14 (−0.53) | 717 | 128,687 | <0.001 |
| Febrile neutropenia | 3.88 (3.56–4.23) | 3.87 (1082.08) | 3.84 (3.57) | 1.94 (0.28) | 513 | 128,891 | <0.001 |
| Platelet count decreased | 2.29 (2.1–2.5) | 2.28 (367.49) | 2.28 (2.12) | 1.19 (−0.48) | 512 | 128,892 | <0.001 |
| White blood cell count decreased | 2.22 (2.03–2.42) | 2.22 (335.81) | 2.21 (2.05) | 1.14 (−0.52) | 505 | 128,899 | <0.001 |
| Leukopenia | 4.77 (4.36–5.21) | 4.76 (1439.94) | 4.71 (4.37) | 2.24 (0.57) | 491 | 128,913 | <0.001 |
| Blister | 4.06 (3.7–4.45) | 4.04 (1039.98) | 4.01 (3.72) | 2 (0.34) | 458 | 128,946 | <0.001 |
| Bone marrow failure | 7.76 (6.99–8.6) | 7.74 (2094.29) | 7.61 (6.97) | 2.93 (1.26) | 364 | 129,040 | <0.001 |
| Skin fissures | 11.95 (10.76–13.27) | 11.92 (3514.84) | 11.6 (10.62) | 3.54 (1.87) | 362 | 129,042 | <0.001 |
| Intestinal obstruction | 4.42 (3.96–4.93) | 4.41 (849.9) | 4.37 (3.99) | 2.13 (0.46) | 326 | 129,078 | <0.001 |
| Myelosuppression | 8.37 (7.49–9.36) | 8.35 (2022.41) | 8.2 (7.47) | 3.04 (1.37) | 319 | 129,085 | <0.001 |
| Hepatic function abnormal | 4.18 (3.74–4.68) | 4.17 (743.17) | 4.14 (3.77) | 2.05 (0.38) | 311 | 129,093 | <0.001 |
| Hypokalemia | 3.21 (2.87–3.59) | 3.2 (459.87) | 3.18 (2.9) | 1.67 (0) | 306 | 129,098 | <0.001 |
| Colitis | 4.08 (3.64–4.57) | 4.08 (694.03) | 4.04 (3.68) | 2.02 (0.35) | 302 | 129,102 | <0.001 |
| Arteriospasm coronary | 41.03 (36.38–46.26) | 40.94 (10,403.24) | 37.15 (33.6) | 5.22 (3.55) | 295 | 129,109 | <0.001 |
BCPNN, Bayesian confidence propagation neural network; MGPS, multi-item gamma Poisson shrinker; PRR, proportional reporting ratio; PT, preferred term; ROR, reporting odds ratio.
Figure 5.
Forest map of the first 30 PTs.
PT, preferred term.
Event occurrence time
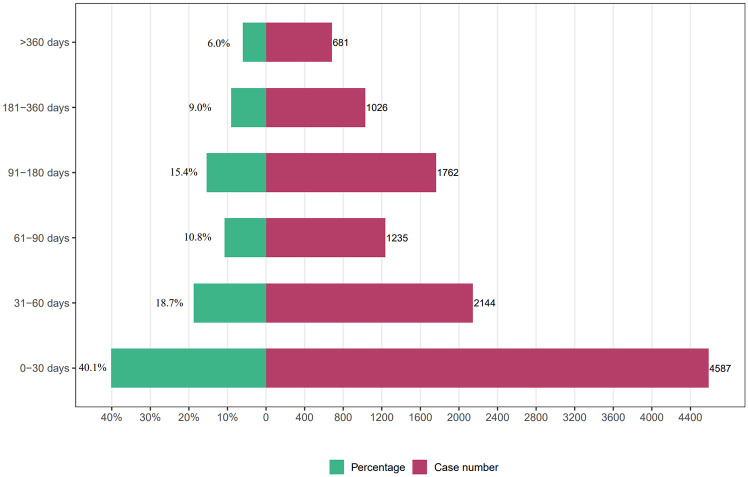
Regarding the timing of AEs, we collected 11,435 reports documenting the occurrence time of AEs (Figure 6). Our analysis revealed that 40.1% (n = 4587) of the AEs occurred within 1 month of initiating capecitabine treatment. Furthermore, AEs were observed within 60–90 days, 90–180 days, and 180–360 days, accounting for 18.7% (n = 2144), 10.8% (n = 1235), 15.4% (n = 1762), and 9.0% (n = 1026) respectively. Moreover, 6% (n = 681) of AEs occurred after 1 year of using capecitabine.
