Abstract
Central post-stroke pain (CPSP) is a chronic neuropathic pain syndrome that commonly occurs after cerebral stroke, and it severely impairs the daily activities of stroke patients. A number of fundamental and clinical studies support the theory that CPSP is mainly caused by ischemic and hemorrhagic injury of the spinal-thalamic-cortical neural pathway. However, the underlying reasons of CPSP genesis and development are far from clear. In recent years, the majority of research focused on microglia, the main resident immune cells of the central nervous system, which highlighted its critical role in the regulation of CPSP. The present article concentrated on exciting discoveries of microglia in mediating CPSP from the perspectives of their bioactive factors, cellular receptors, and signaling pathways, in order to offer a convenient and easy-to-digest overview. In addition, the potential and challenges of several agents in clinical translation of CPSP treatment was discussed based on recent preclinical studies.
Keywords: Central post-stroke pain, microglia, neuroinflammation, thalamic hemorrhage
Introduction
Cerebral stroke is the primary cause of adult long-term sensorimotor dysfunction and disability in China and countries worldwide. Central post-stroke pain (CPSP) is one of the most common complications of stroke, and is characterized by long-term burning, tingling, and electric shock-like pain in stroke-affected limbs. Clinical studies explained that the incidence of CPSP in stroke patients is in the range of 11% to 35%, which is closely related with the raises, countries and regions investigated.1,2 CPSP is unbearable and seriously affects mood, sleep, and social functions of patients.3–5 More seriously, long-term CPSP can lead to self-mutilation, and even push patients to suicide.
Currently, there are multiple drugs that are employed for the treatment of CPSP. For instance, the antiepileptic drugs pregabalin and gabapentin have been thoroughly tested and used.6–8 However, their side effects, including dizziness, somnolence, edema, and weight gain are frequently reported. Interestingly, well-recognized analgesics, including diclofenac and morphine, are not recommended as first-line drugs, suggesting the unique pathogenesis of CPSP.9,10 Indeed, although the symptoms of CPSP are similar with that of other neuropathic pain and paresthesia in clinical, the accumulating fundamental studies demonstrated that its pathological mechanism is quite different. 11 People believed that CPSP is mainly caused by ischemic or hemorrhagic injury within spinal-thalamic-cortical neural pathway. 12 In clinical, the incidence of CPSP reaches to 80% if injury occurred in thalamus, which is why CPSP was originally called “thalamic pain”. 13
In the fundamental studies, the majority of evidence demonstrates that neuronal abnormal hyperexcitability,14,15 the defunctness of inhibitory neurons due to injury, 16 and the aseptic inflammation followed by immune response after stroke,17,18 are the underlying reasons causing CPSP. Of note, we found that most studies described the regulation of CPSP by microglia with direct or indirect ways, reflecting by that 17 out of 28 fundamental research papers of CPSP were relevant to microglia based on our bibliographic retrieval (Figure 1). Specifically, people found that either depletion of microglia or cell-specific mediation of multiple targets on microglia, including receptors, channels, signaling factors and kinases, can suppress ectopic neuronal activities along spinal-thalamic-cortical neural pathway, and eventually alleviate allodynia in CPSP model, which highlighted the importance of microglia in the genesis and development of CPSP. However, there is few focused reviews upon our search. We herein synthesized recent mechanical studies of CPSP relevant to microglia, which may outline future research directions into the mysteries of CPSP pathology. In addition, we discussed recent progress of the treatment strategies from the perspective of fundamental studies, which will contribute to assess their practical applicability in the future.
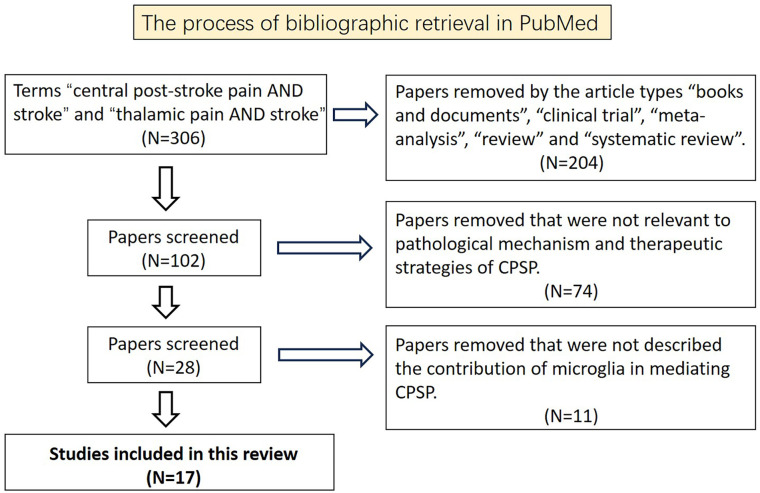
Figure 1.
The process of bibliographic retrieval in PubMed.
Literature search and organization
This bibliographic retrieval was organized on 6 October 2024. We searched the full text articles using the terms “central post-stroke pain AND stroke” and “thalamic pain AND stroke” in PubMed database. The search time limit was set to 2024. A total of 306 full-text papers were found from the search, in which 102 papers were obtained by excluding the article types “books and documents,” “clinical trial,” “meta-analysis,” “review,” and “systematic review.” In addition, we checked throughout the remaining papers and eventually obtained 28 papers that are relevant to pathological mechanism and therapeutic strategies of CPSP. Among the 28 papers, 17 papers mentioned the regulation of CPSP by microglia (Figure 1).
