Abstract
Background:
Although the approval of immunotherapy in patients with extensive-stage small-cell lung cancer (ES-SCLC) has significantly improved the patient’s prognosis, synchronous chemoradiotherapy has always been the standard treatment for limited-stage small-cell lung cancer (LS-SCLC).
Objectives:
Immuno-combined and radio-combined therapy in LS-SCLC has been applied in clinical practice, but what is the best for LS-SCLC?
Design:
This was a retrospective cohort study.
Methods:
Patients with LS-SCLC from January 2019 to December 2023 were retrospectively screened and divided into three groups according to the initial treatment regimen whether included immune-combined and radio-combined treatment. Univariate and multivariate Cox regression were used to analyze the predictors affecting the survival of LS-SCLC, and the progression pattern of patients and the occurrence of adverse events (AEs) were also recorded.
Results:
In this study, the median overall survival (OS) was 15.8 months, not yet reached (NR) and NR, and the median progression-free survival (PFS) was 11.7, 20.9, and 18.9 months in the immunotherapy combined chemotherapy (N = 34), immune combined chemoradiotherapy (N = 26), and chemoradiotherapy (N = 53) groups, respectively. OS and PFS were significantly prolonged in the radio-combined groups compared with the non-radio-combined group, and there was no significant difference between the radio-combined groups, namely immunotherapy combined chemoradiotherapy and chemoradiotherapy groups. In this study, we also constructed some indexes to predict prognosis for LS-SCLC, derived neutrophil and lymphocyte ratios were significantly associated with worse survival, and systemic inflammatory index and neuron-specific enolase (NSE) levels were significantly associated with shorter PFS. The primary organs of progression remained the lung and brain, the main immune-related AE was hypothyroidism, and the radiation-related AE was pneumonia.
Conclusion:
Radiation-combined therapy still plays an important role in LS-SCLC in the era of immunotherapy, and clinicians cannot abandon the use of radiation therapy in the initial treatment plan for LS-SCLC.
Keywords: immune checkpoint inhibitors, limited stage, radiation therapy, small-cell lung cancer
Introduction
Lung cancer remains the leading cause of tumorigenesis and death, and although the incidence of advanced disease has decreased, the diagnosis at the local stage has increased significantly.1,2 Small-cell lung cancer (SCLC) accounts for only about 15% of the total number of lung cancers; however, the 5-year survival rate is less than 7% and worse for extensive-stage (ES) SCLC as defined by the Veterans Administration Lung Study Group (VALSG).3,4 Etoposide plus platinum-based treatment is still the standard regimen for SCLC no matter for limited stage (LS) SCLC or ES-SCLC. Although the initial treatment has a high tumor response rate, patients are prone to chemoresistance, which may be related to intratumoral heterogeneity and molecular complexity.5–7 Thoracic radiotherapy combined with chemotherapy helps to improve the tumor control rate and survival rate of patients with LS-SCLC and has always been recommended as the standard treatment mode.8,9 But even with active treatment, LS-SCLC has a median overall survival (OS) of less than 30 months and 5-year survival rates of 20%–30%.10,11
As a highly malignant tumor, SCLC requires the exploration of new treatment modalities to improve prognosis. In recent years, immune checkpoint inhibitors (ICIs) have made modest progress in ES-SCLC, and IMpower133 and CASPIAN results showed that chemotherapy combined with atezolizumab or durvalumab prolonged the survival outcome of ES-SCLC compared with chemotherapy alone.12,13 The STIMULI trial showed that consolidation of dual immune checkpoint inhibition after concurrent chemoradiation with LS-SCLC did not improve progression-free survival (PFS) and OS compared with standard regimens. 14 However, the recent ADRIATIC study demonstrated a favorable survival benefit for durvalumab compared with placebo as consolidation therapy following concurrent chemoradiation for LS-SCLC. 15 In addition to immune consolidation therapy, the addition of immunotherapy to induction therapy is another exploratory paradigm for LS-SCLC. A phase I/II trial found that concurrent chemoradiotherapy combined with pembrolizumab achieved good efficacy and safety in LS-SCLC. 16 A series of phase II and III studies exploring chemoradiotherapy combined with immunotherapy are being carried out, and based on the status of chemoradiotherapy in LS-SCLC, how to effectively combine it with immunotherapy is the focus of future research. 17
Since the approval of immunotherapy in ES-SCLC, immunotherapy in patients with LS-SCLC has been prescribed sometimes in clinical practice. Currently, data on the efficacy of immunotherapy in LS-SCLC are lacking and it is yet unclear whether immuno-combined radiotherapy is better or not. This study proposed to investigate the best treatment options for LS-SCLC patients by analyzing the different treatment patterns.