Figure 6.
Time to event onset (day).
Discussion
Previous research on AEs to capecitabine primarily relied on clinical studies and case reports. Nevertheless, strict trial designs and stringent inclusion criteria might have led to the underreporting of rare AEs, resulting in incomplete drug safety information. 15 Using a large dataset of real-world samples, our study analyzed the AEs associated with capecitabine over the past two decades, utilizing the FAERS database. In addition, we investigated the disparities between AEs reported in drug instructions and those observed in real-world settings, identifying both rare and potential AEs.
Over the past 20 years, there has been an increasing trend in the occurrence of AEs associated with capecitabine, particularly since 2017. We believe that possible reasons for this trend include (1) the expansion of indications for capecitabine and an increase in its utilization and (2) improvements in the recording and reporting system, leading to a higher number of adverse reaction reports being uploaded to the FAERS database. AEs associated with capecitabine are more prevalent in women (58.9%), likely due to its use in both single-drug chemotherapy and combined chemotherapy for breast cancer.16,17 Interestingly, contrary to common perception, AE reports for patients under 65 years old (33.6%) were higher than those for elderly patients over 65 years old (28.5%), possibly reflecting the current trend of younger onset of breast cancer.18,19 Furthermore, the majority of AE reports come from patients in the United States and Europe, aligning with the epidemiological patterns of breast cancer. Moreover, capecitabine is currently approved by the FDA for adjuvant chemotherapy and advanced treatment of colorectal and gastric cancer.20,21
As anticipated, our research results confirmed the presence of three SOCs associated with capecitabine: blood and lymphatic system disorders, gastrointestinal disorders, and cardiac disorders. 22 We found that the AEs of these three types of SOCs were roughly consistent with those mentioned in the instructions for capecitabine. Simultaneously, we observed potential intestinal obstruction during capecitabine treatment. This could be associated with another type of gastrointestinal disorder known as colitis. A reported case by Pow-Anpongkul et al. 23 suggests that capecitabine usage resulted in colitis-induced intestinal obstruction and subsequent thickening of the intestinal wall. Furthermore, case reports have documented instances of paralytic intestinal obstruction, which researchers suspect to be linked to drug-induced autonomic neuropathy.24,25 In cardiac disorders, we also observed a rare AE: penetrating aortic ulcer, a severe aortic disease that can lead to a series of serious complications including aortic dissection and aortic aneurysm.26,27 Therefore, caution should also be exercised during the use of capecitabine to prevent this AE.
Reports indicate that patients using capecitabine have undergone surgical and medical procedures, which might be influenced by the surgical interventions. Varied factors, such as surgeons, surgical methods, and instruments, can contribute to anastomotic fistulas, anastomotic complications, and restenosis following vascular angioplasty. 28 Thus, the ADE signal derived solely from this study cannot adequately reflect the impact of surgical and medical procedures. In addition, the occurrence of neoplasms benign, malignant, and unspecified (including cysts and polyps) may also be associated with treatment failure in tumors. Capecitabine is commonly utilized in the treatment of metastatic breast cancer, colorectal cancer, and gastric cancer. It is evident that patients exhibit elevated tumor markers, metastasis, metastatic pancreatic neuroendocrine tumor, and metastases to bone, which are attributed to the local invasion and distant metastases of the tumor itself.5,29 Therefore, these effects cannot be solely attributed to the AEs caused by capecitabine.