The general profile of microglia in mediating brain injury after stroke
Microglia are resident macrophages in the central nervous system and are widely distributed in the brain and spinal cord, accounting for 5%–20% of gliocyte numbers. Microglia play critical roles in development and diseases by regulating inflammation, immune surveillance, and clearing up apoptotic cells and debris 19 . Researchers found that cerebral ischemia and cerebral hemorrhage can cause a central inflammatory response accompanied by the activation of gliocytes. 20 Based on the types of cytokines produced by microglia and the consequences of their release, activated microglia are usually divided into cytotoxic phenotype (M1) and neuroprotective phenotype (M2). 21 M1 microglia are considered to exacerbate post-stroke injury by producing pro-inflammatory factors such as tumor necrosis factor (TNF), interleukin (IL)-1β, interferon-γ, IL-6, reactive oxygen species, matrix metalloproteinase-9, and matrix metalloproteinase-3. 22 Conversely, the M2 phenotype produce IL-10, transforming growth factor-β, insulin-like growth factor, and vascular endothelial growth factor, which exert anti-inflammatory effects, repair damaged nerves, promote angiogenesis, and help with functional recovery after stroke. 23
The role and mechanisms of microglia in mediating CPSP
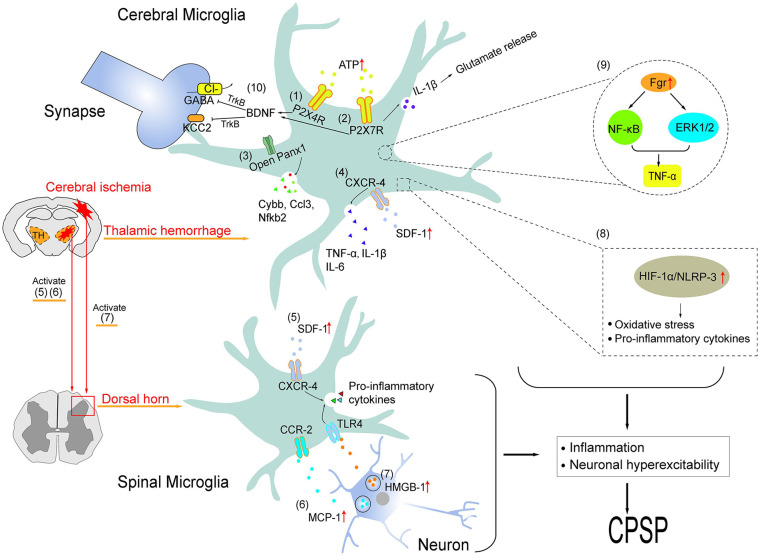
The immune system can interact with the nervous system causing persistent pain. 24 Studies accumulated over the years proved that microglia contribute to the genesis and development of CPSP, and the underlying reasons tend to stand out over time. In a mouse model of thalamic hemorrhage (TH), Hanada found that selective inhibitor of M1-phenotype microglia, minocycline, significantly ameliorated the mechanical and thermal allodynia. Yamashita’s team pointed out that the hyperactivity occurring in the thalamocortical circuit and the ectopic axonal projection within the somatosensory cortex were the underlying reasons of allodynia after TH. 14 Global depletion of microglia by daily feeding PLX3397, the CSF1 receptor inhibitor, effectively prevented the overactivity of ectopic neurons and allodynia in TH mice. These results collectively suggest that microglia contribute to the genesis and development of CPSP on the whole. Based on the above cognition, people further understood the mechanisms involved, which were herein elaborated in terms of surface receptors, signal-transducing factors and kinases et al (Table 1, Figure 2).
Table 1.
Recent findings of microglial signaling in CPSP mediation.
| Cellular targets | Key findings | References |
|---|---|---|
| Purinergic receptors | ||
| * (1) P2X4 | Intra-thalamic injection of autologous blood leads to the mechanical allodynia and the expression of P2X4 within the microglia of peri-lesion tissues. Either P2X4 blocker or adrenergic antidepressants and antiepileptics reverses the pain behavior. | Lu 30 |
| (2) P2X7 | Knockout P2X7 suppresses thermal and mechanical pain behavior induced by intra-thalamic injection of type IV collagenase, which are accompanied with lower expression of thalamic GFAP, Iba1 and BDNF, as well as increased intracellular [Cl−], compared to wild type mice. TH-lesioned tissue generates the high amount of ATP, which promotes microglial IL-1β release via acting on P2X7 receptors. IL-1β further enhances neuronal glutamate release, resulting in a higher frequency of neuron bursting in response to nociceptive stimulation. |
Huang
33
Kuan 15 |
| (3) Panx-1 | TH induces Panx-1 channel opening on microglia cell membrane within the peri-lesion area, which promotes the release of pro-inflammatory factors, and eventually induces pain behavior. | Bu 36 |
| Chemotactic factors | ||
| (4) (5) SDF-1 | At the acute phase of TH, HIF-1α level is increased in the microglial cytoplasm of peri-lesion tissue. HIF-1 complex binds to HRE in the DNA sequence and then induces either SDF-1 or CXCR-4 expression. At the late phase of TH, released SDF-1 acts on functional CXCR-4 through autocrine and paracrine pathway, which results in the release of pro-inflammatory cytokines, and subsequently increase neuronal excitation. TH induces abnormal activation of microglia and the elevation of SDF-1 and CXCR-4 at the spinal dorsal horn. Intrathecal administration of microglial and CXCR-4 inhibitors blocks the expression of SDF-1 and CXCR-4, as well as mechanical pain hypersensitivity. |
Yang
17
Liang 41 |
| (6) MCP-1 | The expression of MCP-1 was increased within the neurons of spinal dorsal horn throughout the acute phase and late phase after TH. Neutralizing MCP-1 by antibodies alleviates spinal microglial activation and pain behavior. | Yang 43 |
| Inflammatory mediators | ||
| (7) HMGB-1 | Cerebral ischemia stimulates the secretion of HMGB-1 protein from spinal neurons. At the acute phase of stroke, neutralizing HMGB-1 or antagonizing TLR-4 prevented microglial depolarization, NOS activity and mechanical allodynia. In addition, pharmacological inhibition of NOS also causes an analgesic effect in mice. | Matsuura 45 |
| (8) HIF-1α | TH induces microglial depolarization, HIF-1α expression, the construction of inflammasome NLRP-3 and pro-inflammatory factors release within thalamic injury region. These effects as well as pain behavior can be reversed by genetic deletion of HIF-1α. | Shi 46 |
| Kinases and others | ||
| (9) FGR | At the acute phase of TH, microglial FGR was transcriptionally upregulated, which promotes TNF-α production via activating NF-κB–ERK1/2 signaling pathway. These effects as well as hyperalgesia can be abolished by either knock down or pharmacological inhibition of FGR. | Huang 49 |
| (10) BDNF | At the late phase after TH, the ATP released by lesion tissue act on microglial P2X4 receptor, which caused BDNF production. Released BDNF over-bound TrkB receptors of thalamic neurons, which induced downstream inhibition of KCC2 and GABA receptors, and consequently enhanced neuronal activity in response to nociceptive inputs. | Shih 16 |
Annotation: the numbers in parentheses are used to direct the signaling in Figure 2.
Figure 2.
Schematic illustration of microglial regulations of central post-stroke pain via multiple mechanisms at the supraspinal and spinal levels, which was categorized and described in detail in Table 1.
