Abstract
Background
Pancreatic ductal adenocarcinoma (PDAC) represents a major clinical challenge due to its tumor microenvironment, which exhibits immune-suppressive properties that facilitate cancer progression, metastasis, and therapy resistance. Interleukin 1 (IL-1) signaling has been implicated as a driver in this process. Mechanistically, both IL-1α and IL-1β bind to the IL-1 receptor type 1, forming a complex with IL-1-receptor accessory protein (IL1RAP), which triggers downstream signaling pathways. The IL1RAP blocking antibody nadunolimab is currently in clinical development, but the precise consequences of inhibiting IL-1 signaling in PDAC remains elusive.
Methods
To evaluate the biological relevance of blocking IL1RAP using nadunolimab in a PDAC animal model, human PDAC cells and cancer-associated fibroblasts (CAFs) were co-transplanted into mice. To study the underlying mechanisms of IL1RAP blockade ex vivo, co-cultured PDAC cells and CAFs were treated with nadunolimab prior to RNA sequencing. Migration assays were performed to assess how nadunolimab affects interactions between CAFs and myeloid immune cells. Finally, to establish a clinical correlation between IL1RAP expression and nadunolimab treatment effects, we analyzed tumor biopsies from a clinical phase I/II study in which nadunolimab was administered to patients.
Results
In the xenograft mouse model, nadunolimab exhibited antitumor effects only when human CAFs were co-transplanted with PDAC cells. IL-1 stimulation induced CAFs to secrete chemokines that recruited neutrophils and monocytes. The secretion of this chemokine and the migration of myeloid cells were inhibited by nadunolimab. Media conditioned by IL-1-stimulated CAFs sustained a neutrophil population with a tissue invasion phenotype, an effect that was reversed by nadunolimab. In a cohort of metastatic late-stage PDAC patients receiving nadunolimab as monotherapy, high IL1RAP expression in tumors was associated with extended progression-free survival.
Conclusions
Our study demonstrates that targeting IL1RAP on CAFs inhibits IL-1-induced chemokine secretion and recruitment of neutrophils and monocytes, thereby counteracting the immunosuppressive microenvironment in PDAC. These findings highlight the therapeutic potential of targeting IL1RAP in PDAC.
Keywords: Neutrophil, Cytokine, Monoclonal antibody, Monocyte
WHAT IS ALREADY KNOWN ON THIS TOPIC
IL-1 contributes to the formation of the immunosuppressive tumor microenvironment of pancreatic ductal adenocarcinoma (PDAC).
IL1RAP is a coreceptor for the IL-1 receptor and a candidate therapeutic target in PDAC.
WHAT THIS STUDY ADDS
Antibody-based targeting of IL1RAP blocks IL-1α and IL-1β signaling in cancer-associated fibroblasts, which prevents the release of chemokines and myeloid cell chemotaxis.
While high IL1RAP expression in PDAC is associated with shorter survival in patients treated with standard of care, it was linked to prolonged progression-free survival in patients that received the IL1RAP-targeting antibody nadunolimab.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
This study increases our understanding of how IL1RAP blockade disrupts the immunosuppressive microenvironment in PDAC, highlighting the potential of IL1RAP inhibition as a promising therapeutic approach for treatment of PDAC patients.
Background
Pancreatic cancer is a highly aggressive disease associated with poor outcomes. The predominant form of pancreatic cancer is pancreatic ductal adenocarcinoma (PDAC), which has a 5-year survival below 5%.1 Within the complex tumor microenvironment (TME), cancer-associated fibroblasts (CAFs) play a crucial role, contributing substantially to the tumor’s composition and behavior. Among several functions, the CAFs secrete cytokines and growth factors that promote tumor cell proliferation, survival, invasiveness, and therapy resistance.2,4 The pancreatic TME also comprises infiltrating immune cells dominated by myeloid cells such as neutrophils and monocytes/macrophages, while cytotoxic T cells are sparse.2
Interleukin-1 (IL-1) signaling plays a key role in shaping the TME by regulating CAF behavior.5,8 However, a consensus on whether primarily IL-1α or IL-1β regulates CAFs in PDAC has still not been reached.5,11 Downstream mediators of IL-1 signaling, such as IL-6 and LIF, promote the inflammatory TME by regulating cytokine secretion, supporting processes like angiogenesis and immune regulation as well as promoting metastasis.3 5 12 13 Mechanistically, IL-1 signals through a receptor heterodimer of interleukin-1 receptor type I (IL1R1) and interleukin-1 receptor accessory protein (IL1RAP), ultimately leading to NFκB activation.14,17 IL1RAP plays a critical role also for IL-33 and IL-36 signaling.18
In cancer, IL1RAP was initially identified as upregulated on leukemia stem cells and allows for selective targeting of this cell population.19,22 Given its elevated expression across multiple cancers, and the role of IL-1 signaling in tumor progression, IL1RAP has emerged as a promising therapeutic target also in solid tumors.23,25 Nadunolimab (CAN04) is a fully humanized IL1RAP-targeting antibody and exerts anticancer effects by two independent mechanisms. It blocks signaling by IL-1α and IL-1β24 26 and induces FcɣR-mediated killing by immune effector cells. In PDAC, inhibition of IL-1 signaling by nadunolimab in combination with chemotherapy increases the antitumor activity in mice, however, the role of the TME for these effects was not investigated.27 Nadunolimab is well tolerated by patients24 and is currently evaluated for treatment of PDAC in a phase I/II clinical trial (NCT03267316).
In this study, we found that IL-1, secreted by PDAC cells, stimulates CAFs to release chemokines that induce chemotaxis of monocytes and neutrophils. Nadunolimab reversed these effects and decreased neutrophil survival. High IL1RAP expression in PDAC patients treated with nadunolimab as a monotherapy was associated with extended progression-free survival. These findings underscore a role for IL1RAP-mediated signaling in the immunosuppressive TME, supporting the potential of nadunolimab for treatment of PDAC.
Methods
PDAC xeno-transplantations in mice
Approximately 3 weeks after subcutaneous transplantation of 2.7×106 BxPC-3 alone or mixed with 5.3×106 CAFs into BALB/c nude mice, when tumors reached the size of 70–130 mm3, the mice were randomized into treatment groups. The mice received intraperitoneal injections of either mouse IgG2a isotype control or a mouse IgG2a variant of nadunolimab (anti-human IL1RAP) at a dose of 10 mg/kg, administered twice on the first day and then twice weekly. Tumor growth and mouse weight were continuously measured.
BxPC-3 and CAF co-cultures
CAF08 cells were acquired from Vitro Biopharma and maintained in MSC-Gro Pancreatic Stellate CAFs Maintenance Medium (Vitro Biopharma). BxPC-3 cells were acquired from ATCC. For preparation of conditioned media, we used RPMI media (Gibco) supplemented with 10% heat-inactivated fetal bovine serum and 1% Penicillin/streptomycin (Hyclone). For co-cultures, 250,000 BxPC-3 cells and 500,000 CAFs were seeded separately or mixed in 6 well plates with 3 mL medium supplemented with 20 µg/mL nadunolimab (Cantargia AB) or matching human IgG1 isotype. Co-cultures were also performed with antibodies neutralizing IL-1α and IL-1β, both of human IgG1 isotype and used at 20 µg/mL and produced at Innovagen, or with anakinra (500 ng/mL (Swedish Orphan Biovitrum)). For preparation of CAF conditioned media, 500,000 CAFs were seeded per well in 6-well plates in a final volume of 3 mL RPMI media (Gibco) supplemented with 10% heat-inactivated fetal bovine serum and 1% Penicillin/streptomycin (Hyclone) and incubated for 24 hours to adhere. The media was exchanged, and cultures were supplemented with IL-1 (0.1 ng/mL IL-1α and 0.1 ng/mL IL-1β (Peprotech)) and 20 µg/mL of antibodies, as indicated in figures, and cultured for an additional 24 hours before the media was harvested.