Skin and subcutaneous tissue disorders are noteworthy among all AEs. Skin and subcutaneous tissue disorders associated with capecitabine usage may manifest as fingerprint loss, dermatoglyphic anomaly, blister, and dry skin, and these symptoms may be attributed to the hand–foot syndrome, as stated in the instructions. It is noteworthy that our study did not observe the occurrence of the severe skin reactions of Stevens–Johnson syndrome and toxic epidermal necrolysis, which are explicitly mentioned in the instructions.30–33 We believe that this may be related to its rare probability and characteristic manifestations, and in the event of these two serious skin reactions, capecitabine should be immediately discontinued and permanently banned, which may be why we did not find them in our study. 34
In addition, metabolism and nutrition disorders including paresthesia, hypoesthesia, dehydration, abnormal liver function, hypokalemia, and may also be an AE to capecitabine, which targets TYMS with FU as its final product in the human body, thereby inhibiting folate function, which is an important mechanism for its tumor inhibition. However, it is important to note that folate is a vital micronutrient for the human body, and deficiencies in functional folate can lead to various metabolic and nutritional disorders. 35 In a clinical trial for advanced gastric cancer, Obermannova et al. 36 observed an increased incidence of severe vitamin D deficiency when cetuximab and capecitabine were combined, potentially due to capecitabine-induced folate deficiency.
In our study on hepatobiliary disorders, we identified both non-cirrhotic portal hypertension and abnormal liver function. Of particular concern is non-cirrhotic portal hypertension, which is not mentioned in the instructions. Zhang et al. 37 reported the occurrence of non-cirrhotic portal hypertension and isolated gastric variceal bleeding in a postoperative chemotherapy regimen combining oxaliplatin and capecitabine for colon cancer. Therefore, when using capecitabine, it is essential to remain vigilant for non-cirrhotic portal hypertension and the potential occurrence of gastrointestinal bleeding.
Limitations
Our findings demonstrate a potential association between the use of capecitabine and the occurrence of AEs, and we cannot deny its limitations. First, the FAERS database contains reports from diverse sources, including healthcare professionals and consumers, which can lead to inconsistencies and potential errors. Moreover, we encountered missing information in the collected reports, possibly introducing confounding factors into the research findings. In addition, as our data exclusively relied on the FAERS database, there might be deviations from the actual incidence rate. Nonetheless, our research has provided valuable guidance for clinical practice and enhanced clinicians’ awareness of potential AEs.
Conclusion
In conclusion, we conducted a comprehensive analysis of AEs associated with capecitabine using the FAERs database and found that the results were largely consistent with the AEs listed in the drug instructions. In addition, we identified previously unreported AEs, such as intestinal obstruction in gastrointestinal disorders, penetrating aortic ulcer in cardiac disorders, and non-cirrhotic portal hypertension in hepatobiliary disorders. The identification of these previously unknown AEs supplements the findings of previous small-sample clinical studies from a specific perspective. Moreover, it underscores the importance of continuous research and improvement of ADE information to enhance the therapeutic efficacy of drugs and facilitate their optimal use in clinical practice. In future research, researchers are encouraged to enhance the investigation of AEs associated with capecitabine through the collection of real-world data and the integration of diverse databases. Furthermore, the inclusion of emerging research programs, such as pharmacogenomics, can provide valuable insights into AEs associated with capecitabine.
Supplemental Material
Supplemental material, sj-docx-1-taw-10.1177_20420986241303428 for Adverse drug events associated with capecitabine: a real-world pharmacovigilance study based on the FAERS database by Rongqiang Liu, Yukai Chen, Shi-Nan Wu, Wangbin Ma, Zhendong Qiu, Jianguo Wang, Ximing Xu, Chen Chen and Weixing Wang in Therapeutic Advances in Drug Safety
Supplemental material, sj-docx-2-taw-10.1177_20420986241303428 for Adverse drug events associated with capecitabine: a real-world pharmacovigilance study based on the FAERS database by Rongqiang Liu, Yukai Chen, Shi-Nan Wu, Wangbin Ma, Zhendong Qiu, Jianguo Wang, Ximing Xu, Chen Chen and Weixing Wang in Therapeutic Advances in Drug Safety
Acknowledgments
None.