Receptors and ion channels
Purinergic receptors
Adenosine triphosphate (ATP) is a common neurotransmitter in the central and peripheral nervous systems. The classic receptors of ATP are purinoceptor families, which include P1, P2X, and P2Y 25 . It is generally believed that upregulation of the ligand-gated non-selective cation channel P2X4 in microglia contributes to the genesis of multiple pain disorders.26,27 For instance, Trang found that perfusion of ATP evoked P2X4 receptor opening, which caused the release and synthesis of brain-derived neurotrophic factor (BDNF) via activating p38-mitogen-activated protein kinase in cultured primary microglia. 28 After nerve injury, released BDNF acts on neuronal TrkB receptors in the spinal cord dorsal horn, leading to neuronal hyperexcitability and allodynia. 29 It is worth noting that P2 receptors are also involved in regulating CPSP. Lu found a robust expression of P2X4 receptors on microglia rather than neurons in the peri-lesion site 7 days after thalamic hemorrhage in rats, accompanied with mechanical allodynia. 30 Administration of either P2X4 blocker or adrenergic antidepressants and antiepileptics significantly prevented the genesis of pain behavior, suggesting that microglial P2X4 also mediate CPSP. In addition, P2X7 receptors are involved in the inflammatory response and microglial activation within the central nervous system. 31 Activated P2X7 receptors are believed to participate in nociceptive transmission and neuropathic pain. 32 Kuan found that ATP was released after TH, and the high amounts of ATP acted on microglial P2X7 receptors, promoting IL-1β secretion. 15 ATP and IL-1β then enhanced glutamate release from neuronal synapses, resulting in hyperexcitation of neurons along the thalamocingulate neural pathway in response to injury stimulation, and eventually caused allodynia. Either pharmacological blocking P2X7 receptors using Brilliant Blue G or knock out P2X7 significantly alleviated abnormal pain behavior and microglial polarization/expression as well as BDNF production around thalamic lesion area.15,33 These studies suggest that purinoceptors, especially P2X4 and P2X7, are great potential targets for developing anti-CPSP drugs.
Pannexins (Panx)-1
Panx, including Panx-1-3, belong to a family of ATP release channels that are extensively expressed in a variety of cell types in the peripheral and central nervous systems. 34 In which, Panx-1 has been demonstrated to regulate multiple neurological disorders. 35 Recently, our lab’s study revealed that Panx-1 was centrally expressed in thalamic microglia and neurons at the acute phase of TH in mice. 36 Blocking Panx-1 channel using either the small-molecule compound carbenoxolone or inhibitory peptide 10Panx significantly alleviated TH-induced mechanical and temperature hyperalgesia of the contralateral hind paw, as well as thalamic tissue injury. In addition, carbenoxolone also effectively prevented TH-induced genetic expression of pro-inflammatory factors, such as CYBB, CCL3, and NFKB2, and their receptors, which was accompanied by protection against neurite collapse around thalamic lesion region. Depletion of microglia by PLX3397 together with application of carbenoxolone had no additional effect on TH-induced allodynia compared with either application alone, suggesting that microglial Panx-1 has a dominant effect in exacerbating CPSP and thalamic tissue injury. Notably, it’s not uncommon to find that overwhelming majority of receptors/channels investigated in CPSP are ATP permeable or ATP binding, prompting that diminishing ATP or abrogating its receptors/channels on microglia is important in mediating CPSP.
Chemotactic factors
Stromal cell-derived factor (SDF)-1
Chemokines are a class of small cytokines or signaling proteins secreted by cells that can participate in the regulation of cell migration and inflammatory responses through receptor binding pathways. SDF-1 is a member of the CXC chemokine family and is expressed in a variety of cells in both peripheral and central nervous systems. 37 SDF-1 can bind to CXCR-4 and exert biological functions in multiple pain disorders, including bone cancer pain, ischemia-reperfusion inflammatory pain, and neuropathic pain.38–40 Chen’s lab found that, under TH condition, the accumulation of hypoxia inducible factor (HIF)-1α was induced within thalamic neurons and glial cells (microglia and astrocyte). 17 Activated HIF-1 complex bound to hypoxia response element, which formed a promotor complex and then initiated the transcription of SDF-1 and CXCR-4 in glial cells. CXCR-4 was then translocated to the glial membrane, where it interacted with autocrine and paracrine SDF-1, resulting in the release of pro-inflammatory cytokines, including TNF-α, IL-1β and IL-6, which subsequently increased neuronal excitation and allodynia. Injection of CXCR-4 antagonist AMD3100 into the rat thalamus inhibited the activation of neurons and glial cells as well as the inflammatory response, which integrally alleviated TH-induced allodynia. Furthermore, it was found that TH-induced spinothalamic tract degeneration as well as secondary neuronal death and neuroinflammation in the spinal dorsal horn were associated with aberrant glial activation and SDF-1 upregulation. 41 Intrathecal injection of CXCR-4 inhibitors significantly ameliorated glial activation and mechanical allodynia in the CPSP model. The above results suggest that the SDF-1–CXCR-4 axis plays an important role in CPSP by mediating neuroinflammation at the supraspinal and spinal levels.
Monocyte chemotactic protein (MCP)-1
MCP-1, as a chemotactic cytokine, can serve as a regulator in the neuroinflammatory cascade and neurodegeneration. MCP-1 can activate microglia and participate in the early response to axotomy. 42 Yang found that, under physiological condition, MCP-1 and its receptor CCR-2 dominantly expressed on neurons, meanwhile CCR-2 partially co-expressed with Iba1 (microglia marker) in the spinal dorsal horn of rats. 43 At the late phase (28 days) after TH, the expression of MCP-1 was dramatically increased within neurons, which was accompanied with the up-regulation of c-fos and Iba1. Intrathecal injection of MCP-1 inhibitors or neutralizing antibodies significantly alleviated allodynia development as well as the up-regulation of c-fos and Iba1 after TH. These results suggest that neuron-derived MCP-1 may contribute to microglial activation via MCP-1–CCR-2 axis, which consequently leads to the hyperexcitability of spinal neurons and CPSP.