Chemotaxis assays
Concentrated leucocytes were obtained from healthy blood donors in the morning and cells were prepared within 8 hours after blood collection. Peripheral blood mononuclear cells (PBMCs) and granulocytes were separated by centrifugation with Lymphoprep (Stemcell Technologies). PBMCs and low density (LD) granulocytes were collected from the interphase and normal density (ND) granulocytes from the erythrocyte pellet, subsequently, erythrocytes were lysed with ACK lysing buffer (Thermo Fisher Scientific). The cells used in the assays were incubated with Trustain Fc block (Biolegend) and prestained with antibodies prior to experiments. Co-culture conditioned media was added to 24 well plates (undiluted for PBMC analysis, diluted 1:2 for monocytes and LD neutrophils, and 1:4 for ND neutrophils). The prepared leucocytes were applied in PET 3 µm pore diameter Millicell cell culture inserts (Merck), for 1 hour (ND neutrophils) or 3 hours (PBMCs and LD neutrophils). Cells that migrated through the insert into the media were collected and analyzed by flow cytometry after addition of CountBright beads (Thermo Fisher Scientific) and Propidium Iodide (Becton Dickinson) in PBS. For reagents used for flow cytometry, see online supplemental table 1.
Results
IL1RAP has broad expression in pancreatic tumors and is associated with poor outcomes
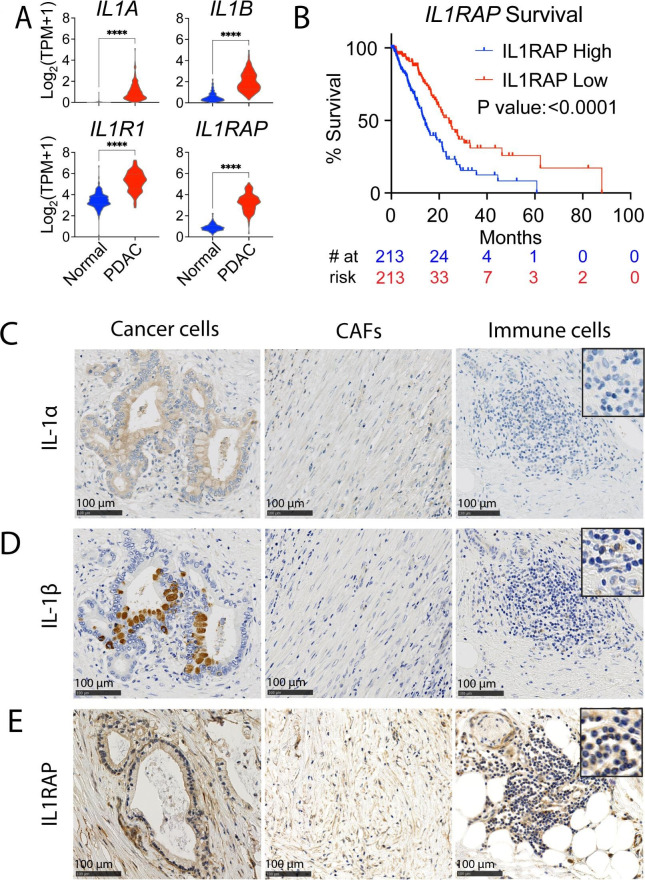
To assess the expression of IL-1-related genes in pancreatic tumors, we used global gene expression data from TCGA-PDAC and normal pancreas samples (GTEx).28 29 Expression of IL1A, IL1B, IL1R1 and IL1RAP was significantly higher in pancreatic tumors compared with normal pancreas tissue (figure 1A). Whereas the expression of IL1A, IL1B or IL1R1 did not significantly correlate to patient outcome, high IL1RAP expression was associated with poor survival (online supplemental figure S1A,B). Consistent with this finding, high IL1RAP expression was strongly associated with worse survival also in the larger Know Your Tumor PDAC patient cohort predominantly consisting of stage III and IV patients30 31 (figure 1B and online supplemental figure S1C). Moreover, IL1RAP was significantly upregulated in stage IV PDAC patients and associated with poor outcomes within this group (online supplemental figure S1D). Since IL1RAP is essential also for IL-33 and IL-36 signaling, we investigated their expression in the tumors. While IL36 expression was not detected in most samples, IL33 expression was higher in PDAC than in normal pancreas but did not correlate with survival (online supplemental figure S1E,F). For a more detailed assessment of IL-1 expression in PDAC tumors, we performed immunohistochemistry on tumor biopsies from patients. The epithelial tumor cells displayed uniform expression of IL-1α while CAFs and immune cells had weak or no expression (figure 1C). For IL-1β, a few tumor cells spread across the tumor showed strong expression, and the stroma was negative, with expression on some infiltrating immune cells (figure 1D). Moreover, the majority of both epithelial tumor cells, CAFs and immune cells stained positive for IL1RAP (figure 1E). The ubiquitous IL1RAP expression on cancer and stroma cells in the PDAC tumors, and association with poor outcome, suggest that targeting IL1RAP could have therapeutic potential in PDAC.
Figure 1. IL1RAP is broadly expressed in pancreatic tumors. (A) Gene expression data from GTEx and TCGA public datasets, comparing gene expression in normal pancreatic tissue (N=167) with pancreatic ductal adenocarcinoma (PDAC) tumors (N=150). (B) Survival analysis of PDAC patients grouped by median IL1RAP expression in the Know Your Tumor data set. (C–E) IHC stains for IL-1α, IL-1β, and IL1RAP in a PDAC tumor, showing expression on cancer cells (left images), cancer-associated fibroblasts (CAFs) (middle images), and immune cells (right images). The images shown are representative stains out of five tumors tested. A 2.5× magnification of selected cells is shown in the upper right corner. Significance calculated by Mann-Whitney test in (A) and by Log-rank (Mantel-Cox) test in (B). Significance levels are indicated as ****p<0.0001. IHC, Immunohistochemistry.
Targeting IL1RAP has CAF-dependent anticancer effects
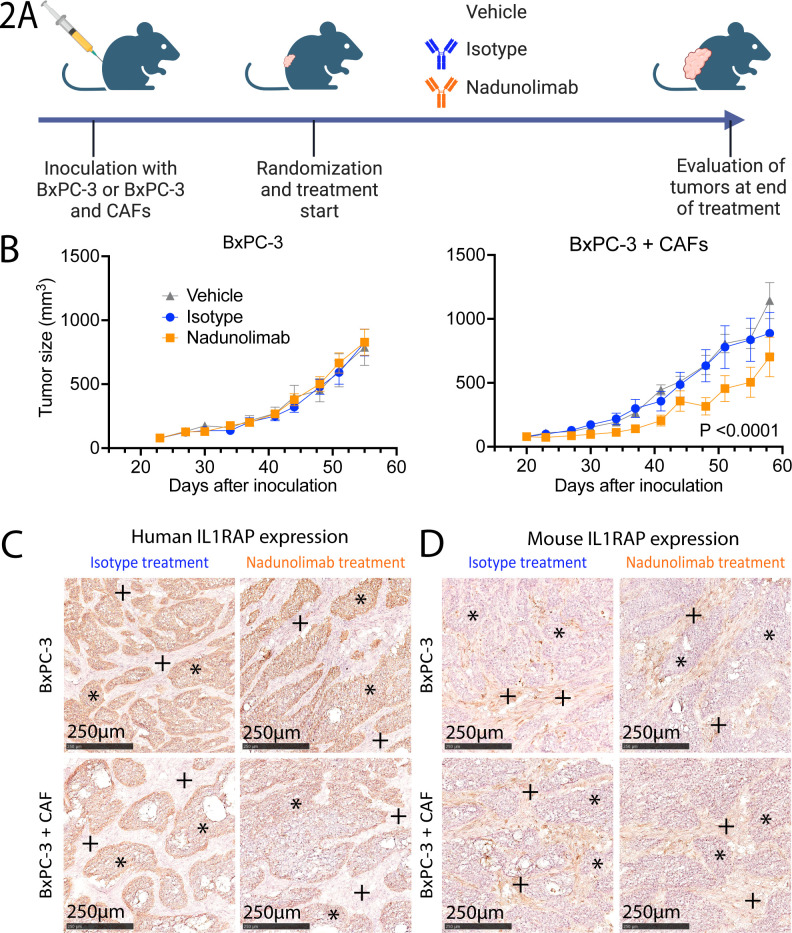
To evaluate the therapeutic potential of targeting IL1RAP, we established subcutaneous PDAC xenografts using the human PDAC cell line BxPC-3 either alone or cotransplanted with PDAC patient-derived CAFs (figure 2A). IL1RAP expression was confirmed on both BxPC-3 cells and CAFs (online supplemental figure S2). Mice with established tumors were treated with a mouse Fc-variant of the IL1RAP antibody nadunolimab, which exhibited no discernible effect in mice transplanted with BxPC-3 cells alone. In contrast, tumor growth was significantly reduced in mice transplanted with a mix of BxPC-3 cells and CAFs (figure 2B). These findings suggest that blocking IL1RAP disrupted critical interactions between human CAFs and BxPC-3 cells. At the experimental endpoint, the tumors displayed similar morphology across all groups with BxPC-3 cells staining positive for human IL1RAP while stromal cells almost exclusively expressed murine IL1RAP (figure 2C,D). This indicates that human CAFs had been replaced by mouse stromal cells at the experimental endpoint. Collectively, our data indicate that the nadunolimab treatment results in a suppression of tumor growth, an effect that was dependent on human CAFs in the tumors.