Footnotes
ORCID iDs: Rongqiang Liu  https://orcid.org/0000-0001-7993-8891
https://orcid.org/0000-0001-7993-8891
Shi-Nan Wu  https://orcid.org/0000-0002-6992-9059
https://orcid.org/0000-0002-6992-9059
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Rongqiang Liu, Department of Hepatobiliary Surgery, Renmin Hospital of Wuhan University, Wuhan, Hubei, China.
Yukai Chen, Cancer Center, Renmin Hospital of Wuhan University, Wuhan, Hubei, China.
Shi-Nan Wu, Eye Institute of Xiamen University, School of Medicine, Xiamen University, Xiamen, Fujian, China.
Wangbin Ma, Department of Hepatobiliary Surgery, Renmin Hospital of Wuhan University, Wuhan, Hubei, China.
Zhendong Qiu, Department of Hepatobiliary Surgery, Renmin Hospital of Wuhan University, Wuhan, Hubei, China.
Jianguo Wang, Department of Hepatobiliary Surgery, Renmin Hospital of Wuhan University, Wuhan, Hubei, China.
Ximing Xu, Department of Hepatobiliary Surgery, Renmin Hospital of Wuhan University, Wuhan, Hubei 430060, China.
Chen Chen, Department of Hepatobiliary Surgery, Renmin Hospital of Wuhan University, Wuhan, Hubei 430060, China.
Weixing Wang, Department of Hepatobiliary Surgery, Renmin Hospital of Wuhan University, Wuhan, Hubei 430060, China.
Declarations
Ethics approval and consent to participate: This article does not contain any studies with human or animal participants. There are no human participants in this article and informed consent is not required.
Consent for publication: Not applicable.
Author contributions: Rongqiang Liu: Data curation; Formal analysis; Methodology; Writing – original draft.
Yukai Chen: Data curation; Formal analysis; Methodology; Writing – original draft.
Shi-Nan Wu: Data curation; Formal analysis; Methodology; Writing – original draft.
Wangbin Ma: Supervision; Validation Zhendong Qiu Supervision; Validation.
Zhendong Qiu: Supervision; Validation.
Jianguo Wang: Supervision; Validation.
Ximing Xu: Conceptualization; Project administration; Writing – review & editing.
Chen Chen: Conceptualization; Project administration; Writing – review & editing.
Weixing Wang: Conceptualization; Project administration; Writing – review & editing.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Competing interests: The authors declare that there is no conflict of interest.
Availability of data and materials: The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: FAERS (https://www.fda.gov/drugs/questions-and-answers-fdas-adverse-event-reporting-system-faers/fda-adverse-event-reporting-system-faers-public-dashboard).
References
- 1. Walko CM, Lindley C. Capecitabine: a review. Clin Ther 2005; 27: 23–44. [DOI] [PubMed] [Google Scholar]
- 2. Rich TA, Shepard RC, Mosley ST. Four decades of continuing innovation with fluorouracil: current and future approaches to fluorouracil chemoradiation therapy. J Clin Oncol 2004; 22: 2214–2232. [DOI] [PubMed] [Google Scholar]
- 3. Adjei AA. A review of the pharmacology and clinical activity of new chemotherapy agents for the treatment of colorectal cancer. Br J Clin Pharmacol 1999; 48: 265–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhang W, Wang Y, Jiang X, et al. Newly identified adverse events for gemcitabine using the Food and Drug Administration Adverse Event Reporting System. Expert Opin Drug Saf 2024; 23: 917–923. [DOI] [PubMed] [Google Scholar]