Inflammatory mediators
High-mobility group box (HMGB)-1
HMGB-1 is a highly conserved nuclear protein and an inflammatory factor that is becoming one of the foci of intensive care medicine research. Researchers recently found that cerebral immune cells and necrotic cells can release HMGB-1 in respond to inflammatory signals after cerebral ischemia, which leads to blood–brain barrier breakdown and further aggravates post-stroke complications. 44 In the model of bilateral carotid artery occlusion in mice, Matsuura found that ischemic stress stimulated the expression of HMGB-1 protein on spinal neurons at the acute phase. 45 Neutralizing HMGB-1 or antagonizing its well-known receptors toll like receptor (TLR)-4 prevented microglial depolarization, NOS activity and mechanical allodynia. In addition, pharmacological inhibition of NOS also caused an analgesic effect in mice. These data suggest that neuronal HMGB-1 may induce the pro-inflammatory depolarization of spinal microglia via acting on TLR-4, which eventually cause CPSP.
HIF-1α
HIF-1α, an oxygen-dependent transcriptional activator, can act as the initiator of neuroinflammation following TH and is involved in the genesis of CPSP by driving microglia activation and the expression of pro-inflammatory cytokines as described above. 17 In addition, Shi recently revealed another HIF-1α-mediated inflammatory signaling that promoted CPSP with comorbid anxiety and depression. They found that TH induced the release of pro-inflammatory cytokines, the expression of microglia marker and NOD-like receptor thermal protein domain associated protein (NLRP)-3 in the peri‑thalamic lesion tissues, as well as CPSP and anxiety‑/depression‑like behaviors, which were significantly reversed by pharmacological inhibition of glia, NLRP-3 or HIF-1α. Interestingly, HIF-1α inhibitors also suppressed the expression of NLRP-3, suggesting that TH may promote CPSP with complications via activating HIF-1α–NLRP3 inflammatory signaling of thalamic microglia. 46
Kinases and others
FGR
FGR non-receptor tyrosine kinase belongs to the SRC family of kinases, which includes c-SRC, c-YES, FYN, c-FGR, LYN, HCK, LCK, and BLK. These kinases participate in cell growth, proliferation, metabolism, differentiation, and migration.47,48 Huang found that pharmacological inhibition or knockdown of FGR significantly prevented the expression of Iba1, rather than GFAP within the hemorrhagic thalamus of mice, suggesting that FGR contributes to the activation of microglia under this condition. 49 An immunofluorescent staining study further demonstrated that FGR was co-expressed with P65, extracellular signal-regulated kinase (ERK)1/2, and TNF-α within microglia. The inhibition of FGR prevented phosphorylated activation of ERK1/2, the intracellular aggregation of P65, and TNF-α production. Given TNF-α can trigger peripheral neuropathic pain by acting as a pro-inflammatory cytokine,50,51 author postulates that microglial FGR can be transcriptionally upregulated by NF-κB–ERK1/2 signaling and further induces and maintains hyperalgesia after TH. 45 This study also found that TH induced the expression of LYN, whereas LYN was unaffected by FGR knockdown, suggesting that LYN may also participate in the genesis and development of CPSP. However, questions regarding the pathological function of LYN, and whether LYN is of microglia original need to be further addressed in a CPSP model.
BDNF
BDNF is originally considered as one of the neurotrophins associated with neuronal maintenance, survival, plasticity, and neurotransmitter regulation. However, accumulating studies evidence that BDNF concentration in serum and pathological tissues is positively correlated with mechanical and thermal hyperpathia in human and animal subjects.52,53 Of note, Shih’s study pointed out that BDNF regulated CPSP induced by TH. 16 In detail, they found that the protein level of BDNF rapidly increased in the thalamic lesion tissue accompanied with the expression of CD11b (microglia marker) and P2X4, compared with the sham-operative group in rats. Inhibition of TrkB, the well-known receptor of BDNF, by either antibodies or antagonist alleviated hyperpathia and reduced KCC2 expression. In addition, application of the agonist of GABA receptor performed similar effect with that of inhibiting P2X4 in pain behavior. Based on the main findings, author concluded that after thalamus-hemorrhagic injury, released ATP activated microglia via acting on P2X4 receptors, which caused BDNF production. BDNF bound to TrkB receptors of excitatory neuron and induced downstream inhibition of KCC2 and GABA receptors, which consequently disinhibited to the lateral thalamic inhibitory inputs and led to neuron sensitivity to afferent noxious stimulation. However, the author also admits that there are several research gaps need to be addressed further. One of the main issues is that the cell source of BDNF was not experimental evidenced, although overexpression of BDNF by proliferating microglia is the key factor in mediating neuropathic pain in other disorders. 29 The second but not the last is that how BDNF–TrkB axis reduced neuronal KCC2 expression in thalamus remains unclear.
Other factors and recent treatment advances
With the development of RNA sequencing and bioinformatics analyses, dynamic changes to genes at the levels of tissue and cellular subtypes can be investigated, which can reveal key factors involved in mediating CPSP. Huang used bulk RNA sequencing and single-nucleus RNA sequencing to detect the mRNA expression of thalamic tissue in TH mice. 54 They found that TH resulted in an altered cellular composition, in which a significant decrease in the number of neuron and astrocyte markers, and an increase in microglia markers. In addition, three key genes, Apoe, Abca1, and Hexb, were identified, each of which is closely related to macrophages, T cells, related chemokines, immune stimulators, and receptors. Further gene ontology enrichment analysis suggested that such genes may mediate CPSP pathology by regulating protein export from nucleus and protein sumoylation within thalamic microglia and peripheral infiltrating immune cells. However, experimental studies are required to explore the pathological functions of these genes in CPSP models.
Stroke exhibits significant sexual dimorphism. 55 Young women commonly have a lower incidence of stroke than young men, whereas postmenopausal women have a higher risk of stroke, higher mortality rate, and worse prognosis than men, which is, at least partially, due to the differential expression of genes located on the X chromosome. 56 Qi’s several studies showed that X chromosome escapee genes are involved in sexual dimorphism of ischemic brain injury through the epigenetic modification of inflammatory genes.56–58 For instance, kdm5c and kdm6a mRNA levels were significantly higher in cerebral microglia of aged females than males after stroke, observed on either postmortem human brain tissue or mice brain tissue. 56 Knock-down kdm5c/kdm6a significantly suppressed the expression of pro-inflammatory factors, and up-regulated the anti-inflammatory factors in neonatal microglia. Interestingly, clinical studies showed that females tend to exhibit higher pain ratings after stroke than males, which raises the possibility that the differential expression of X chromosome-encoded genes may contribute to the sexual dimorphism of CPSP through mediating the inflammatory polarization of microglia.