Methods
Study design and patient participation
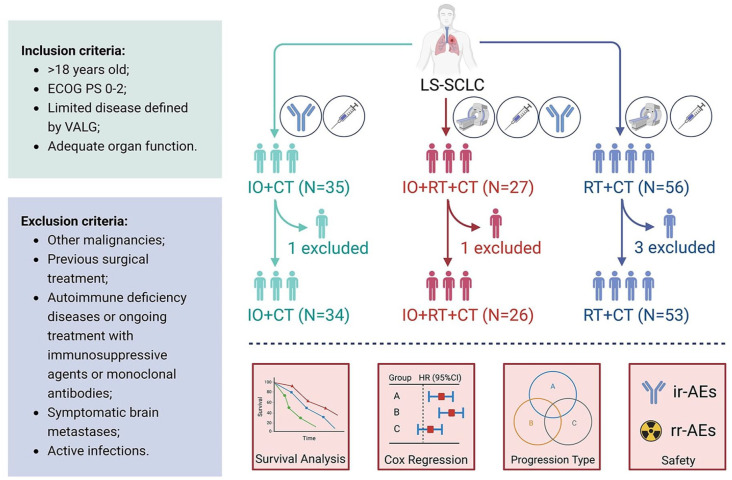
This was a retrospective study comparing the efficacy of immuno-combined treatment versus radio-combined treatment in patients with LS-SCLC, which was approved by the Institutional Review Board of Beijing Chest Hospital, Capital Medical University. We retrospectively reviewed patients with histopathologically or pathologically diagnosed SCLC in our hospital from January 2019 to December 2023, intrathoracic disease surrounded by a single radiation field, and no metastasis at other distant sites defined as LS-SCLC. Patients were included if they were >18 years of age, had an Eastern Cooperative Oncology Group (ECOG) performance status of 0–2, had limited disease as defined by the VALSG, and had adequate organ function. At the same time, the following patients were excluded: other malignancies; previous surgical treatment; autoimmune deficiency diseases or ongoing treatment with immunosuppressive agents or monoclonal antibodies; symptomatic brain metastases; and active infections. The reporting of this study conforms to the STROBE statement. 18
Treatment and follow-up
Patients received four to six cycles of chemotherapy, with the main chemotherapy regimen consisting of etoposide (100 mg/m2, intravenous infusion, days 1–3) combined with cisplatin (75 mg/m2, intravenous infusion, day 1) or carboplatin (AUC = 5–6, intravenous infusion, day 1). Radiotherapy (45 Gy, 1.5 Gy, once a day/3 weeks) was performed during chemotherapy, and thoracic radiotherapy was started as concurrent chemoradiotherapy in the early stage and sequential chemoradiotherapy in the late stage of chemotherapy. Immunotherapy regimens involved in this study included anti-PD-L1 (atezolizumab and durvalumab) and PD-1 antibodies (sintilimab, tislelizumab, camrelizumab, and serplulimab), and immunotherapy was started concurrently with chemotherapy, with the continuation of immunotherapy after six cycles at the discretion of the treating physician and the patient for a maximum of 12 months. In addition, prophylactic cranial irradiation (PCI) (25 Gy/2.5 Gy/10 f) was allowed at the discretion of the attending physician. There may be patients with LS-SCLC who cannot tolerate or refuse radiotherapy in clinical practice, and some patients are scheduled for immunochemotherapy translational research, so such patients do not receive standard chemoradiotherapy. The patient cohorts were divided into three groups according to the initial treatment regimen: immunotherapy combined chemotherapy (IO + CT), immunotherapy combined chemoradiotherapy (IO + RT + CT), and chemoradiotherapy (RT + CT).
Demographic and tumor imaging data were collected before treatment, and serum albumin (ALB), lactate dehydrogenase (LDH), C-reactive protein (CRP), and tumor markers including neuron-specific enolase (NSE) and progastrin-releasing peptide (Pro-GRP) were also recorded, and neutrophil-to-lymphocyte ratio (NLR) and derived NLR (dNLR, neutrophil count/(white blood cell count − neutrophil count)), systemic inflammatory index (SII, neutrophil count * platelet count/lymphocyte count) were calculated.19,20
The primary objective of this study was to compare the tumor response and patient survival time of the first-line regimen of LS-SCLC. The initial time was determined as the date of initiation of chemotherapy, PFS ended on the date of progressive disease (PD) documented in RECIST v1.1 or last follow-up, and OS ended on the date of death or last follow-up. Tumor response was documented and objective response rate (ORR) and disease control rate (DCR) were calculated. ORR was defined as the proportion of patients with complete response (CR) and partial response (PR) after tumor treatment; DCR was defined as the proportion of patients with response (CR + PR) and stable disease (SD). Safety is also an outcome, and the Common Terminology Criteria for Adverse Events version 5.0 describes the type and severity of the safety profile, focusing on immune-related adverse events (ir-AEs) and radiation-related adverse events (rr-AEs). Patients were followed every 3 months, and tumor information of the chest and abdomen, brain, and bone was examined by a variety of imaging modalities to record progression. Also, for patients who progressed after treatment, we documented the pattern of disease progression and treatment after progression.
Statistical analyses
All statistical analyses were performed using R (version 4.0.5, R Core Team, New Zealand). Variables were described by frequency (proportion) or median (interquartile range), and differences between groups were compared using the Chi-square test and t-test or Mann–Whitney U test. Univariate Cox regression was used to analyze the association between clinical parameters and patient survival, and relevant variables with p < 0.1 were included in multivariate Cox to explore independent factors for LS-SCLC. Kaplan–Meier analysis and Log-Rank test were used to compare survival data between cohorts. For clusters with little difference in prognosis, we performed subgroup analyses to identify populations with potential benefits. p < 0.05 was considered statistically significant.
Results
Patient characteristics
From January 2019 to December 2023, 118 patients were screened and 113 patients with LS-SCLC were finally included. Patients’ baseline characteristics are shown in Table 1, and we provided treatment information for ICIs (Table S1). Median age was 64 years (range 57–68 years); 83 (73.5%) males and 30 (26.5%) females. Most patients were current or former smokers (n = 76, 67.3%) and had an ECOG score of 0–1 (n = 110, 97.3%). Of these, only six patients were treated with PCI, and the most common immune regimens were atezolizumab (n = 14) and durvalumab (n = 20). All patients received at least one cycle of platinum-based chemotherapy and were divided into IO + CT (n = 34), IO + RT + CT (n = 26), and RT + CT (n = 53) groups according to the use of immune-combined and radio-combined treatment, and the baseline characteristics of patients were balanced among the three groups (Table 1, Table S2, and Figure 1).
Table 1.
Baseline characteristics by treatment group for patients with limited-stage small-cell lung cancer.