- 5. Guo M, Shu Y, Chen G, et al. A real-world pharmacovigilance study of FDA adverse event reporting system (FAERS) events for niraparib. Sci Rep 2022; 12: 20601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yang Z, Lv Y, Yu M, et al. GLP-1 receptor agonist-associated tumor adverse events: a real-world study from 2004 to 2021 based on FAERS. Front Pharmacol 2022; 13: 925377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhou C, Peng S, Lin A, et al. Psychiatric disorders associated with immune checkpoint inhibitors: a pharmacovigilance analysis of the FDA Adverse Event Reporting System (FAERS) database. EClinicalMedicine 2023; 59: 101967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Trillenberg P, Sprenger A, Machner B. Sensitivity and specificity in signal detection with the reporting odds ratio and the information component. Pharmacoepidemiol Drug Saf 2023; 32: 910–917. [DOI] [PubMed] [Google Scholar]
- 9. Rothman KJ, Lanes S, Sacks ST. The reporting odds ratio and its advantages over the proportional reporting ratio. Pharmacoepidemiol Drug Saf 2004; 13: 519–523. [DOI] [PubMed] [Google Scholar]
- 10. Bate A. Bayesian confidence propagation neural network. Drug Saf 2007; 30: 623–625. [DOI] [PubMed] [Google Scholar]
- 11. Heo SJ, Jung I. Extended multi-item gamma Poisson shrinker methods based on the zero-inflated Poisson model for postmarket drug safety surveillance. Stat Med 2020; 39: 4636–4650. [DOI] [PubMed] [Google Scholar]
- 12. Sakaeda T, Tamon A, Kadoyama K, et al. Data mining of the public version of the FDA Adverse Event Reporting System. Int J Med Sci 2013; 10: 796–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Brown EG, Wood L, Wood S. The medical dictionary for regulatory activities (MedDRA). Drug Saf 1999; 20: 109–117. [DOI] [PubMed] [Google Scholar]
- 14. Tong A, Flemming K, McInnes E, et al. Enhancing transparency in reporting the synthesis of qualitative research: ENTREQ. BMC Med Res Methodol 2012; 12: 181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kim HS, Lee S, Kim JH. Real-world evidence versus randomized controlled trial: clinical research based on electronic medical records. J Korean Med Sci 2018; 33: e213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Masuda N, Lee SJ, Ohtani S, et al. Adjuvant capecitabine for breast cancer after preoperative chemotherapy. N Engl J Med 2017; 376: 2147–2159. [DOI] [PubMed] [Google Scholar]
- 17. Blum JL, Jones SE, Buzdar AU, et al. Multicenter phase II study of capecitabine in paclitaxel-refractory metastatic breast cancer. J Clin Oncol 1999; 17: 485–493. [DOI] [PubMed] [Google Scholar]
- 18. Rosenberg SM, Ruddy KJ, Tamimi RM, et al. BRCA1 and BRCA2 mutation testing in young women with breast cancer. JAMA Oncol 2016; 2: 730–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Anders CK, Johnson R, Litton J, et al. Breast cancer before age 40 years. Semin Oncol 2009; 36: 237–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cunningham D, Lang I, Marcuello E, et al. Bevacizumab plus capecitabine versus capecitabine alone in elderly patients with previously untreated metastatic colorectal cancer (AVEX): an open-label, randomised phase 3 trial. Lancet Oncol 2013; 14: 1077–1085. [DOI] [PubMed] [Google Scholar]
- 21. Bang YJ, Kim YW, Yang HK, et al. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomised controlled trial. Lancet 2012; 379: 315–321. [DOI] [PubMed] [Google Scholar]
- 22. Park JY. Analysis of data on capecitabine-related adverse drug reactions from the Korean adverse event reporting system database. Eur J Oncol Nurs 2018; 34: 55–60. [DOI] [PubMed] [Google Scholar]
- 23. Pow-Anpongkul P, Chu PG, Kidambi TD. Capecitabine-induced enteritis leading to small bowel obstruction. Gastroenterology 2019; 156: e8–e9. [DOI] [PubMed] [Google Scholar]