In recent years, multiple compounds and molecules have been demonstrated therapeutic potential for CPSP through preclinical experiments. For example, Zhang found that the crosstalk between endoplasmic reticulum stress and neuroinflammation induced by TH promoted the genesis of CPSP. Either administration of epoxyeicosatrienoic acids or selective inhibition of soluble epoxy hydrolase by TPPU significantly alleviated the activation of microglial endoplasmic reticulum stress, neuroinflammation, and CPSP in rats. 18 They conclude that targeting epoxyeicosatrienoic acid signaling may not only curtail stroke-induced nerve injury but also produce an analgesic effect. This finding was also supported by Wan’s recent paper, in which the abirritation of epoxyeicosatrienoic acids in multiple chronic neuropathic pain disorders, including CPSP, have been proven without apparent side effects in animal models. 59 These results collectively offer a potential in the clinical translation.
In addition, Infantino found that a co-ultramicronized combination of N-palmitoylethanolamine and luteolin reduced microglial activation and altered the activation patterns of microglia at TH peri-lesion sites, which was reflected by the decrease in microglial M1-type and increase in M2-type, accompanied with the reduction in mechanical hypersensitivity after TH. 60 Palmitoylethanolamine is an endocannabinoid-like lipid mediator with known anti-inflammatory and analgesic effects, which have been proven by multiple clinical trials although this substance has not been approved by the U.S. Food and Drug Administration. 61 Another component of the co-ultramicronized combination, luteolin, was experimental demonstrated as an effective natural agent in management of chronic pain. 62 However, given the observation that the analgesic effect of luteolin could be inhibited by pretreatment with μ-opioid receptor antagonist, naloxone, in a rat neuropathic model, 62 whether continuous administration of luteolin could lead to drug dependence and addiction via affecting μ-opioid receptor signaling needs to be cautiously considered.
Furthermore, in clinical studies, researchers have observed that CPSP patients often have a severe sleep disorder comorbidity, and that the disruption of circadian rhythms and sleep deprivation can cause neuronal hyperexcitability, which might worsen chronic pain. Using a TH-induced CPSP mice model, Kaur found that administration of exogenous melatonin (30 mg/kg) significantly reversed the decrease of blood melatonin after TH. Importantly, melatonin ameliorated the pain resulting from distorted sleep-activity behavior, and this effect can persist weeks after melatonin administration was discontinued. 63 Mechanically, Kaur’s following-up study suggested that melatonin reduced the level of inflammatory cytokines, including TNF-α, IL-6 and IL-1β, via, at least partially, suppressing pro-inflammatory polarization of microglia in a rat CPSP model. 64 However, given the inhibitory effects of melatonin to the production of estrogen 65 and the known beneficial effect of estrogen in mediating brain injury and complications after stroke, the CPSP female cohort needs to be well-designed in clinical trials. In conclusion, the findings collectively suggest that these chemical/drugs deserved to be preferentially considered in further translational studies.
Discussion and conclusion
In this review, we summarized main mechanisms of microglia in mediating CPSP, and recent research progress in the treatment of CPSP based on animal experiments. In clinical, it is widely accepted that damage to the nociceptive afferent regions along spinothalamic pathway is associated with CPSP. 13 In fundamental studies, due to the limitation of experimental approach, thalamic hemorrhage and bilateral carotid artery occlusion are the most common ways to construct CPSP models. In either animal models, people found that the activation of microglia in cerebral and spinal tissues is accompanied with sensory abnormalities and nociceptive hyperalgesia after stroke. Importantly, global depletion of microglia significantly alleviates CPSP, highlighting the importance of microglia in this disorder. 10
According to the studies described above, this article classifies current mechanistic studies relevant to microglia, including ligands and receptors, inflammatory mediators, and kinases. Among the ligands and receptors, it is clear that ATP relative receptors/channels, including P2X4, P2X7 and Panx-1,66,67 and chemokines relative receptors, including CXCR-4 and CCR-2, 68 are most frequently reported. Under physiological condition, the level of intracellular ATP is typically higher than that in the extracellular space, which provides energy to cellular metabolism. However, intracellular ATP can be extremely released from necrotic and apoptotic cells under pathological conditions. In the set of CPSP, without doubt, people demonstrated that stroke stress induce the activation of ATP-permeable channels Panx-1, and ATP receptors on microglia, which further promotes neuron hypersensitivity through a series of downstream signaling.15,16,30,33,36 In this case, cell-type specific targeting ATP relative channels/receptors might be the directions in the treatment of CPSP. In addition, multiple chemokines-receptor axis was also reported, including SDF-1–CXCR-4 and MCP-1–CCR-2.17,43 The hyperfunction of above chemokines axis are positively associated with pro-inflammatory factors production and proliferation of microglia, which is believed to be the underlying reasons of CPSP. So interruption of these targets might be also the potential strategy in CPSP treatment.
Another set of main finding is the inflammatory depolarization of microglia in mediating CPSP. As the major resident immune cells of central nervous system, microglia can be rapidly activated in response to tissue oxidative stress, infection and injury, and performs polymorphic phenotypes depending on the disorder progress and cell locations. 69 In CPSP models, dominantly studies suggest that microglia tend to manifest pro-inflammatory phenotype, and consequently worsens pain sensitization via producing inflammasome (NLRP-3 complex) and pro-inflammatory factors (NF-κB, IL-1β and TNF-α et al.).17,45,46,49 The accumulated inflammatory mediators within the cerebral lesion region or remote spinal dorsal horn can diffusively affect adjacent glial cells and enhance the neuronal excitability in response to peripheral stimulation, which eventually cause the genesis and development of allodynia. In this case, eliminating pro-inflammatory polarization, as well as related factors of inflammation signaling, including P2X7, Panx-1, CXCR-4, TLR-4, SDF-1, Fgr and HIF-1α et al, on microglia will be the strategy in CPSP treatment. The above results also collectively enlighten us that more efforts may preferentially perform on the up-stream and down-stream pathways of these factors, especially that have been reported in stroke and brain injury, for future mechanistic research and drug development of CPSP. Of note, whether the anti-inflammatory depolarization of microglia is functional in CPSP remains unclear due to the limited study.