| Characteristics | Overall (N = 113) | IO + CT (N = 34) | IO + RT + CT (N = 26) | RT + CT (N = 53) | p |
|---|---|---|---|---|---|
| Sex (%) | 0.447 | ||||
| Female | 30 (26.5) | 7 (20.6) | 6 (23.1) | 17 (32.1) | |
| Male | 83 (73.5) | 27 (79.4) | 20 (76.9) | 36 (67.9) | |
| Smoke (%) | 0.728 | ||||
| Former/current | 76 (67.3) | 23 (67.6) | 19 (73.1) | 34 (64.2) | |
| Never/unknown | 37 (32.7) | 11 (32.4) | 7 (26.9) | 19 (35.8) | |
| ECOG (%) | 0.872 | ||||
| PS0-1 | 110 (97.3) | 33 (97.1) | 25 (96.2) | 52 (98.1) | |
| PS2 | 3 (2.7) | 1 (2.9) | 1 (3.8) | 1 (1.9) | |
| T (%) | 0.576 | ||||
| T1 | 10 (8.8) | 3 (8.8) | 2 (7.7) | 5 (9.4) | |
| T2 | 41 (36.3) | 13 (38.2) | 13 (50.0) | 15 (28.3) | |
| T3 | 25 (22.1) | 6 (17.6) | 6 (23.1) | 13 (24.5) | |
| T4 | 37 (32.7) | 12 (35.3) | 5 (19.2) | 20 (37.7) | |
| N (%) | 0.242 | ||||
| N0 | 8 (7.1) | 1 (2.9) | 1 (3.8) | 6 (11.3) | |
| N1 | 16 (14.2) | 5 (14.7) | 2 (7.7) | 9 (17.0) | |
| N2 | 70 (61.9) | 23 (67.6) | 15 (57.7) | 32 (60.4) | |
| N3 | 19 (16.8) | 5 (14.7) | 8 (30.8) | 6 (11.3) | |
| TNM (%) | 0.115 | ||||
| I–II | 16 (14.2) | 4 (11.8) | 1 (3.8) | 11 (20.8) | |
| III | 97 (85.8) | 30 (88.2) | 25 (96.2) | 42 (79.2) | |
| PCI (%) | 6 (5.3) | 2 (5.9) | 1 (3.8) | 3 (5.7) | 0.930 |
| Age (median (IQR)) | 64.0 (57.0, 68.0) | 64.0 (56.0, 66.0) | 61.0 (56.2, 66.8) | 65.0 (58.0, 69.0) | 0.299 |
| BMI (median (IQR)) | 25.4 (23.6, 27.0) | 25.2 (24.2, 27.8) | 25.4 (22.6, 26.1) | 25.7 (23.6, 27.0) | 0.567 |
| ALB (median (IQR)) | 41.2 (38.1, 42.7) | 41.2 (38.7, 42.6) | 41.0 (38.8, 42.7) | 40.9 (38.0, 42.8) | 0.954 |
| LDH (median (IQR)) | 173.0 (153.0, 205.0) | 162.0 (151.0, 177.0) | 184.5 (154.5, 205.0) | 183.0 (158.0, 208.0) | 0.142 |
| CRP (median (IQR)) | 3.6 (1.2, 9.5) | 4.2 (1.3, 10.0) | 4.6 (1.7, 11.1) | 3.4 (1.1, 6.9) | 0.604 |
| NLR (median (IQR)) | 2.3 (1.7, 3.3) | 2.4 (1.6, 3.6) | 2.2 (1.8, 2.9) | 2.4 (1.8, 3.3) | 0.972 |
| SII (median (IQR)) | 566.0 (376.0, 779.0) | 569.5 (344.0, 732.8) | 562.0 (471.0, 735.2) | 560.0 (376.0, 841.0) | 0.658 |
| dNLR (median (IQR)) | 1.7 (1.3, 2.2) | 1.8 (1.1, 2.4) | 1.7 (1.4, 1.9) | 1.7 (1.3, 2.3) | 0.946 |
| NSE (median (IQR)) | 22.3 (15.1, 32.6) | 21.9 (14.1, 28.0) | 17.9 (13.3, 28.1) | 26.6 (17.2, 40.6) | 0.191 |
| pro-GRP (median (IQR)) | 549.5 (130.4, 1388.0) | 489.9 (101.8, 1306.9) | 860.7 (117.2, 2527.2) | 460.3 (153.2, 1373.6) | 0.559 |
ALB, albumin; BMI, body mass index; CRP, C-reactive protein; CT, chemotherapy; dNLR, derived neutrophil-to-lymphocyte ratio; ECOG PS, Eastern Cooperative Oncology Group performance status; IO, immunotherapy; IQR, Interquartile range; LDH, lactate dehydrogenase; NLR, neutrophil-to-lymphocyte ratio; NSE, neuron-specific enolase; PCI, prophylactic cranial irradiation; pro-GRP, progastrin-releasing peptide; RT, radiotherapy; SII, systemic inflammatory index.
Figure 1.
Flow chart of the study design. Patients with LS-SCLC were screened according to established inclusion and exclusion criteria and divided into immune combined chemotherapy group (N = 34), immune combined chemoradiotherapy group (N = 26), and chemoradiotherapy group (N = 53) according to the treatment used. Survival information, prognostic factors, progression patterns, and safety of patients were also recorded and analyzed.
CT, chemotherapy; ECOG PS, Eastern Cooperative Oncology Group performance status; IO, immunotherapy; ir-AE, immune-related adverse event; LS-SCLC, limited-stage small-cell lung cancer; rr-AE, radiation-related adverse event; RT, radiotherapy; VALG, Veterans Administration Lung Study Group.
Efficacy analysis
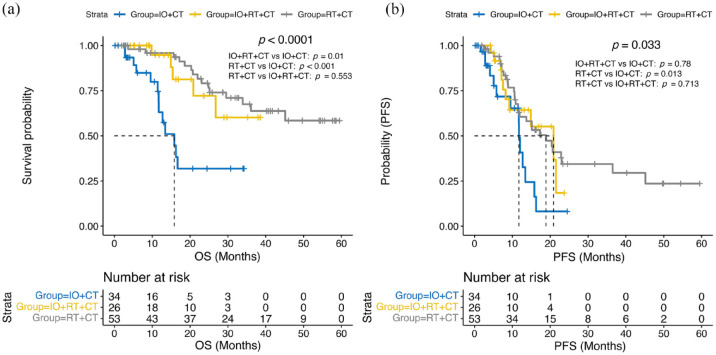
As of March 2024, 33 patients had died with median OS not reached (NR) (95% confidence interval (CI): 29.5–NR); 53 patients had disease progression or died with median PFS of 15.1 months (95% CI: 11.9–20.9). Median OS was 15.8 months (95% CI: 11.7–NR) in the IO + CT group and NR in the IO + RT + CT group (95% CI: 26.8–NR), nor the RT + CT group (95% CI: 36.1–NR) (Figure 2(a) and Table S3). Univariate Cox regression analysis showed that the hazard ratio (HR) for disease death in the IO + RT + CT group was 0.26 (95% CI, 0.09–0.72; p = 0.01) compared with IO + CT, while the HR for death in the RT + CT group was 0.21 (95% CI, 0.09–0.46; p < 0.001; Table 2). The median PFS for patients receiving immunotherapy, immune combined with radiotherapy, and radiotherapy during chemotherapy was 11.7 (95% CI, 9.5–NR), 20.9 (95% CI, 9.17–NR), and 18.9 (95% CI, 11.9–45.1) months, respectively (Figure 2(b) and Table S3), and the HRs for disease progression for chemoradiotherapy combined with immunotherapy and chemoradiotherapy compared with chemotherapy combined with immunotherapy were 0.48 (95% CI, 0.21–1.09; p = 0.078) and 0.43 (95% CI, 0.22–0.84; p = 0.013), respectively (Table 2). Patients receiving radiotherapy tended to have longer OS (p < 0.0001) and PFS (p = 0.0094) compared with LS-SCLC without radiotherapy (Figure S1).