Figure 2. Nadunolimab elicits a CAF-dependent antitumor effect. (A) Experimental outline of in vivo experiments with BxPC-3 cells transplanted subcutaneously into mice, alone or mixed with CAFs before injection. The recipient mice were subsequently divided into groups (N=9–10) and treated with a murine IgG2a variant of the human-specific IL1RAP antibody nadunolimab, or matching isotype control (10 mg/kg, twice per week). Tumor volumes were monitored, and tumor tissue harvested at experimental endpoint (created with BioRender.com). (B) Tumor size of mice receiving BxPC-3 cells alone (left panel) or mixed with CAFs (right panel). Mean tumor volume and SE of the mean are plotted. Significance between isotype and nadunolimab-treated groups over the treatment period is indicated and calculated by two-way ANOVA test of the three treatment arms with Tukey correction. (C–D) IHC stain of tumors at the experimental endpoint, using human specific (C) and mouse specific (D) IL1RAP antibodies. *BxPC-3-regions, +CAF-regions. ANOVA, analysis of variance; CAF, cancer-associated fibroblast; IHC, immunohistochemistry.
IL1RAP inhibition primarily affects CAFs
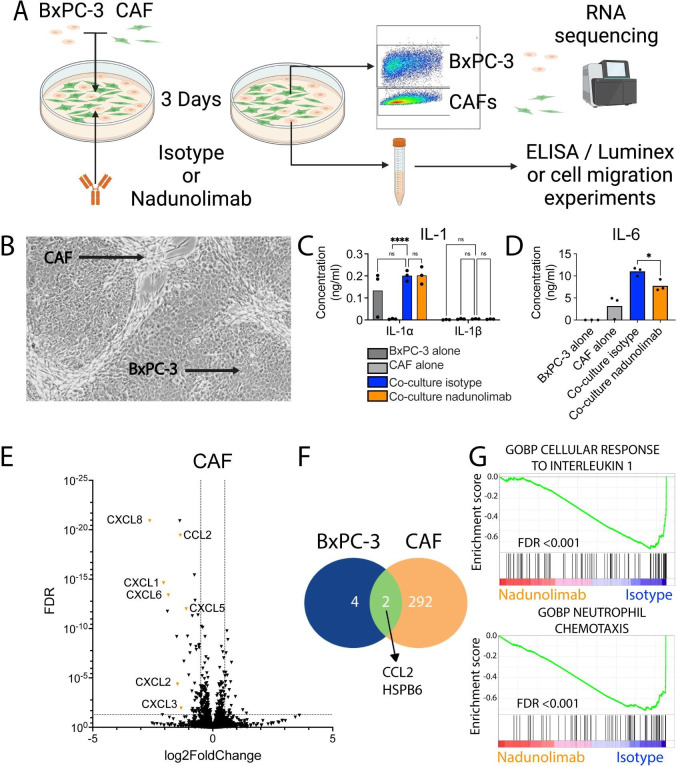
To elucidate how IL1RAP blockage impact CAF and PDAC interactions, a co-culture system was established (figure 3A). The co-cultures developed distinct regions composed of CAFs and BxPC-3 cells, resembling the cellular architecture found in primary tumors (figure 3B). Notably, the co-culture media contained approximately 50-fold more IL-1α than IL-1β, and nadunolimab did not affect the IL-1 concentrations (figure 3C). Separate cultures of CAFs and BxPC-3 cells indicated that IL-1α primarily originated from BxPC-3 cells (figure 3C). IL-33 and IL-36γ were not detected in the cultures suggesting that they did not influence CAF and PDAC interactions in this model (data not shown). Importantly, IL-6 and LIF secretion, which are known downstream targets of IL-1 and important for TME formation,32 were both significantly reduced with nadunolimab (figure 3D, online supplemental figure S3A), confirming the inhibition of IL-1 signaling. In contrast, the level of TGF-β1, another important CAF regulator,5 33 remained unchanged (online supplemental figure S3B).
Figure 3. Nadunolimab treatment primarily affects cancer-associated fibroblasts (CAFs). (A) The pancreatic ductal adenocarcinoma (PDAC) cell line BxPC-3 and CAFs were cultured separately or in a mixed co-culture, with 20 µg/mL nadunolimab or isotype control antibody for 3 days. The BxPC-3 cells and CAFs were sorted by flow cytometry based on EpCAM expression, and followed by RNA sequencing. Cell culture media was harvested for further experiments (created with BioRender.com). (B) Image of a co-culture with CAF and BxPC-3 cells indicated. (C) Concentration of IL-1 in media from single and co-cultures with nadunolimab or isotype control. (D) Concentration of IL-6 in media. (E) Volcano plot of genes dysregulated in CAFs from co-cultures treated with nadunolimab. (F) Number of genes significantly dysregulated (FDR<0.01) following nadunolimab treatment. (G) GSEA enrichment plots showing gene sets negatively enriched in CAFs following nadunolimab treatment. Significance in (C) and (D) was calculated using one-way ANOVA with Šidák correction and the indicated comparisons and bar shows mean value with N=3. To identify significantly dysregulated gens in (E) and (F), we used a Wald test with Benjamini-Hochberg multiple test correction. Significance levels are indicated as *p<0.05, ****p<0.0001. ANOVA, analysis of variance; FDR, false discovery rate; GOBP, gene ontology biological processes; GSEA, gene set enrichment analysis; ns, not significant.
To investigate how IL1RAP inhibition influenced the co-cultured cells, we performed RNA sequencing of sorted BxPC-3 cells and CAFs following nadunolimab treatment (figure 3A). Nadunolimab treatment resulted in only six differentially expressed genes in BxPC-3 cells, while 294 genes were differentially expressed in CAFs (false discovery rate, FDR<0.01, figure 3E,F, online supplemental figure S3C, and online supplemental table S2). These data show that IL1RAP inhibition predominantly affected the CAFs. To extrapolate biological relevance of the gene expression changes, we conducted gene set enrichment analysis. In line with IL1RAP being essential for IL-1 signaling, a gene set of cellular response to IL-1 was among the most significant negatively enriched gene sets in the CAFs (FDR<0.001, figure 3G), but not significantly enriched in BxPC-3 cells (FDR: 0.26, online supplemental figure S3D). In CAFs, 137 gene sets were negatively enriched on nadunolimab treatment, encompassing various gene sets related to myeloid cell migration, including neutrophil chemotaxis (FDR<0.001, figure 3G and online supplemental table S3). These findings highlight that IL1RAP inhibition primarily affected CAFs and suggests that myeloid cell chemotaxis is inhibited by IL1RAP blockade.