In addition to function as inflammatory regulator, more and more researchers found that microglia can mediate brain injury and functional outcome after stroke by phagocytosis.70–72 The engulfment of microglia to apoptotic neurons was commonly believed to protect against neuronal impairments and promote functional recovery after cerebral ischemia.70,71 Differently, elimination of synapses by reactive microglia worsened neurobehavioral deficit after cerebral ischemia/hemorrhage. 72 However, whether the phagocytic function of microglia contribute to CPSP is completely unknown. We noticed that the well-known regulators of CPSP, for instance mitogen-activated protein kinases 49 and CXCR-4, 17 were previously reported to promote the engulfment of microglia to neurons,73,74 which raises the possibility that microglial phagocytosis may be functional in CPSP either.
In summary, CPSP is one of the untreatable stroke complications that is receiving increasing attention. Activated microglia in the supraspinal regions and spinal cord can aggravate CPSP through, at least in part, neuroinflammation, chemokines and ATP related receptors/channels. These finding suggests that intervening the corresponding targets could be preferentially considered in the therapeutic strategy of CPSP.
Footnotes
Abbreviation: ATP, adenosine triphosphate;
BDNF, brain-derived neurotrophic factor
CPSP, central post-stroke pain;
ERK, extracellular signal-regulated kinase;
HIF, hypoxia inducible factor;
HMGB, high-mobility group box;
IL, interleukin;
MCP, monocyte chemotactic protein;
NF-κB, nuclear factor kappa-B;
NLRP, nod-like receptor thermal protein domain associated protein;
Panx, pannexins;
SDF, stromal cell-derived factor;
TH, thalamic hemorrhage;
TNF, tumor necrosis factor;
TLR, toll like receptor;
Author’s contribution: H.C., X.Q. and F.B. conceived and designed the study; Y.L., Z.D., S.L. and G.L. wrote the manuscript. X.Q., F.B., Y.L., L.X., L.L. and L.H. proofread the manuscript; All authors approved the final manuscript.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was funded by Shenzhen High-level Hospital Construction Fund; Sanming Project of Medicine in Shenzhen (SZZYSM 202111011); Natural Science Foundation of Shenzhen (JCYJ20220531091808020, JCYJ20230807094810022, JCYJ20230807094817035, JCYJ20230807094801002, JCYJ20220531092406014).
Ethics approval: Not applicable
Informed consent: Not applicable
ORCID iD: Fan Bu  https://orcid.org/0000-0002-0051-6037
https://orcid.org/0000-0002-0051-6037
References
- 1. Widar M, Ahlström G. (2002) Disability after a stroke and the influence of long-term pain on everyday life. Scand J Caring Sci 16: 302–310. [DOI] [PubMed] [Google Scholar]
- 2. Liampas A, Velidakis N, Georgiou T, et al. (2020) Prevalence and management challenges in central post-stroke neuropathic pain: A systematic review and meta-analysis. Adv Ther 37: 3278–3291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jönsson A-C, Lindgren I, Hallström B, et al. (2006) Prevalence and intensity of pain after stroke: a population based study focusing on patients’ perspectives. J Neurol Neurosurg Psychiatry 77: 590–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Widar M, Ahlström G, Ek A-C. (2004) Health-related quality of life in persons with long-term pain after a stroke. J Clin Nurs 13: 497–505. [DOI] [PubMed] [Google Scholar]
- 5. Widar M, Ek A-C, Ahlström G. (2024) Coping with long-term pain after a stroke. J Pain Symptom Manage 27: 215–225. [DOI] [PubMed] [Google Scholar]
- 6. Attal N, Brasseur L, Parker F, et al. (1998) Effects of gabapentin on the different components of peripheral and central neuropathic pain syndromes: a pilot study. Eur Neurol 40: 191–200. [DOI] [PubMed] [Google Scholar]
- 7. Backonja M, Glanzman RL. (2003) Gabapentin dosing for neuropathic pain: evidence from randomized, placebo-controlled clinical trials. Clin Ther 25: 81–104. [DOI] [PubMed] [Google Scholar]
- 8. Kim JS, Bashford G, Murphy KT, et al. (2011) Safety and efficacy of pregabalin in patients with central post-stroke pain. Pain 152: 1018–1023. [DOI] [PubMed] [Google Scholar]
- 9. Dworkin RH, O’Connor AB, Backonja M, et al. (2007) Pharmacologic management of neuropathic pain: evidence-based recommendations. Pain 132: 237–251. [DOI] [PubMed] [Google Scholar]
- 10. Hanada T, Kurihara T, Tokudome M, et al. (2014) Development and pharmacological verification of a new mouse model of central post-stroke pain. Neurosci Res 78: 72–80. [DOI] [PubMed] [Google Scholar]
- 11. Cheng Y, Wu B, Huang J, et al. (2023) Research Progress on the Mechanisms of Central Post-Stroke Pain: A Review. Cell Mol Neurobiol 43: 3083–3098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Treister AK, Hatch MN, Cramer SC, et al. (2017) Demystifying poststroke pain: From etiology to treatment. PM R 9: 63–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Klit H, Finnerup NB, Jensen TS. (2009) Central post-stroke pain: clinical characteristics, pathophysiology, and management. Lancet Neurol 8: 857–868. [DOI] [PubMed] [Google Scholar]
- 14. Hiraga S-I, Itokazu T, Hoshiko M, et al. (2020) Microglial depletion under thalamic hemorrhage ameliorates mechanical allodynia and suppresses aberrant axonal sprouting. JCI Insight 5: 131801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kuan Y-H, Shih H-C, Tang S-C, et al. (2015) Targeting P(2)X(7) receptor for the treatment of central post-stroke pain in a rodent model. Neurobiol Dis 78: 134–145. [DOI] [PubMed] [Google Scholar]
- 16. Shih H-C, Kuan Y-H, Shyu B-C. (2017) Targeting brain-derived neurotrophic factor in the medial thalamus for the treatment of central poststroke pain in a rodent model. Pain 158: 1302–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yang F, Luo W-J, Sun W, et al. (2017) SDF1-CXCR4 signaling maintains central post-stroke pain through mediation of glial-neuronal interactions. Front Mol Neurosci 10: 226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Liu T, Li T, Chen X, et al. (2021) EETs/sEHi alleviates nociception by blocking the crosslink between endoplasmic reticulum stress and neuroinflammation in a central poststroke pain model. J Neuroinflammation 18: 211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Saijo K, Glass CK. (2011) Microglial cell origin and phenotypes in health and disease. Nat Rev Immunol 11: 775–787. [DOI] [PubMed] [Google Scholar]