Figure 2.
(a) KM curves of OS in different treatment groups of patients with LS-SCLC. (b) KM curves of PFS in different treatment groups of patients with LS-SCLC.
CT, chemotherapy; IO, immunotherapy; LS-SCLC, limited-stage small-cell lung cancer; OS, overall survival; PFS, progression-free survival; RT, radiotherapy.
Table 2.
Univariate Cox regression analysis of overall survival and progression-free survival in patients with limited-stage small-cell lung cancer.
| Characteristics | Overall survival | Progression-free survival | ||
|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | |
| Group (IO + RT + CT vs IO + CT) | 0.26 (0.09–0.72) | 0.01 | 0.48 (0.21–1.09) | 0.078 |
| Group (RT + CT vs IO + CT) | 0.21 (0.09–0.46) | <0.001 | 0.43 (0.22–0.84) | 0.013 |
| Sex (male vs female) | 1.26 (0.55–2.9) | 0.59 | 0.88 (0.48–1.61) | 0.681 |
| Age | 1.01 (0.97–1.05) | 0.576 | 1.01 (0.97–1.04) | 0.69 |
| Smoke (never/unknown vs former/current) | 0.92 (0.43–1.98) | 0.835 | 1.21 (0.68–2.16) | 0.517 |
| BMI | 1.04 (0.94–1.16) | 0.459 | 1.02 (0.93–1.11) | 0.706 |
| T (T3–4 vs T1–2) | 0.86 (0.43–1.71) | 0.66 | 0.9 (0.52–1.55) | 0.703 |
| N (N1-3 vs N0) | 3.15 (0.43–23.04) | 0.259 | 1.65 (0.4–6.81) | 0.49 |
| TNM (III vs I-II) | 1.25(0.44–3.57) | 0.673 | 0.79 (0.35–1.78) | 0.575 |
| ALB | 1.04 (0.94–1.15) | 0.446 | 0.989 (0.913–1.07) | 0.775 |
| LDH | 1 (0.997–1.003) | 0.865 | 1.001 (0.999–1.003) | 0.311 |
| CRP | 1.013 (0.999–1.028) | 0.069 | 1.007 (0.994–1.02) | 0.311 |
| NLR | 1.193 (0.951–1.496) | 0.128 | 1.142 (0.944–1.38) | 0.172 |
| SII | 1.001 (1–1.002) | 0.194 | 1.001 (1–1.001) | 0.097 |
| dNLR | 1.405 (1.012–1.951) | 0.042 | 1.246 (0.938–1.655) | 0.129 |
| NSE | 1 (0.991–1.009) | 0.96 | 1.006 (0.999–1.013) | 0.071 |
| pro-GRP | 1 (1–1) | 1 | 1 (1–1) | 0.647 |
ALB, albumin; BMI, body mass index; CI, confidence interval; CRP, C-reactive protein; CT, chemotherapy; dNLR, derived neutrophil-to-lymphocyte ratio; HR, hazard ratio; IO, immunotherapy; LDH, lactate dehydrogenase; NLR, neutrophil-to-lymphocyte ratio; NSE, neuron-specific enolase; pro-GRP, progastrin-releasing peptide; RT, radiotherapy; SII, systemic inflammatory index.
A total of 79 patients were treated with combined thoracic radiation, and the median OS and median PFS were NR in patients treated with concurrent chemoradiotherapy and sequential chemoradiotherapy, and no statistically significant differences were observed between the two groups (p = 0.23 and p = 0.82; Figure S2). With a total of 60 LS-SCLC patients receiving immuno-combined treatment therapy, median OS (NR vs 15.4 months, p = 0.091) and median PFS (14.9 vs 12.7 months, p = 0.19) were longer in the cohort of PD-L1 inhibitor-treated patients than PD-1 inhibitor-treated patients, although this difference did not reach statistical significance (Figure S3). In addition, 10 patients were maintained with immunotherapy after 4–6 cycles of immunochemotherapy, and the median OS and PFS had not yet been reached, while the median OS and median PFS in the group without immunomaintenance were 20.9 and 11.7 months, respectively (Figure S4).
Given that the survival benefit was difficult to discern between IO + RT + CT and RT + CT groups, we performed subgroup analyses to obtain potentially beneficial LS-SCLC patients. Exploratory subgroup analyses showed that survival differences in OS and PFS were not observed in almost all subgroups (Figure S5).
One hundred five patients in the patient cohort were evaluable for response to treatment, and 74.1% of patients achieved an objective response according to RECIST1.1 criteria (PR: N = 75); 28 (26.7%) had SD, 2 (1.9%) had PD, and the DCR was 98.1%. Among 27 patients treated with immune combined chemotherapy, ORR and DCR were 66.7% and 96.3%, respectively; while among 25 patients treated with immune combined chemoradiotherapy, ORR was 60% and DCR was 96%; ORR (79.2%) and DCR (100%) in chemoradiotherapy group were slightly higher than immune-combined groups, but the statistical difference was NR (Table S3).