IL1RAP blockade on CAFs reduces IL-1-induced secretion of chemokines
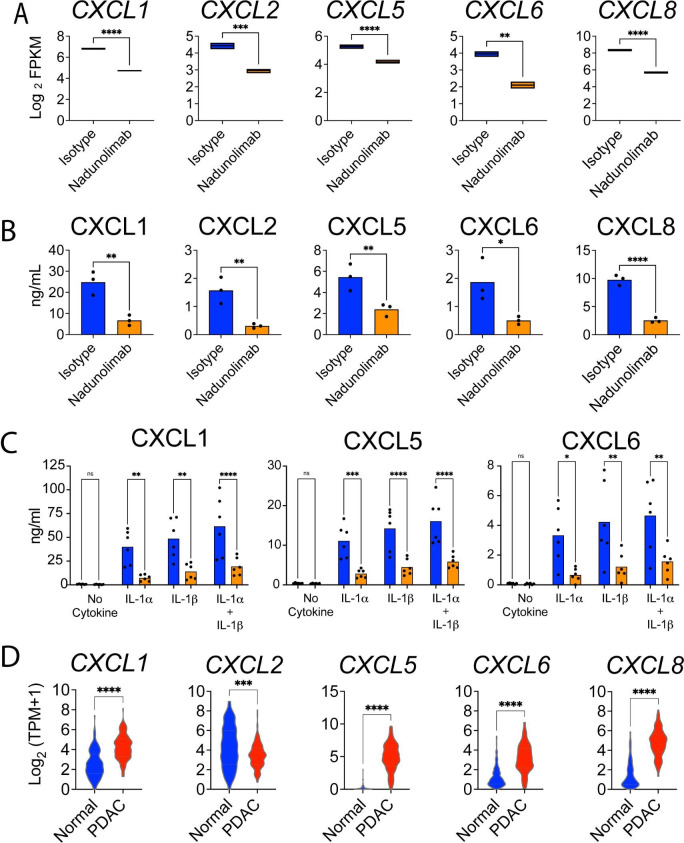
Consistent with the suppression of neutrophil migration, the chemokines, CXCL1, CXCL2, CXCL5, CXCL6 and CXCL8, ligands for CXCR1 and CXCR2, were all downregulated in CAFs following nadunolimab treatment (figure 3E and figure 4A). This transcriptional downregulation translated into a decrease in the corresponding neutrophil chemoattractants in the co-culture media (figure 4B and online supplemental figure S4A). Given the presence of both IL-1α and IL-1β in pancreatic tumors (figure 1), we next investigated if they affected the secretion of these chemokines. Both IL-1α and IL-1β induced secretion of CXCL1, CXCL2, CXCL5, CXCL6, and CXCL8 by CAFs, confirming that they are regulated by IL-1. This response was reversed by nadunolimab (figure 4C and online supplemental figure S3B). To evaluate the clinical relevance of these observations, we compared the expression of the CXCL chemokines in the GTEx and TCGA-PDAC patient data sets. All except CXCL2 showed significantly elevated expression in pancreatic tumors compared with healthy pancreas (figure 4D) but were not significantly associated with shorter survival (online supplemental figure S4C,D). These findings suggest that signaling through the IL1RAP/IL1R1 complex on CAFs induces the expression of multiple chemokines, which can be blocked by nadunolimab.
Figure 4. IL-1-induced CAF secretion of CXCR1/2 ligands is suppressed by IL1RAP blockage. (A) RNA expression of genes encoding chemokines in CAF and BxPC-3 co-cultures treated with nadunolimab or isotype control (average of N=4 with boxes showing minimum and maximum values; data from the RNA sequencing experiment are presented in figure 3). (B) Concentration of corresponding chemokines in the media from CAF and BxPC-3 co-cultures treated with nadunolimab or isotype control. The full data set is shown in online supplemental figure S4A. (C) CXCL1, CXCL5 and CXCL6 levels in media after 24 hours culture of CAFs, stimulated with 0.1 ng/mL IL-1α and IL-1β in combination with nadunolimab or isotype control (20 µg/mL). (D) Expression of CXCL chemokines in normal pancreas (N=167) and PDAC tumors (N=150) in the GTEx and TCGA data sets. Significance is calculated by one-way ANOVA test with Šidák correction in (A–C) and by Mann-Whitney test in (D). Mean values in A to C are shown with N=3-6. Significance levels are indicated as *p<0.05; **p<0.01; ***p<0.001; ****p<0.0001. ANOVA, analysis of variance; CAF, cancer-associated fibroblast. FPKM, fragments per million; PDAC, pancreatic ductal adenocarcinoma; ns, not significant. TPM, transcripts per million.
Inhibition of IL1RAP reduces neutrophil recruitment and survival mediated by CAFs
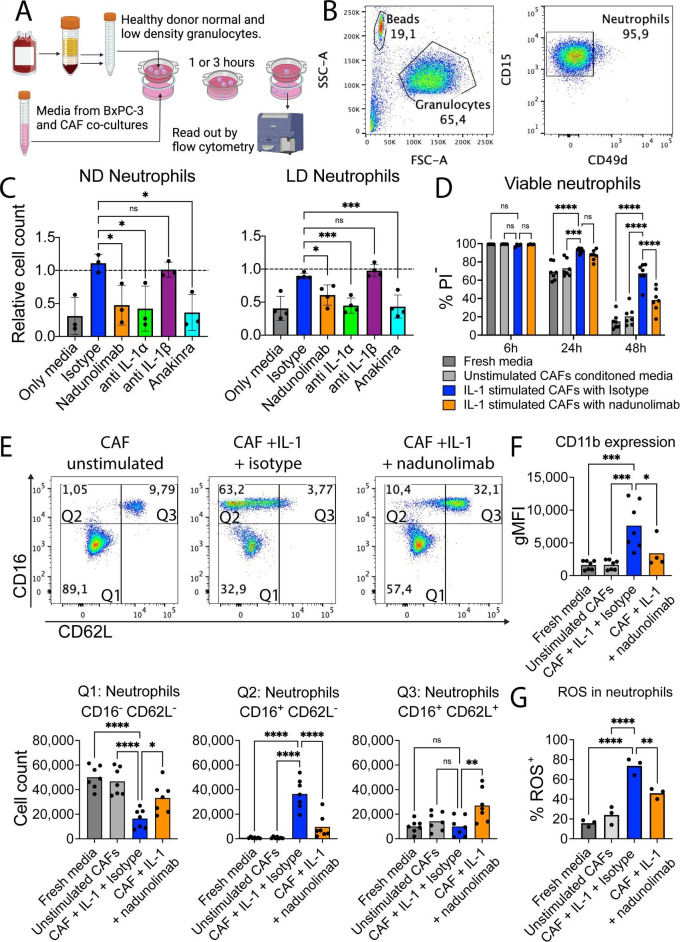
Infiltration of neutrophils into PDAC tumors is generally associated with poor outcomes.34 35 Since several of the chemokines that were downregulated by IL1RAP inhibition are important for neutrophil migration we conducted a chemotaxis assay using healthy neutrophils and co-culture media to evaluate the impact of nadunolimab on neutrophil migration (figure 5A,B). Consistent with the increased secretion of chemokines on IL-1 stimulation of CAFs, the BxPC-3 and CAF co-culture media induced migration of both ND and LD neutrophils, an effect that was reversed by addition of nadunolimab in the co-culture (figure 5C). A similar reduction in migration was observed with the addition of an IL-1α neutralizing antibody or Anakinra, an IL-1 receptor antagonist which blocks both IL-1α and IL-1β. In contrast, blocking of IL-1β had no discernible impact on neutrophil migration (figure 5C).
Figure 5. Blockage of IL1RAP on cancer-associated fibroblasts (CAFs) reduces neutrophil chemotaxis, survival, and activation. (A) Outline of the migration assay using normal density (ND) and low density (LD) neutrophils from healthy blood. After separation based on density, erythrocytes were lysed, and granulocytes were collected. Cells were stained with fluorescently labeled antibodies and allowed to migrate over a cell culture insert toward conditioned media, followed by cell counting by flow cytometry (created with BioRender.com). (B) Representative pseudo-color plots from the neutrophil migration assay. Neutrophils were gated as CD15+CD49D− cells from the granulocyte population in FSC and SSC. (C) Neutrophil chemotaxis using nadunolimab, IL-1 blocking antibodies (20 µg/mL), and anakinra (500 ng/mL) during the media conditioning. Each experiment was normalized to media from co-cultures without addition of antibodies (N=4). (D) Viability of healthy donor ND neutrophils after culture in CAF-conditioned media diluted 1:4 (N=4 or 7). (E) Flow cytometry of CD16 and CD62L expression on neutrophils after 24 hours culture. Upper panels show pseudo-color plots of the neutrophil populations. Lower panels show quantification of neutrophil numbers in the respective gates from the top panels (N=7). (F) CD11b expression on neutrophils after 24 hours culture with CAF conditioned media (N=7). (G) Percentage of neutrophils with ROS production after 24 hours culture with CAF conditioned media (N=3). Mean values are shown, and significance was calculated using one-way analysis of variance (ANOVA) with Dunnet correction except in (D) where two-way ANOVA with Dunnet correction was used, with the indicated pair-wise comparisons. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001. CAF, cancer-associated fibroblast; FSC-A, forward scatter area; gMFI, geometric mean fluorescence intensity; ns, not significant. PI, propidium iodide; ROS, reactive oxygen species; SSC-A, side scatter area.