- 20. Ellis A, Bennett DLH. (2013) Neuroinflammation and the generation of neuropathic pain. Br J Anaesth 111: 26–37. [DOI] [PubMed] [Google Scholar]
- 21. Tang Y, Le W. (2016) Differential Roles of M1 and M2 Microglia in Neurodegenerative Diseases. Mol Neurobiol 53: 1181–1194. [DOI] [PubMed] [Google Scholar]
- 22. Yenari MA, Kauppinen TM, Swanson RA. (2010) Microglial activation in stroke: therapeutic targets. Neurotherapeutics 7: 378–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Qin C, Zhou L-Q, Ma X-T, et al. (2019) Dual Functions of Microglia in Ischemic Stroke. Neurosci Bull 35: 921–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Calvo M, Dawes JM, Bennett DLH. (2012) The role of the immune system in the generation of neuropathic pain. Lancet Neurol 11: 629–642. [DOI] [PubMed] [Google Scholar]
- 25. Abbracchio MP, Burnstock G. (1994) Purinoceptors: are there families of P2X and P2Y purinoceptors? Pharmacol Ther 64: 445–475. [DOI] [PubMed] [Google Scholar]
- 26. Tsuda M, Shigemoto-Mogami Y, Koizumi S, et al. (2023) P2X4 receptors induced in spinal microglia gate tactile allodynia after nerve injury. Nature 424: 778–783. [DOI] [PubMed] [Google Scholar]
- 27. Ulmann L, Hatcher JP, Hughes JP, et al. (2008) Up-regulation of P2X4 receptors in spinal microglia after peripheral nerve injury mediates BDNF release and neuropathic pain. J Neurosci 28: 11263–11268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Trang T, Beggs S, Wan X, et al. (2009) P2X4-receptor-mediated synthesis and release of brain-derived neurotrophic factor in microglia is dependent on calcium and p38-mitogen-activated protein kinase activation. J Neurosci 29: 3518–3528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Coull JAM, Beggs S, Boudreau D, et al. (2005) BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature 438: 1017–1021. [DOI] [PubMed] [Google Scholar]
- 30. Lu H-F, Xu C-Y, Zhang L, et al. (2018) A new central post-stroke pain rat model: autologous blood injected thalamic hemorrhage involved increased expression of P2X4 receptor. Neurosci Lett 687: 124–130. [DOI] [PubMed] [Google Scholar]
- 31. Monif M, Burnstock G, Williams DA. (2010) Microglia: proliferation and activation driven by the P2X7 receptor. Int J Biochem Cell Biol 42: 1753–1756. [DOI] [PubMed] [Google Scholar]
- 32. Pelegrin P, Surprenant A. (2009) The P2X(7) receptor-pannexin connection to dye uptake and IL-1beta release. Purinergic Signal 5: 129–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Huang ACW, Shih H-C, Shyu BC. (2024) The P2X7 Hypothesis of Central Post-Stroke Pain. Int J Mol Sci; 25. Epub ahead of print 14 June 2024. DOI: 10.3390/ijms25126577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bruzzone R, Hormuzdi SG, Barbe MT, et al. (2003) Pannexins, a family of gap junction proteins expressed in brain. Proc Natl Acad Sci U S A 100: 13644–13649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shestopalov VI, Slepak VZ. (2014) Molecular pathways of pannexin1-mediated neurotoxicity. Front Physiol 5: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bu F, Li Y, Lan S, et al. (2023) Blocking pannexin-1 channels alleviates thalamic hemorrhage-induced pain and inflammatory depolarization of microglia in mice. ACS Chem Neurosci 14: 2548–2559. [DOI] [PubMed] [Google Scholar]
- 37. Réaux-Le Goazigo A, Van Steenwinckel J, Rostène W, et al. (2013) Current status of chemokines in the adult CNS. Prog Neurobiol 104: 67–92. [DOI] [PubMed] [Google Scholar]
- 38. Li X-Q, Zhang Z-L, Tan W-F, et al. (2016) Down-Regulation of CXCL12/CXCR4 expression alleviates ischemia-reperfusion-induced inflammatory pain via inhibiting glial TLR4 activation in the spinal cord. PLoS One 11: e0163807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Shen W, Hu X-M, Liu Y-N, et al. (2014) CXCL12 in astrocytes contributes to bone cancer pain through CXCR4-mediated neuronal sensitization and glial activation in rat spinal cord. J Neuroinflammation 11: 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Luo X, Tai WL, Sun L, et al. (2016) Crosstalk between astrocytic CXCL12 and microglial CXCR4 contributes to the development of neuropathic pain. Mol Pain; 12. Epub ahead of print 2016. DOI: 10.1177/1744806916636385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Liang T, Chen X-F, Yang Y, et al. (2022) Secondary damage and neuroinflammation in the spinal dorsal horn mediate post-thalamic hemorrhagic stroke pain hypersensitivity: SDF1-CXCR4 signaling mediation. Front Mol Neurosci 2022; 15: 911476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Muessel MJ, Klein RM, Wilson AM, et al. (2002) Ablation of the chemokine monocyte chemoattractant protein-1 delays retrograde neuronal degeneration, attenuates microglial activation, and alters expression of cell death molecules. Brain Res Mol Brain Res 103: 12–27. [DOI] [PubMed] [Google Scholar]
- 43. Yang F, Jing J-J, Fu S-Y, et al. (2023) Spinal MCP-1 Contributes to Central Post-stroke Pain by Inducing Central Sensitization in Rats. Mol Neurobiol 60: 2086–2098. [DOI] [PubMed] [Google Scholar]
- 44. Zhang J, Takahashi HK, Liu K, et al. (2011) Anti-high mobility group box-1 monoclonal antibody protects the blood-brain barrier from ischemia-induced disruption in rats. Stroke 42: 1420–1428. [DOI] [PubMed] [Google Scholar]
- 45. Matsuura W, Harada S, Liu K, et al. (2018) Evidence of a role for spinal HMGB1 in ischemic stress-induced mechanical allodynia in mice. Brain Res 1687: 1–10. [DOI] [PubMed] [Google Scholar]
- 46. Shi Z-M, Jing J-J, Xue Z-J, et al. (2023) Stellate ganglion block ameliorated central post-stroke pain with comorbid anxiety and depression through inhibiting HIF-1α/NLRP3 signaling following thalamic hemorrhagic stroke. J Neuroinflammation 20: 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lohman AW, Weilinger NL, Santos SM, et al. (2019) Regulation of pannexin channels in the central nervous system by Src family kinases. Neurosci Lett 2019; 695: 65–70. [DOI] [PubMed] [Google Scholar]