Prognostic factor analysis
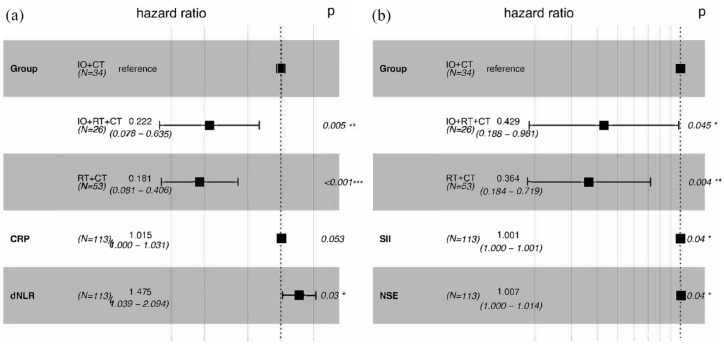
Univariate Cox regression results showed that the treatment group, CRP level, and dNLR were associated with patient survival, and the treatment group, SII, and NSE were associated with disease progression (Table 2). Multivariate analysis suggested that IO + RT + CT (HR: 0.222, 95% CI: 0.078–0.635, p = 0.005) and RT + CT (HR: 0.181, 95% CI: 0.081–0.406, p < 0.001) were independent protective factors for OS compared with IO + CT, similarly, IO + RT + CT (HR: 0.429, 95% CI: 0.188–0.981, p = 0.045) and RT + CT (HR: 0.364, 95% CI: 0.184–0.719, p = 0.004) were also independent predictors of PFS (Figure 3(a)). In addition, patients with higher dNLR had shorter survival times (HR: 1.475, 95% CI: 1.039–2.094, p = 0.03), faster progression in patients with higher SII (HR: 1.001, 95% CI: 1.000–1.001, p < 0.001), and higher NSE levels (HR: 1.007, 95% CI: 1.000–1.014, p = 0.031; Figure 3(b)).
Figure 3.
(a) Forest plot of multivariate Cox regression analysis for overall survival in patients with limited-stage small-cell lung cancer. (b) Forest plot of multivariate Cox regression analysis for progression-free survival in patients with limited-stage small-cell lung cancer.
*** represents p < 0.001, ** represents p < 0.01, while * represents p < 0.05.
CRP, C-reactive protein; CT, chemotherapy; dNLR, derived neutrophil–lymphocyte ratio; IO, immunotherapy; NSE, neuron-specific enolase; RT, radiotherapy; SII, systemic immune-inflammatory index.
Maxstat maximum rank statistic was used to select the cutoff values of the above continuous variables, and the cutoff values of dNLR, SII, and NSE were 2.55, 833, and 24.64, respectively. According to the above cutoff values, the patient population was divided into two groups and Kaplan-Meier curves were plotted (Figure S6).
Progression
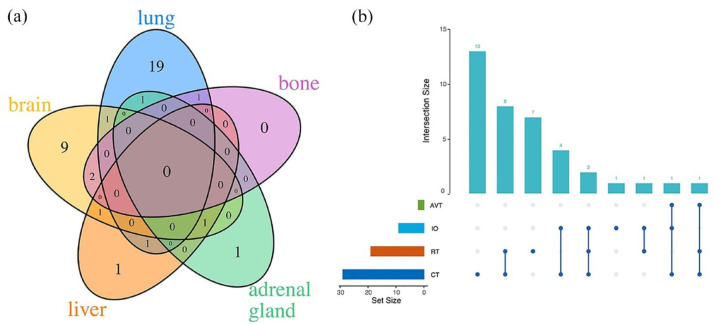
Meanwhile, the pattern of disease progression in patients was documented in Figure 4, with progression occurring mainly locally in the lung (N = 23, 57.5%), and the common sites of distant metastases involved were the brain (N = 14, 35.0%) and liver (N = 4, 10.0%). Most patients developed progression at one site, but eight patients had metastases at multiple sites. In addition, we documented the patient’s treatment regimen after progression, with chemotherapy predominating (N = 13), followed by radiotherapy combined with chemotherapy (N = 8) and radiotherapy alone (N = 7). Table S4 documents the site of progression and treatment after progression in each of the three arms.
Figure 4.
(a) The organs of progression of limited-stage small-cell lung cancer. (b) The treatment regimen after progression of limited-stage small-cell lung cancer.
AVT, antivascular therapy; CT, chemotherapy; IO, immunotherapy; RT, radiotherapy.
Safety
All patients were included in the safety analysis, and Table S5 shows treatment-related toxicities. Four patients experienced ir-AEs, one patient receiving immune combination chemotherapy developed hypothyroidism and neuromuscular disease, and the remaining three were all in the IO + RT + CT group (two hypothyroidism and one allergic reaction). In addition, 34 patients experienced rr-AEs, the most common of which were radiation pneumonitis (N = 24) and radiation esophagitis and pharyngitis (N = 13), no treatment-related deaths were identified, and most AEs were self-limiting and resolved with symptomatic treatment.
Discussion
Median PFS in this cohort of patients was 15.1 months, median OS was not yet reached, but at least longer than 29.5 months, ORR was 74.1%, and DCR was 98.1%, which is consistent with previous studies of active treatment with LS-SCLC.8–11 We speculate on the potential effect of immunotherapy on LS-SCLC based on its better performance in non-small-cell lung cancer (NSCLC) and ES-SCLC, but the conclusions reached by the two prospective studies are inconsistent.16,14 A national cancer database-based study found that immunotherapy, as part of the initial regimen for LS-SCLC, did not prolong patient survival. 21 However, another small study supports anti-PD-L1 as a supplement to chemoradiation for LS-SCLC. 22 The ADRIATIC study rewrote the LS-SCLC treatment landscape to drive more exploration of treatment modalities for LS-SCLC immunotherapy.15,17 Among the three groups in this study, survival was poor in the immune combined chemotherapy group, with median OS and PFS of only 15.8 and 11.7 months, which may be related to the fact that the treatment used in this group could not be compared with the standard regimen. Survival time was longer in either the ICI plus chemoradiotherapy or chemoradiotherapy groups; however, the difference between the two groups was not significant. Our study did not succeed in challenging the standard regimen for LS-SCLC, that is, the addition of immunotherapy did not improve the prognosis of patients.