To assess whether cytokines secreted by CAFs also affected the maintenance of neutrophils, we cultured neutrophils in media from CAFs stimulated with IL-1α and IL-1β. This media promoted neutrophil survival (figure 5D), and the emergence of a CD16+CD62L− population with increased CD11b expression, consistent with neutrophil activation and increased migratory capacity (figure 5E,F and online supplemental figure S5). Reactive oxygen species (ROS), a key part of neutrophil activation, was higher in the neutrophils exposed to conditioned media from IL-1 stimulated CAFs (figure 5G). These effects were inhibited by the addition of nadunolimab (figure 5D–G and online supplemental figure S5). In contrast, direct IL-1 stimulation of neutrophils did not induce these changes (online supplemental figure S6), showing that the observed neutrophil phenotype was CAF-mediated. These observations suggest that IL-1 stimulation causes CAFs to secrete factors that induce neutrophil chemotaxis, activation and survival, effects that are blocked by IL1RAP inhibition.
Nadunolimab suppresses CAF-mediated monocyte recruitment
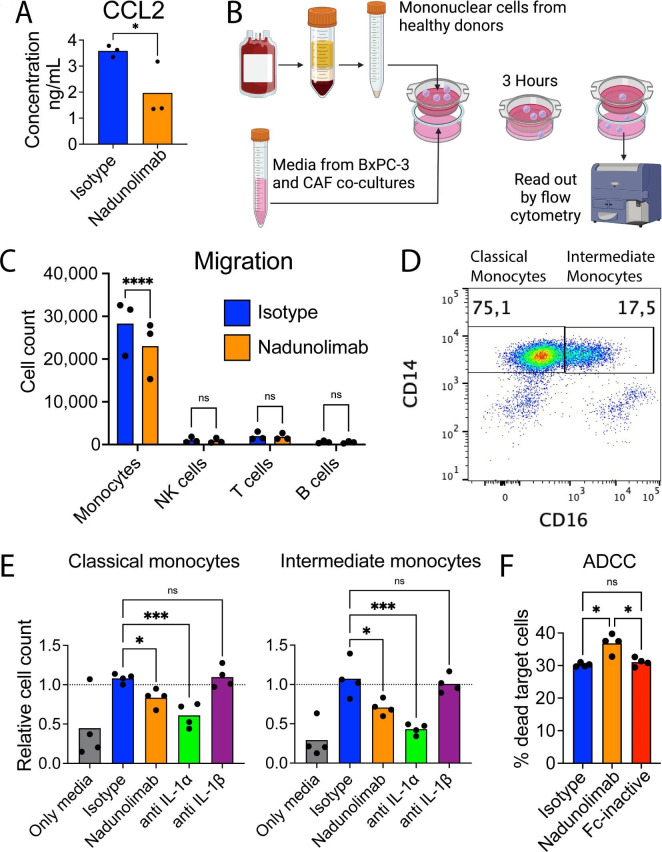
Not only neutrophil attractants were reduced following nadunolimab treatment. CCL2, a monocyte chemoattractant, was also downregulated by nadunolimab, both in BxPC-3 cells and CAFs resulting in reduced CCL2 levels in the media (figure 3F, figure 6A and online supplemental figure S7). To investigate the impact of nadunolimab on the migration of monocytes, we used peripheral blood mononuclear cells (PBMCs) with conditioned media from BxPC-3 and CAF co-cultures (figure 6B). Despite monocytes only constituting about 10% of the PBMC fraction, the vast majority of migrated PBMCs were monocytes, and addition of nadunolimab to the co-culture reduced the monocyte migration (figure 6C). We then investigated whether the monocyte migration was induced by IL-1α or IL-1β and if classical (CD14+CD16−) and intermediate (CD14+CD16+) monocytes were differentially affected (figure 6D). While blocking IL-1β had no significant impact on monocyte migration, blocking of IL1RAP or IL-1α suppressed the chemotaxis of both classical and intermediate monocytes (figure 6E).
Figure 6. Nadunolimab reduces monocyte chemotaxis and induces antibody-dependent cellular cytotoxicity (ADCC). (A) Concentration of CCL2 in supernatants of BxPC-3 and CAF co-cultures. See online supplemental figure S7 for the full analysis. (B) Outline of a transwell migration assay with PBMCs, stained with fluorescently labeled antibodies and allowed to migrate toward co-culture conditioned media with nadunolimab or isotype antibody added to the co-culture. Migrated cells were assessed and counted by flow cytometry (created with BioRender.com). (C) Counts of migrated monocytes (CD14+), NK cells (CD56+), T cells (CD3+), and B cells (CD19+). (D) Representative plot of CD14 and CD16 expression on monocytes with the gating strategy used to assess classical and intermediate monocyte populations in the migration assay. (E) Monocyte migration with co-culture conditioned media and blockage of IL1RAP or IL-1 in the BxPC-3 and CAF co-cultures. Mean values are shown with N=3 or 4. Experiments were normalized to media from co-cultures without addition of antibodies. (F) Percentage of dead target cells after an ADCC assay with BxPC-3 target cells, monocytes (CD14 enriched PBMCs), and antibodies at 20 µg/mL. Significance calculated by one-way ANOVA with Šidák correction in (A) and (C), Dunnet correction in (E) and (F), and indicated as *p<0.05; ***p<0.001; ****p<0.0001. ANOVA, analysis of variance; CAF, cancer-associated fibroblast; ns, not significant. NK, natural killer; PBMCs, peripheral blood mononuclear cells.
Given that monocytes have been reported to elicit antibody-dependent cellular cytotoxicity (ADCC) in a CD16-dependent manner,36 we sought to investigate whether nadunolimab, a FcɣR enhanced antibody, can induce monocyte mediated ADCC. Consistent with our hypothesis, nadunolimab induced ADCC of BxPC-3 cells, whereas an Fc inactive variant of nadunolimab did not induce cell death (figure 6F). These findings collectively suggest that while blockade of IL-1 signaling reduces monocyte chemotaxis, nadunolimab can also induce monocyte-mediated killing of PDAC cells.
High IL1RAP expression is associated with extended progression-free survival in PDAC patients treated with nadunolimab
To evaluate the clinical relevance of targeting IL1RAP, we obtained biopsies from a phase I /II clinical trial (CANFOUR; NCT03267316) where nadunolimab as monotherapy was evaluated in 23 metastatic late stage PDAC patients that in most cases were refractory PDAC (table 1 and online supplemental figure S8). All patients received at least one dose of nadunolimab as monotherapy. Treatment with nadunolimab was safe and well tolerated, in line with the previously published phase one dose-escalation study.24 Stable disease was reported for 3 patients, 15 patients had progressive disease, and 5 were not evaluable.
Table 1. Demographic information for patients receiving nadunolimab as monotherapy.