- 24. Mak G, Ward R, Shehabi Y, et al. Use of neostigmine in capecitabine-induced paralytic ileus. Tumori 2013; 99: e225–e228. [DOI] [PubMed] [Google Scholar]
- 25. Laudadio L, Biondi E, D’Ostilio N, et al. Paralytic ileus associated with capecitabine. Tumori 2008; 94: 742–745. [DOI] [PubMed] [Google Scholar]
- 26. Depetris I, Marino D, Bonzano A, et al. Fluoropyrimidine-induced cardiotoxicity. Crit Rev Oncol Hematol 2018; 124: 1–10. [DOI] [PubMed] [Google Scholar]
- 27. Evangelista A, Moral S, Ballesteros E, et al. Beyond the term penetrating aortic ulcer: a morphologic descriptor covering a constellation of entities with different prognoses. Prog Cardiovasc Dis 2020; 63: 488–495. [DOI] [PubMed] [Google Scholar]
- 28. Lawrence W., Jr. Technologic innovations in surgery: a philosophic reflection on their impact on operations for cancer. J Surg Oncol 2009; 100: 163–168. [DOI] [PubMed] [Google Scholar]
- 29. Shu Y, Ding Y, Dai B, et al. A real-world pharmacovigilance study of axitinib: data mining of the public version of FDA adverse event reporting system. Expert Opin Drug Saf 2022; 21: 563–572. [DOI] [PubMed] [Google Scholar]
- 30. Jadhav P, Rogers JE, Shroff R. A case report—Stevens-Johnson syndrome as an adverse effect of capecitabine. J Gastrointest Cancer 2018; 49: 349–350. [DOI] [PubMed] [Google Scholar]
- 31. Karthikeyan K, Sameera KV, Shaji S, et al. Capecitabine induced Steven-Johnson syndrome: a rare case report. J Oncol Pharm Pract 2022; 28: 250–254. [DOI] [PubMed] [Google Scholar]
- 32. Sendur MA, Kilickap S. Stevens-Johnson syndrome after treatment with capecitabine. Clin Oncol (R Coll Radiol) 2008; 20: 202–203. [DOI] [PubMed] [Google Scholar]
- 33. Ahn HR, Lee SK, Youn HJ, et al. Stevens-Johnson syndrome and concurrent hand foot syndrome during treatment with capecitabine: a case report. World J Clin Cases 2021; 9: 4279–4284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gerull R, Nelle M, Schaible T. Toxic epidermal necrolysis and Stevens-Johnson syndrome: a review. Crit Care Med 2011; 39: 1521–1532. [DOI] [PubMed] [Google Scholar]
- 35. Jennings BA, Willis G. How folate metabolism affects colorectal cancer development and treatment; a story of heterogeneity and pleiotropy. Cancer Lett 2015; 356: 224–230. [DOI] [PubMed] [Google Scholar]
- 36. Obermannova R, Valik D, Hasenclever D, et al. High prevalence of severe hypovitaminosis D in patients with advanced gastric cancer treated with first-line chemotherapy with or without anti-EGFR-directed monoclonal antibody (EXPAND trial) showing no prognostic impact. Eur J Cancer 2019; 116: 107–113. [DOI] [PubMed] [Google Scholar]
- 37. Zhang X, Gao YY, Song DZ, et al. Isolated gastric variceal bleeding related to non-cirrhotic portal hypertension following oxaliplatin-based chemotherapy: a case report. World J Gastroenterol 2022; 28: 3524–3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-taw-10.1177_20420986241303428 for Adverse drug events associated with capecitabine: a real-world pharmacovigilance study based on the FAERS database by Rongqiang Liu, Yukai Chen, Shi-Nan Wu, Wangbin Ma, Zhendong Qiu, Jianguo Wang, Ximing Xu, Chen Chen and Weixing Wang in Therapeutic Advances in Drug Safety
Supplemental material, sj-docx-2-taw-10.1177_20420986241303428 for Adverse drug events associated with capecitabine: a real-world pharmacovigilance study based on the FAERS database by Rongqiang Liu, Yukai Chen, Shi-Nan Wu, Wangbin Ma, Zhendong Qiu, Jianguo Wang, Ximing Xu, Chen Chen and Weixing Wang in Therapeutic Advances in Drug Safety