Subgroup analysis in this study suggests that immunotherapy is not indicated for LS-SCLC with a smoking history, which may contradict previous studies. Smoking is well known to be strongly associated with the development of SCLC, and tobacco-induced high tumor mutation burden (TMB) provides an opportunity for ICI therapy.23–26 However, exploratory subgroup analysis of IMpower 133 did not suggest an association of TMB with the benefit from atezolizumab in ES-SCLC. 12 TMB is not routinely tested clinically, and documentation of smoking history in this study was based on written cases, resulting in a low proportion of SCLC patients with documented smoking history, thus temporarily failing to demonstrate a relationship between smoking and TMB levels and efficacy of immunotherapy and requiring further investigation in SCLC.
Currently, the timing of thoracic radiotherapy for LS-SCLC, the efficacy of PD-L1 versus PD-1 inhibitors, and whether there is immune maintenance are inconclusive. A meta-analysis of seven randomized trials showed that LS-SCLC with early radiotherapy tended to have longer OS, especially when overall treatment time was shorter. 27 Concurrent chemoradiotherapy is associated with better survival than sequential chemoradiotherapy; however, some studies do not support this, and in addition, patient tolerance to toxicities needs to be considered simultaneously.10,28 In addition to serplulimab, other PD-1 inhibitors combined with chemotherapy failed to improve patient survival in ES-SCLC, while PD-L1 combined with chemotherapy has been approved for first-line treatment.12,13,29–31 Another meta-analysis on ES-SCLC showed that the efficacy of the combination chemotherapy was equivalent, but anti-PD-L1 combination chemotherapy tended to reduce the risk of death in the subgroup with brain metastases. 32 Immunologic maintenance therapy with PD-L1 antibodies has now demonstrated efficacy in patients with extensive-stage disease, but consolidation therapy with nivolumab–ipilimumab has not been shown to delay patient progression after chemoradiation in LS-SCLC.12–14 We found that OS and PFS tended to be longer in patients with immune maintenance, but the sample size was small and such results should be interpreted with caution.
It is not only that the treatment modality may affect the survival time of patients, but may also be influenced by other factors, such as inflammatory parameters, patient status, and tumor markers. Inflammatory markers based on blood specimens are closely related to the prognosis of SCLC while reflecting the intensity of systemic anti-tumor immune response. 33 Baseline NLR and dNLR have predictive value for lung cancer immunotherapy, and NLR after 6 weeks of treatment initiation is a biomarker of early response in patients.34–36 As an important component of the tumor microenvironment, neutrophils are involved in the growth and invasion of tumor cells. 37 dNLR was calculated based on leukocyte and neutrophil levels, and this study suggests that the higher the dNLR, the shorter the survival. SII, a biomarker used to assess systemic immune-inflammatory status, has been found to predict prognosis in many types of tumors, and an association with disease progression in SCLC was similarly observed in this study. 19 NSE is convenient to detect using blood and is used as an SCLC-specific tumor marker, and high levels of NSE are associated with shorter OS and PFS in SCLC. 38 This finding was similarly observed in SCLC studies receiving immunotherapy, except that NSE was not truncated in this study and presented as a continuous variable. 39 This may be associated with NSE promoting vascular endothelial growth factor and promoting tumor metastasis, and the underlying mechanism warrants further investigation. 40
This study has the following limitations: first, the retrospective design makes it impossible to obtain tumor biomarkers, although PD-LI expression and TMB levels have not demonstrated their predictive value in SCLC,12,30 and imperfect recording of electronic medical records leads to the occurrence of excursions; second, some patients in our study did not receive standard care because the patient’s physical condition and safety issues need to be considered comprehensively in clinical practice; furthermore, single-center studies have limited interpretation of the results, and sample size should be expanded to analyze differences in efficacy as well as to explore more subpopulations of possible benefit.
Conclusion
Although immunotherapy is widely used in NSCLC and ES-SCLC, its effect has not yet expanded to LS-SCLC. However, our study did not find that the addition of immunotherapy prolonged survival in patients with LS-SCLC receiving standard chemoradiotherapy, but further exploration of the management of this combination modality in multicenter randomized clinical trials is needed.
Supplemental Material
Supplemental material, sj-docx-1-tam-10.1177_17588359241307191 for Immuno-combined treatment versus radio-combined treatment in limited-stage small-cell lung cancer by Li Tong, Xiaomi Li, Mingming Hu, Minghang Zhang, Yishuo Wang, Kai Zhang, Qunhui Wang, Tongmei Zhang and Baolan Li in Therapeutic Advances in Medical Oncology
Supplemental material, sj-docx-2-tam-10.1177_17588359241307191 for Immuno-combined treatment versus radio-combined treatment in limited-stage small-cell lung cancer by Li Tong, Xiaomi Li, Mingming Hu, Minghang Zhang, Yishuo Wang, Kai Zhang, Qunhui Wang, Tongmei Zhang and Baolan Li in Therapeutic Advances in Medical Oncology
Acknowledgments
We thank all participants for their endeavors and contributions to this study. In addition, we thank the website for Figure 1 (https://www.biorender.com/).
Footnotes
ORCID iD: Tongmei Zhang  https://orcid.org/0000-0003-4271-3773
https://orcid.org/0000-0003-4271-3773
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Li Tong, Department of Oncology, Beijing Chest Hospital, Capital Medical University, Beijing, China; Laboratory for Clinical Medicine, Capital Medical University, Beijing, China.
Xiaomi Li, Department of Oncology, Beijing Chest Hospital, Capital Medical University, Beijing, China; Department of Oncology, Beijing Tuberculosis and Thoracic Tumor Research Institute, Beijing, China.
Mingming Hu, Department of Oncology, Beijing Chest Hospital, Capital Medical University, Beijing, China; Laboratory for Clinical Medicine, Capital Medical University, Beijing, China.
Minghang Zhang, Department of Oncology, Beijing Chest Hospital, Capital Medical University, Beijing, China.
Yishuo Wang, Department of Oncology, Beijing Chest Hospital, Capital Medical University, Beijing, China.
Kai Zhang, Department of Oncology, Beijing Chest Hospital, Capital Medical University, Beijing, China; Department of Oncology, Beijing Tuberculosis and Thoracic Tumor Research Institute, Beijing, China.