| IL1RAP high (n=5) | IL1RAP low (n=10) | |
| Age (years); mean (range) | 63 (61–64) | 62 (40–81) |
| Sex (female/male); n (%) | 2 (40%) / 3 (60%) | 2 (20%) / 8 (80%) |
| ECOG 0/1; n (%) | 3 (60%) / 2 (40%) | 3 (30%) / 7 (70%) |
| Location of metastases at study entry; n (%) | ||
| Liver | 3 (60%) | 9 (90%) |
| Lymph node | 2 (40%) | 3 (30%) |
| Other sites | 3 (60%) | 6 (60%) |
| No of previous lines of treatment | ||
| 1 | 3 (60%) | 4 (40%) |
| 2 | 1 (20%) | 4 (40%) |
| ≥3 | 1 (20%) | 2 (20%) |
ECOG, Eastern Cooperative Oncology Group
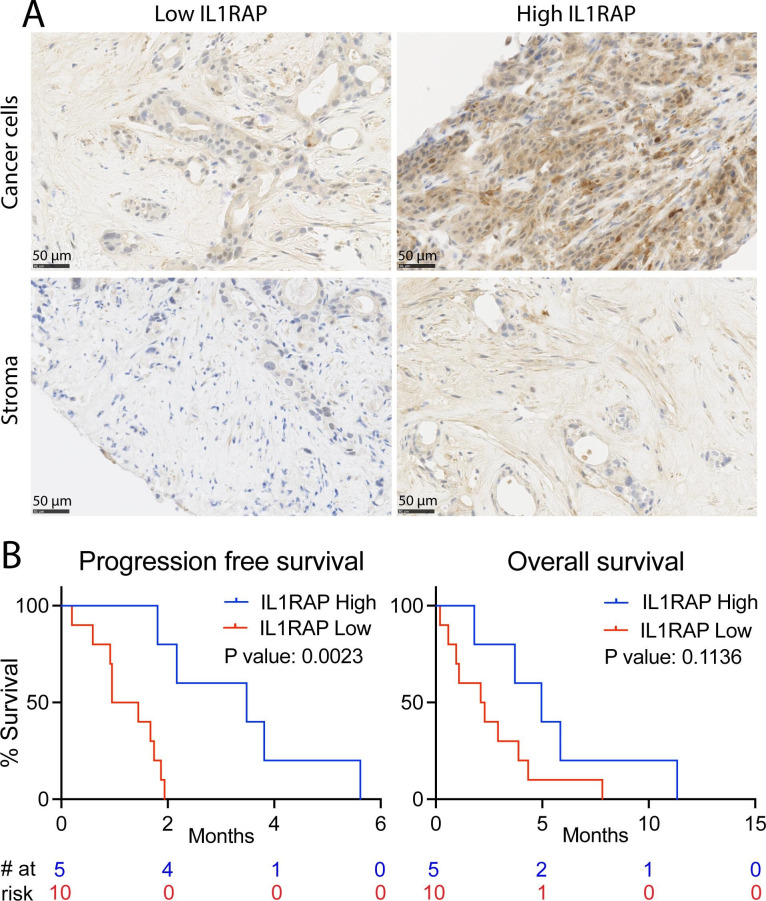
Pretreatment biopsies from 17 of these 23 patients were stained for the expression of IL1RAP using immunohistochemistry. Patients were grouped based on high or low IL1RAP staining of the tumors (figure 7A). Notably, high IL1RAP expression on tumor cells and stroma combined, was associated with a significantly increased progression-free survival (median: 3.5 vs 1.2 months, p=0.0023) and showed a trend toward extended overall survival (median: 5.0 vs 2.2 months, p=0.1136) with a follow-up of 11.5 months (figure 7B). Although based on a small number of patients, these findings suggest that high levels of IL1RAP in PDAC tumors is associated with clinical benefit for patients treated with nadunolimab. This is especially noteworthy considering that high IL1RAP expression is associated with worse outcome in patients receiving standard treatment (figure 1).25
Figure 7. High IL1RAP expression is associated with longer survival in nadunolimab-treated patients. (A) Representative histology slides where cancer cells (upper panels) and stroma cells (lower panels). Tumor and stroma cells were scored as having low IL1RAP expression (Tumor cell H-score ≤100, and stroma staining intensity assessment ≤1, left panels) or high IL1RAP expression (Tumor cell H-score >100 and stroma staining intensity assessment >1, right panels). (B) Progression-free and overall survival of PDAC patients receiving nadunolimab as monotherapy, with survival analysis based on low or high combined IL1RAP expression in the tumor. The combined score was defined as high when both the tumor cells and stromal cells scored high according to the above criteria (N=5). All other samples were categorized as having a low combined score (N=10). Significance in the survival analysis was tested by log-rank (Mantel-Cox) test.
Discussion
The stromal component within PDAC is a key element of the TME with IL-1 signaling fostering an immunosuppressive state that contributes to tumor progression and therapy resistance.9 IL-1α, secreted by PDAC tumor cells, plays a pivotal role by influencing both tumorigenesis and metastatic processes.5 6 8 37 38 Similarly, IL-1β produced by tumor cells and infiltrating immune cells is associated with inflammation, immunosuppression, and poor prognosis.7 9 11 However, due to their redundant signaling, strategies solely targeting IL-1α or IL-1β will likely not be sufficient to efficiently block IL-1 signaling in tumors. In contrast, targeting IL1RAP has the potential to completely inhibit IL-1 signaling and constitutes a promising new target for cancer therapy. In addition to anakinra, which also blocks IL-1 signaling and is explored as a therapeutic agent in PDAC,38 39 antibody-based targeting IL1RAP has the potential to directly mount an immune attack against the tumor by binding to Fc receptors on immune effector cells. In this study, we found that IL1RAP expression was highest in stage IV patients, indicating an association between IL1RAP expression and advanced disease. Importantly, a strong association between high IL1RAP expression and worse survival was observed within stage IV PDAC patients, further supporting that IL1RAP is a marker for poor prognosis and a candidate therapeutic target.
The observation that nadunolimab suppressed human PDAC growth in a CAF-dependent manner in mice highlighted a connection between IL1RAP in the stroma and early tumor growth in this model. However, the TME in the mice did not support long-term growth of human CAFs, which were replaced by murine stromal cells. Since nadunolimab is not cross-reactive to mouse IL1RAP, we speculate that the CAF-dependent effect observed in vivo were likely related to low levels of remaining human CAFs in the TME. Hence, the full antitumor potential of nadunolimab is likely underestimated in this model. To explore the mechanism of how IL1RAP blockade affects the interaction between PDAC cells and CAFs, we used an in vitro co-culture system predominantly expressing IL-1α. This explains the similarity between antibody-based blocking of IL-1α or IL1RAP with the IL-1 receptor antagonist anakinra in this model and is in accordance with earlier observations that BxPC-3 cells express IL-1α.8
There is an increased recognition of the importance of infiltrating immune cells in PDAC tumors contributing to the immunosuppressive TME.11 34 40 High neutrophil infiltration is associated with poor outcomes,34 35 41 emphasizing the relevance of immune cell dynamics in PDAC. Chemotaxis of neutrophils is driven by several CXCR1 and CXCR2 ligands including CXCL1, CXCL5 and CXCL8,42 all of which were downregulated in CAFs on targeting IL1RAP and led to reduced neutrophil chemotaxis. Among these chemokines, CXCL5 has previously been identified as an independent prognostic factor in PDAC.43 Furthermore, factors secreted by CAFs not only sustained neutrophil viability but also activated them as demonstrated by ROS production and a decrease in CD62L expression. Neutrophil-derived ROS has several functions, including the ability to inhibit T cell proliferation, and indicates that nadunolimab inhibits neutrophil-mediated immune suppression.44 In a clinical context, we anticipate that these effects would lead to decreased neutrophil infiltration, thereby inhibiting the immune suppressive TME.
The reduced chemokine secretion following IL1RAP inhibition included downregulation of CCL2 and resulted in reduced monocyte migration. This effect can have profound implications for tumor biology, as a decrease in CCL2-induced monocyte recruitment to tumors has been linked to reduced tumor growth, decreased levels of monocytic myeloid-derived suppressor cells, mitigation of immune suppression, and enhanced sensitivity to immune checkpoint blockade.45,48 Moreover, the reduction of both CCL2, which signals through CCR2, and CXCR2 ligands by IL1RAP inhibition is encouraging. This is particularly noteworthy as combined inhibition of CCR2 on tumor-associated macrophages and CXCR2 on tumor-associated neutrophils improves response to chemotherapy in PDAC.49 In addition to suppressing monocyte chemotaxis, nadunolimab also facilitated direct tumor cell killing by inducing monocyte-mediated ADCC. Similar ADCC effects have previously been observed with IL1RAP antibodies and NK cells in leukemia.19,21
The observation that high IL1RAP expression in tumors is associated with an extended progression-free survival in late-stage PDAC patients undergoing nadunolimab treatment is promising. This is especially notable considering that high IL1RAP expression is associated with worse outcome in patients receiving standard treatment.25 These results, although based on a rather small cohort of late-stage patients, support a therapeutic effect of nadunolimab in PDAC patients.