- 48. Okada M. (2012) Regulation of the SRC family kinases by Csk. Int J Biol Sci 8: 1385–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Huang T, Fu G, Gao J, et al. (2020) Fgr contributes to hemorrhage-induced thalamic pain by activating NF-κB/ERK1/2 pathways. JCI Insight 5: 139987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kang O-H, Lee G-H, Choi HJ, et al. (2007) Ethyl acetate extract from Angelica Dahuricae Radix inhibits lipopolysaccharide-induced production of nitric oxide, prostaglandin E2 and tumor necrosis factor-alphavia mitogen-activated protein kinases and nuclear factor-kappaB in macrophages. Pharmacol Res 55: 263–270. [DOI] [PubMed] [Google Scholar]
- 51. Hung AL, Lim M, Doshi TL. (2017) Targeting cytokines for treatment of neuropathic pain. Scand J Pain 17: 287–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zhang X, Wang J, Zhou Q, et al. (2011) Brain-derived neurotrophic factor-activated astrocytes produce mechanical allodynia in neuropathic pain. Neuroscience 199: 452–460. [DOI] [PubMed] [Google Scholar]
- 53. Brietzke AP, Antunes LC, Carvalho F, et al. (2019) Potency of descending pain modulatory system is linked with peripheral sensory dysfunction in fibromyalgia: An exploratory study. Medicine (Baltimore) 98: e13477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Huang T, Xiao Y, Zhang Y, et al. (2003) Combination of single-nucleus and bulk RNA-seq reveals the molecular mechanism of thalamus haemorrhage-induced central poststroke pain. Front Immunol 14: 1174008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Petrea RE, Beiser AS, Seshadri S, et al. (2009) Gender differences in stroke incidence and poststroke disability in the Framingham heart study. Stroke 40: 1032–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Qi S, Al Mamun A, Ngwa C, et al. (2021) X chromosome escapee genes are involved in ischemic sexual dimorphism through epigenetic modification of inflammatory signals. J Neuroinflammation 18: 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ngwa C, Al Mamun A, Qi S, et al. (2022) Regulation of microglial activation in stroke in aged mice: a translational study. Aging (Albany NY) 14: 6047–6065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Al Mamun A, Chauhan A, Qi S, et al. (2020) Microglial IRF5-IRF4 regulatory axis regulates neuroinflammation after cerebral ischemia and impacts stroke outcomes. Proc Natl Acad Sci U S A 117: 1742–1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Wan L, Li Z, Liu T, et al. (2020) Epoxyeicosatrienoic acids: Emerging therapeutic agents for central post-stroke pain. Pharmacol Res 159: 104923. [DOI] [PubMed] [Google Scholar]
- 60. Infantino R, Schiano C, Luongo L, et al. (2022) MED1/BDNF/TrkB pathway is involved in thalamic hemorrhage-induced pain and depression by regulating microglia. Neurobiol Dis 164: 105611. [DOI] [PubMed] [Google Scholar]
- 61. Lang-Illievich K, Klivinyi C, Lasser C, et al. (2023) Palmitoylethanolamide in the treatment of chronic pain: A systematic review and meta-analysis of double-blind randomized controlled trials. Nutrients; 15. Epub ahead of print 10 March 2023. DOI: 10.3390/nu15061350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ntalouka F, Tsirivakou A. (2023) Luteolin: A promising natural agent in management of pain in chronic conditions. Front Pain Res (Lausanne) 4: 1114428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kaur T, Shih H-C, Huang ACW, et al. (2022) Modulation of melatonin to the thalamic lesion-induced pain and comorbid sleep disturbance in the animal model of the central post-stroke hemorrhage. Mol Pain 2022; 18: 17448069221127180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kaur T, Huang AC-W, Shyu B-C. (2023) Modulation of melatonin in pain behaviors associated with oxidative stress and neuroinflammation responses in an animal model of central post-stroke pain. Int J Mol Sci; 24. Epub ahead of print 12 March 2023. DOI: 10.3390/ijms24065413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Harrod CG, Bendok BR, Hunt Batjer H. (2005) Interactions between melatonin and estrogen may Regulate cerebrovascular function in women: Clinical implications for the effective use of HRT during menopause and aging. Med Hypotheses 64: 725–735. [DOI] [PubMed] [Google Scholar]
- 66. Bravo D, Maturana CJ, Pelissier T, et al. (2015) Interactions of pannexin 1 with NMDA and P2X7 receptors in central nervous system pathologies: Possible role on chronic pain. Pharmacol Res 101: 86–93. [DOI] [PubMed] [Google Scholar]
- 67. North RA. (2016) P2X receptors. Philos Trans R Soc Lond B Biol Sci; 371. Epub ahead of print 5 August 2016. DOI: 10.1098/rstb.2015.0427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Bacon KB, Harrison JK.(2000) Chemokines and their receptors in neurobiology: perspectives in physiology and homeostasis. J Neuroimmunol 104: 92–97. [DOI] [PubMed] [Google Scholar]
- 69. Colonna M, Butovsky O. (2017) Microglia function in the central nervous system during health and neurodegeneration. Annu Rev Immunol 35: 441–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Cai W, Dai X, Chen J, et al. (2019) STAT6/Arg1 promotes microglia/macrophage efferocytosis and inflammation resolution in stroke mice. JCI Insight 4: 131355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Zhang G, Li Q, Tao W, et al. (2023) Sigma-1 receptor-regulated efferocytosis by infiltrating circulating macrophages/microglial cells protects against neuronal impairments and promotes functional recovery in cerebral ischemic stroke. Theranostics 13: 543–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Shi X, Luo L, Wang J, et al. (2021) Stroke subtype-dependent synapse elimination by reactive gliosis in mice. Nat Commun 12: 6943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. De Maeyer RPH, van de Merwe RC, Louie R, et al. (2020) Blocking elevated p38 MAPK restores efferocytosis and inflammatory resolution in the elderly. Nat Immunol 21: 615–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Gao A, Lin Y, Chai Y, et al. (2024) CXCL12/CXCR4 Axis promotes the chemotaxis and phagocytosis of B cells through the PI3K-AKT signaling pathway in an early vertebrate. J Immunol 2024; ji2300562. [DOI] [PubMed] [Google Scholar]