Qunhui Wang, Department of Oncology, Beijing Tuberculosis and Thoracic Tumor Research Institute, Beijing Chest Hospital, Capital Medical University, No. 9 Beiguan Street, Tongzhou District, Beijing 101149, China; Laboratory for Clinical Medicine, Capital Medical University, Beijing, China.
Tongmei Zhang, Department of Oncology, Beijing Tuberculosis and Thoracic Tumor Research Institute, Beijing Chest Hospital, Capital Medical University, No. 9 Beiguan Street, Tongzhou District, Beijing 101149, China; Laboratory for Clinical Medicine, Capital Medical University, Beijing, China.
Baolan Li, Department of Oncology, Beijing Tuberculosis and Thoracic Tumor Research Institute, Beijing Chest Hospital, Capital Medical University, No. 9 Beiguan Street, Tongzhou District, Beijing 101149, China; Laboratory for Clinical Medicine, Capital Medical University, Beijing, China.
Declarations
Ethics approval and consent to participate: The study was approved by the Ethics Committees of Beijing Chest Hospital, Capital Medical University, Capital Medical University before data collection began (YJS-2022-19). Given its retrospective nature, the institutional committees waived informed consent for the study.
Consent for publication: Not applicable.
Author contributions: Li Tong: Conceptualization; Data curation; Writing – original draft.
Xiaomi Li: Data curation; Formal analysis; Writing – original draft.
Mingming Hu: Data curation; Formal analysis.
Minghang Zhang: Data curation.
Yishuo Wang: Software; Supervision.
Kai Zhang: Methodology; Resources.
Qunhui Wang: Project administration; Writing – review & editing.
Tongmei Zhang: Conceptualization; Writing – review & editing.
Baolan Li: Conceptualization; Writing – review & editing.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Beijing Municipal Public Welfare Development and Reform Pilot Project for Medical Research Institutes (grant number JYY2023-14 and JYY2023-15) to Tongmei Zhang.
The authors declare that there is no conflict of interest.
Availability of data and materials: The corresponding author can provide the datasets used and/or analyzed during the current study upon reasonable request.
References
- 1. Zou K, Sun P, Huang H, et al. Etiology of lung cancer: evidence from epidemiologic studies. J Natl Cancer Center 2022; 2(4): 216–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2022. CA Cancer J Clin 2022; 72(1): 7–33. [DOI] [PubMed] [Google Scholar]
- 3. Byers LA, Rudin CM. Small cell lung cancer: where do we go from here? Cancer 2015; 121(5): 664–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Micke P, Faldum A, Metz T, et al. Staging small cell lung cancer: Veterans Administration Lung Study Group versus International Association for the Study of Lung Cancer—what limits limited disease? Lung Cancer 2002; 37(3): 271–276. [DOI] [PubMed] [Google Scholar]
- 5. Rudin CM, Brambilla E, Faivre-Finn C, et al. Small-cell lung cancer. Nat Rev Dis Primers 2021; 7(1): 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wang J, Zhou C, Yao W, et al. Adebrelimab or placebo plus carboplatin and etoposide as first-line treatment for extensive-stage small-cell lung cancer (CAPSTONE-1): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2022; 23(6): 739–747. [DOI] [PubMed] [Google Scholar]
- 7. Gay CM, Stewart CA, Park EM, et al. Patterns of transcription factor programs and immune pathway activation define four major subtypes of SCLC with distinct therapeutic vulnerabilities. Cancer Cell 2021; 39(3): 346–360.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Warde P, Payne D. Does thoracic irradiation improve survival and local control in limited-stage small-cell carcinoma of the lung? A meta-analysis. J Clin Oncol 1992; 10(6): 890–895. [DOI] [PubMed] [Google Scholar]
- 9. Pignon JP, Arriagada R, Ihde DC, et al. A meta-analysis of thoracic radiotherapy for small-cell lung cancer. N Engl J Med 1992; 327(23): 1618–1624. [DOI] [PubMed] [Google Scholar]
- 10. Takada M, Fukuoka M, Kawahara M, et al. Phase III study of concurrent versus sequential thoracic radiotherapy in combination with cisplatin and etoposide for limited-stage small-cell lung cancer: results of the Japan Clinical Oncology Group Study 9104. J Clin Oncol 2002; 20(14): 3054–3060. [DOI] [PubMed] [Google Scholar]
- 11. Gaspar LE, Gay EG, Crawford J, et al. Limited-stage small-cell lung cancer (stages I–III): observations from the National Cancer Data Base. Clin Lung Cancer 2005; 6(6): 355–360. [DOI] [PubMed] [Google Scholar]
- 12. Horn L, Mansfield AS, Szczęsna A, et al. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med 2018; 379(23): 2220–2229. [DOI] [PubMed] [Google Scholar]
- 13. Goldman JW, Dvorkin M, Chen Y, et al. Durvalumab, with or without tremelimumab, plus platinum-etoposide versus platinum-etoposide alone in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): updated results from a randomised, controlled, open-label, phase 3 trial. Lancet Oncol 2021; 22(1): 51–65. [DOI] [PubMed] [Google Scholar]
- 14. Peters S, Pujol JL, Dafni U, et al. Consolidation nivolumab and ipilimumab versus observation in limited-disease small-cell lung cancer after chemo-radiotherapy—results from the randomised phase II ETOP/IFCT 4-12 STIMULI trial. Ann Oncol 2022; 33(1): 67–79. [DOI] [PubMed] [Google Scholar]
- 15. Cheng Y, Spigel DR, Cho BC, et al. Durvalumab after chemoradiotherapy in limited-stage small-cell lung cancer. N Engl J Med 2024; 391(14): 1313–1327. [DOI] [PubMed] [Google Scholar]
- 16. Welsh JW, Heymach JV, Guo C, et al. Phase 1/2 trial of pembrolizumab and concurrent chemoradiation therapy for limited-stage SCLC. J Thorac Oncol 2020; 15(12): 1919–1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schlick B, Shields MD, Marin-Acevedo JA, et al. Immune checkpoint inhibitors and chemoradiation for limited-stage small cell lung cancer. Curr Treat Options Oncol 2022; 23(8): 1104–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med 2007; 147(8): 573–577. [DOI] [PubMed] [Google Scholar]