Taken together, we found that that cancer cells in PDAC tumors secrete IL-1α and IL-1β, which initiate signaling in CAFs through the IL1R1/IL1RAP receptor complex. This signaling triggers chemokine secretion, which stimulate infiltration and activation of neutrophils and monocytes that contribute to the immunosuppressive TME. Nadunolimab reversed these effects and stimulated effector-cell mediated killing of tumor cells. In a small cohort of PDAC patients treated with nadunolimab, high IL1RAP expression in tumors was associated with increased progression-free survival. These findings increase our understanding of how IL-1 signaling in the TME promotes tumor growth and highlight IL1RAP as a promising therapeutic target in PDAC.
supplementary material
Acknowledgements
We would like to thank the patients and their families for participating in the CANFOUR study and all study staff at the clinical sites. We would also like to thank the patients and staff involved in the Know Your Tumor initiative through PanCAN. This article contains gene expression data obtained from the TCGA Research Network (https://www.cancer.gov/tcga) and Genotype-Tissue Expression (GTEx) project (https://www.gtexportal.org/home/).
Footnotes
Funding: The authors thank the Swedish Cancer Society (22 2158 Pj), the Medical Faculty of Lund University (N/A), the Swedish Research Council (2023-01806), and governmental funding of clinical research within the NHS (National Health Services, Sweden) (2024-0067), for research grants. The CANFOUR trial was sponsored by Cantargia AB (N/A).
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Not applicable.
Ethics approval: This study involves human participants and was approved by EudraCT 2017-001111-36, Belgium; Ethics committee of the Institut Jules Bordet (ref: CE 2706), Denmark; Den Videnskabsetiske Komite E for Region Hovedstaden af Denmark/Committee E of the Committees on Health Research Ethics for the Capital Region of Denmark (ref: : H-17015038), Norway; Regional Committee for Medical and Health Research Ethics (REC South East) (ref: 2017/1069/REC South East B), Netherland; Medical Ethics Review Committee of the Foundation Netherlands Cancer Institute–The Antoni van Leeuwenhoek Hospital (METC AVL) (ref: NL61562.031.17), and Austria; Ethikkommission der Medizinischen Universität Wien (ref: 1812/2018). Participants gave informed consent to participate in the study before taking part.
Data availability free text: Additional information regarding methods used is available in online supplemental materials. TCGA and GTEx data are available through UCSC Xena Browser (https://xena.ucsc.edu/). Know Your Tumor data is available through the PanCAN SPARK platform (www.pancan.org/spark). RNA sequencing and data from in vitro cultures are available on request. The human dData from the CANFOUR trial (NCT03267316) generated in this study are not publicly available due to patient privacy requirements but are available on reasonable request.
Contributor Information
Nils Hansen, Email: nils.hansen@med.lu.se.
Pablo Peña-Martínez, Email: pablo.pena@med.lu.se.
Petter Skoog, Email: Petter.Skoog@cantargia.com.
Katrin Reinbach, Email: katrin.reinbach@med.lu.se.
Finja C Hansen, Email: FinjaHansen@gmx.net.
Susanne Larsson Faria, Email: susanne.larssonfaria@gmail.com.
Caitríona Grönberg, Email: caitriona.gronberg@cantargia.com.
Kawther Abdilleh, Email: Kabdilleh@pancan.org.
Susanne Magnusson, Email: susanne.magnusson@cantargia.com.
Karin von Wachenfeldt, Email: karin@trulylabs.com.
Camilla Rydberg Millrud, Email: camilla.rydberg_millrud@cantargia.com.
David Liberg, Email: david.liberg@cantargia.com.
Marcus Järås, Email: marcus.jaras@med.lu.se.
Data availability statement
Data are available on reasonable request.
References
- 1.Bengtsson A, Andersson R, Ansari D. The actual 5-year survivors of pancreatic ductal adenocarcinoma based on real-world data. Sci Rep. 2020;10:16425. doi: 10.1038/s41598-020-73525-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Muller M, Haghnejad V, Schaefer M, et al. The Immune Landscape of Human Pancreatic Ductal Carcinoma: Key Players, Clinical Implications, and Challenges. Cancers (Basel) 2022;14:995. doi: 10.3390/cancers14040995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morgan A, Griffin M, Kameni L, et al. Medical Biology of Cancer-Associated Fibroblasts in Pancreatic Cancer. Biology (Basel) 2023;12:1044. doi: 10.3390/biology12081044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Truong L-H, Pauklin S. Pancreatic Cancer Microenvironment and Cellular Composition: Current Understandings and Therapeutic Approaches. Cancers (Basel) 2021;13:5028. doi: 10.3390/cancers13195028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Biffi G, Oni TE, Spielman B, et al. IL1-Induced JAK/STAT Signaling Is Antagonized by TGFβ to Shape CAF Heterogeneity in Pancreatic Ductal Adenocarcinoma. Cancer Discov. 2019;9:282–301. doi: 10.1158/2159-8290.CD-18-0710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tjomsland V, Spångeus A, Välilä J, et al. Interleukin 1α sustains the expression of inflammatory factors in human pancreatic cancer microenvironment by targeting cancer-associated fibroblasts. Neoplasia. 2011;13:664–75. doi: 10.1593/neo.11332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang D, Li L, Jiang H, et al. Tumor-Stroma IL1β-IRAK4 Feedforward Circuitry Drives Tumor Fibrosis, Chemoresistance, and Poor Prognosis in Pancreatic Cancer. Cancer Res. 2018;78:1700–12. doi: 10.1158/0008-5472.CAN-17-1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brunetto E, De Monte L, Balzano G, et al. The IL-1/IL-1 receptor axis and tumor cell released inflammasome adaptor ASC are key regulators of TSLP secretion by cancer associated fibroblasts in pancreatic cancer. J Immunother Cancer. 2019;7:45. doi: 10.1186/s40425-019-0521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Das S, Shapiro B, Vucic EA, et al. Tumor Cell–Derived IL1β Promotes Desmoplasia and Immune Suppression in Pancreatic Cancer. Cancer Res. 2020;80:1088–101. doi: 10.1158/0008-5472.CAN-19-2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tjomsland V, Bojmar L, Sandström P, et al. IL-1α expression in pancreatic ductal adenocarcinoma affects the tumor cell migration and is regulated by the p38MAPK signaling pathway. PLoS One. 2013;8:e70874. doi: 10.1371/journal.pone.0070874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caronni N, La Terza F, Vittoria FM, et al. IL-1β+ macrophages fuel pathogenic inflammation in pancreatic cancer. Nature New Biol. 2023;623:415–22. doi: 10.1038/s41586-023-06685-2. [DOI] [PubMed] [Google Scholar]
- 12.van Duijneveldt G, Griffin MDW, Putoczki TL. Emerging roles for the IL-6 family of cytokines in pancreatic cancer. Clin Sci. 2020;134:2091–115. doi: 10.1042/CS20191211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McAndrews KM, Chen Y, Darpolor JK, et al. Identification of Functional Heterogeneity of Carcinoma-Associated Fibroblasts with Distinct IL6-Mediated Therapy Resistance in Pancreatic Cancer. Cancer Discov. 2022;12:1580–97. doi: 10.1158/2159-8290.CD-20-1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li S, Strelow A, Fontana EJ, et al. IRAK-4: A novel member of the IRAK family with the properties of an IRAK-kinase. Proc Natl Acad Sci USA. 2002;99:5567–72. doi: 10.1073/pnas.082100399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brikos C, Wait R, Begum S, et al. Mass Spectrometric Analysis of the Endogenous Type I Interleukin-1 (IL-1) Receptor Signaling Complex Formed after IL-1 Binding Identifies IL-1RAcP, MyD88, and IRAK-4 as the Stable Components. Mol Cell Proteomics. 2007;6:1551–9. doi: 10.1074/mcp.M600455-MCP200. [DOI] [PubMed] [Google Scholar]
- 16.Cullinan EB, Kwee L, Nunes P, et al. IL-1 receptor accessory protein is an essential component of the IL-1 receptor. J Immunol . 1998;161:5614–20. [PubMed] [Google Scholar]
- 17.Korherr C, Hofmeister R, Wesche H, et al. A critical role for interleukin-1 receptor accessory protein in interleukin-1 signaling. Eur J Immunol. 1997;27:262–7. doi: 10.1002/eji.1830270139. [DOI] [PubMed] [Google Scholar]