- 19. Anpalakhan S, Signori A, Cortellini A, et al. Using peripheral immune-inflammatory blood markers in tumors treated with immune checkpoint inhibitors: an INVIDIa-2 study sub-analysis. iScience 2023; 26(11): 107970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yang T, Hao L, Yang X, et al. Prognostic value of derived neutrophil-to-lymphocyte ratio (dNLR) in patients with non-small cell lung cancer receiving immune checkpoint inhibitors: a meta-analysis. BMJ Open 2021; 11(9): e049123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bilani N, Alley E, Elson L, et al. Effect of immunotherapy on overall survival in limited-stage small cell lung carcinoma: a national cancer database analysis. Ther Adv Med Oncol 2021; 13: 1758835920982806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yao N, Qin Z, Ma J, et al. Anti-programmed death ligand 1 immunotherapy in patients with limited-stage small cell lung cancer: a real-world exploratory study. J Chemother 2023; 35(5): 448–454. [DOI] [PubMed] [Google Scholar]
- 23. Varghese AM, Zakowski MF, Yu HA, et al. Small-cell lung cancers in patients who never smoked cigarettes. J Thorac Oncol 2014; 9(6): 892–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rizvi NA, Hellmann MD, Snyder A, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 2015; 348(6230): 124–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hellmann MD, Callahan MK, Awad MM, et al. Tumor mutational burden and efficacy of nivolumab monotherapy and in combination with ipilimumab in small-cell lung cancer. Cancer Cell 2018; 33(5): 853–861.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Liu Q, Zhang J, Guo C, et al. Proteogenomic characterization of small cell lung cancer identifies biological insights and subtype-specific therapeutic strategies. Cell 2024; 187(1): 184–203.e28. [DOI] [PubMed] [Google Scholar]
- 27. De Ruysscher D, Pijls-Johannesma M, Vansteenkiste J, et al. Systematic review and meta-analysis of randomised, controlled trials of the timing of chest radiotherapy in patients with limited-stage, small-cell lung cancer. Ann Oncol 2006; 17(4): 543–552. [DOI] [PubMed] [Google Scholar]
- 28. Kassik MT, Vordermark D, Kornhuber C, et al. Factors associated with overall survival, progression-free survival and toxicity in patients with small cell lung cancer and thoracic irradiation in a clinical real-world setting. Radiat Oncol 2023; 18(1): 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cheng Y, Han L, Wu L, et al. Effect of first-line serplulimab vs placebo added to chemotherapy on survival in patients with extensive-stage small cell lung cancer: the ASTRUM-005 randomized clinical trial. JAMA 2022; 328(12): 1223–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rudin CM, Awad MM, Navarro A, et al. Pembrolizumab or placebo plus etoposide and platinum as first-line therapy for extensive-stage small-cell lung cancer: randomized, double-blind, phase III KEYNOTE-604 study. J Clin Oncol 2020; 38(21): 2369–2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Spigel DR, Vicente D, Ciuleanu TE, et al. Second-line nivolumab in relapsed small-cell lung cancer: CheckMate 331. Ann Oncol 2021; 32(5): 631–641. [DOI] [PubMed] [Google Scholar]
- 32. Yu H, Chen P, Cai X, et al. Efficacy and safety of PD-L1 inhibitors versus PD-1 inhibitors in first-line treatment with chemotherapy for extensive-stage small-cell lung cancer. Cancer Immunol Immunother 2022; 71(3): 637–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hiam-Galvez KJ, Allen BM, Spitzer MH. Systemic immunity in cancer. Nat Rev Cancer 2021; 21(6): 345–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Russo A, Russano M, Franchina T, et al. Neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), and outcomes with nivolumab in pretreated non-small cell lung cancer (NSCLC): a large retrospective multicenter study. Adv Ther 2020; 37(3): 1145–1155. [DOI] [PubMed] [Google Scholar]
- 35. Suh KJ, Kim SH, Kim YJ, et al. Post-treatment neutrophil-to-lymphocyte ratio at week 6 is prognostic in patients with advanced non-small cell lung cancers treated with anti-PD-1 antibody. Cancer Immunol Immunother 2018; 67(3): 459–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Xiong Q, Huang Z, Xin L, et al. Post-treatment neutrophil-to-lymphocyte ratio (NLR) predicts response to anti-PD-1/PD-L1 antibody in SCLC patients at early phase. Cancer Immunol Immunother 2021; 70(3): 713–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mantovani A, Marchesi F, Jaillon S, et al. Tumor-associated myeloid cells: diversity and therapeutic targeting. Cell Mol Immunol 2021; 18(3): 566–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhou M, Wang Z, Yao Y, et al. Neuron-specific enolase and response to initial therapy are important prognostic factors in patients with small cell lung cancer. Clin Transl Oncol 2017; 19(7): 865–873. [DOI] [PubMed] [Google Scholar]
- 39. Li L, Zhang Z, Hu Y. Neuron-specific enolase predicts the prognosis in advanced small cell lung cancer patients treated with first-line PD-1/PD-L1 inhibitors. Medicine (Baltimore) 2021; 100(36): e27029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Liu X, Liu S, Fu J, et al. Knockdown of neuron-specific enolase suppresses the proliferation and migration of NCI-H209 cells. Oncol Lett 2019; 18(5): 4809–4815. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tam-10.1177_17588359241307191 for Immuno-combined treatment versus radio-combined treatment in limited-stage small-cell lung cancer by Li Tong, Xiaomi Li, Mingming Hu, Minghang Zhang, Yishuo Wang, Kai Zhang, Qunhui Wang, Tongmei Zhang and Baolan Li in Therapeutic Advances in Medical Oncology
Supplemental material, sj-docx-2-tam-10.1177_17588359241307191 for Immuno-combined treatment versus radio-combined treatment in limited-stage small-cell lung cancer by Li Tong, Xiaomi Li, Mingming Hu, Minghang Zhang, Yishuo Wang, Kai Zhang, Qunhui Wang, Tongmei Zhang and Baolan Li in Therapeutic Advances in Medical Oncology