- 18.Fields JK, Günther S, Sundberg EJ. Structural Basis of IL-1 Family Cytokine Signaling. Front Immunol. 2019;10:1412. doi: 10.3389/fimmu.2019.01412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Järås M, Johnels P, Hansen N, et al. Isolation and killing of candidate chronic myeloid leukemia stem cells by antibody targeting of IL-1 receptor accessory protein. Proc Natl Acad Sci USA. 2010;107:16280–5. doi: 10.1073/pnas.1004408107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Askmyr M, Ågerstam H, Hansen N, et al. Selective killing of candidate AML stem cells by antibody targeting of IL1RAP. Blood. 2013;121:3709–13. doi: 10.1182/blood-2012-09-458935. [DOI] [PubMed] [Google Scholar]
- 21.Ågerstam H, Karlsson C, Hansen N, et al. Antibodies targeting human IL1RAP (IL1R3) show therapeutic effects in xenograft models of acute myeloid leukemia. Proc Natl Acad Sci U S A. 2015;112:10786–91. doi: 10.1073/pnas.1422749112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Trad R, Warda W, Alcazer V, et al. Chimeric antigen receptor T-cells targeting IL-1RAP: a promising new cellular immunotherapy to treat acute myeloid leukemia. J Immunother Cancer. 2022;10:e004222. doi: 10.1136/jitc-2021-004222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Frenay J, Bellaye P-S, Oudot A, et al. IL-1RAP, a Key Therapeutic Target in Cancer. Int J Mol Sci. 2022;23:14918. doi: 10.3390/ijms232314918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Robbrecht D, Jungels C, Sorensen MM, et al. First-in-human phase 1 dose-escalation study of CAN04, a first-in-class interleukin-1 receptor accessory protein (IL1RAP) antibody in patients with solid tumours. Br J Cancer. 2022;126:1010–7. doi: 10.1038/s41416-021-01657-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang Y, Chen X, Wang H, et al. Innate immune mediator, Interleukin-1 receptor accessory protein (IL1RAP), is expressed and pro-tumorigenic in pancreatic cancer. J Hematol Oncol. 2022;15:70. doi: 10.1186/s13045-022-01286-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fields JK, Kihn K, Birkedal GS, et al. Molecular Basis of Selective Cytokine Signaling Inhibition by Antibodies Targeting a Shared Receptor. Front Immunol. 2021;12:779100. doi: 10.3389/fimmu.2021.779100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rydberg Millrud C, Deronic A, Grönberg C, et al. Blockade of IL-1α and IL-1β signaling by the anti-IL1RAP antibody nadunolimab (CAN04) mediates synergistic anti-tumor efficacy with chemotherapy. Cancer Immunol Immunother. 2023;72:667–78. doi: 10.1007/s00262-022-03277-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lonsdale J, Thomas J, Salvatore M, et al. The Genotype-Tissue Expression (GTEx) project. Nat Genet. 2013;45:580–5. doi: 10.1038/ng.2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cancer Genome Atlas Research Network Electronic address: andrew_aguirre@dfci.harvard.edu, Cancer Genome Atlas Research Network. Integrated Genomic Characterization of Pancreatic Ductal Adenocarcinoma. Cancer Cell. 2017;32:185–203. doi: 10.1016/j.ccell.2017.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abdilleh K, Khalid O, Ladnier D, et al. Pancreatic Cancer Action Network’s SPARK: A Cloud-Based Patient Health Data and Analytics Platform for Pancreatic Cancer. JCO Clin Cancer Inform . 2024;8:e2300119. doi: 10.1200/CCI.23.00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pishvaian MJ, Bender RJ, Halverson D, et al. Molecular Profiling of Patients with Pancreatic Cancer: Initial Results from the Know Your Tumor Initiative. Clin Cancer Res. 2018;24:5018–27. doi: 10.1158/1078-0432.CCR-18-0531. [DOI] [PubMed] [Google Scholar]
- 32.Soler MF, Abaurrea A, Azcoaga P, et al. New perspectives in cancer immunotherapy: targeting IL-6 cytokine family. J Immunother Cancer. 2023;11:e007530. doi: 10.1136/jitc-2023-007530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Öhlund D, Handly-Santana A, Biffi G, et al. Distinct populations of inflammatory fibroblasts and myofibroblasts in pancreatic cancer. J Exp Med. 2017;214:579–96. doi: 10.1084/jem.20162024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang W-Q, Liu L, Xu H-X, et al. Infiltrating immune cells and gene mutations in pancreatic ductal adenocarcinoma. Br J Surg. 2016;103:1189–99. doi: 10.1002/bjs.10187. [DOI] [PubMed] [Google Scholar]
- 35.Ino Y, Yamazaki-Itoh R, Shimada K, et al. Immune cell infiltration as an indicator of the immune microenvironment of pancreatic cancer. Br J Cancer. 2013;108:914–23. doi: 10.1038/bjc.2013.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yeap WH, Wong KL, Shimasaki N, et al. CD16 is indispensable for antibody-dependent cellular cytotoxicity by human monocytes. Sci Rep. 2016;6:34310. doi: 10.1038/srep34310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Melisi D, Niu J, Chang Z, et al. Secreted interleukin-1alpha induces a metastatic phenotype in pancreatic cancer by sustaining a constitutive activation of nuclear factor-kappaB. Mol Cancer Res. 2009;7:624–33. doi: 10.1158/1541-7786.MCR-08-0201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dosch AR, Singh S, Dai X, et al. Targeting Tumor-Stromal IL6/STAT3 Signaling through IL1 Receptor Inhibition in Pancreatic Cancer. Mol Cancer Ther. 2021;20:2280–90. doi: 10.1158/1535-7163.MCT-21-0083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhuang Z, Ju H-Q, Aguilar M, et al. IL1 Receptor Antagonist Inhibits Pancreatic Cancer Growth by Abrogating NF-κB Activation. Clin Cancer Res. 2016;22:1432–44. doi: 10.1158/1078-0432.CCR-14-3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rubin SJS, Sojwal RS, Gubatan J, et al. The Tumor Immune Microenvironment in Pancreatic Ductal Adenocarcinoma: Neither Hot nor Cold. Cancers (Basel) 2022;14:4236. doi: 10.3390/cancers14174236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shen M, Hu P, Donskov F, et al. Tumor-associated neutrophils as a new prognostic factor in cancer: a systematic review and meta-analysis. PLoS One. 2014;9:e98259. doi: 10.1371/journal.pone.0098259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gibellini L, Borella R, Santacroce E, et al. Circulating and Tumor-Associated Neutrophils in the Era of Immune Checkpoint Inhibitors: Dynamics, Phenotypes, Metabolism, and Functions. Cancers (Basel) 15:3327. doi: 10.3390/cancers15133327. n.d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang R, Liu Q, Peng J, et al. CXCL5 overexpression predicts a poor prognosis in pancreatic ductal adenocarcinoma and is correlated with immune cell infiltration. J Cancer. 2020;11:2371–81. doi: 10.7150/jca.40517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hsu BE, Shen Y, Siegel PM. Neutrophils: Orchestrators of the Malignant Phenotype. Front Immunol. 2020;11:1778. doi: 10.3389/fimmu.2020.01778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li X, He G, Liu J, et al. CCL2-mediated monocytes regulate immune checkpoint blockade resistance in pancreatic cancer. Int Immunopharmacol. 2022;106:108598. doi: 10.1016/j.intimp.2022.108598. [DOI] [PubMed] [Google Scholar]
- 46.Gu H, Deng W, Zheng Z, et al. CCL2 produced by pancreatic ductal adenocarcinoma is essential for the accumulation and activation of monocytic myeloid-derived suppressor cells. Immun Inflamm Dis. 2021;9:1686–95. doi: 10.1002/iid3.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sanford DE, Belt BA, Panni RZ, et al. Inflammatory monocyte mobilization decreases patient survival in pancreatic cancer: a role for targeting the CCL2/CCR2 axis. Clin Cancer Res. 2013;19:3404–15. doi: 10.1158/1078-0432.CCR-13-0525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mitchem JB, Brennan DJ, Knolhoff BL, et al. Targeting tumor-infiltrating macrophages decreases tumor-initiating cells, relieves immunosuppression, and improves chemotherapeutic responses. Cancer Res. 2013;73:1128–41. doi: 10.1158/0008-5472.CAN-12-2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nywening TM, Belt BA, Cullinan DR, et al. Targeting both tumour-associated CXCR2+ neutrophils and CCR2+ macrophages disrupts myeloid recruitment and improves chemotherapeutic responses in pancreatic ductal adenocarcinoma. Gut. 2018;67:1112–23. doi: 10.1136/gutjnl-2017-313738. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available on reasonable request.