Abstract
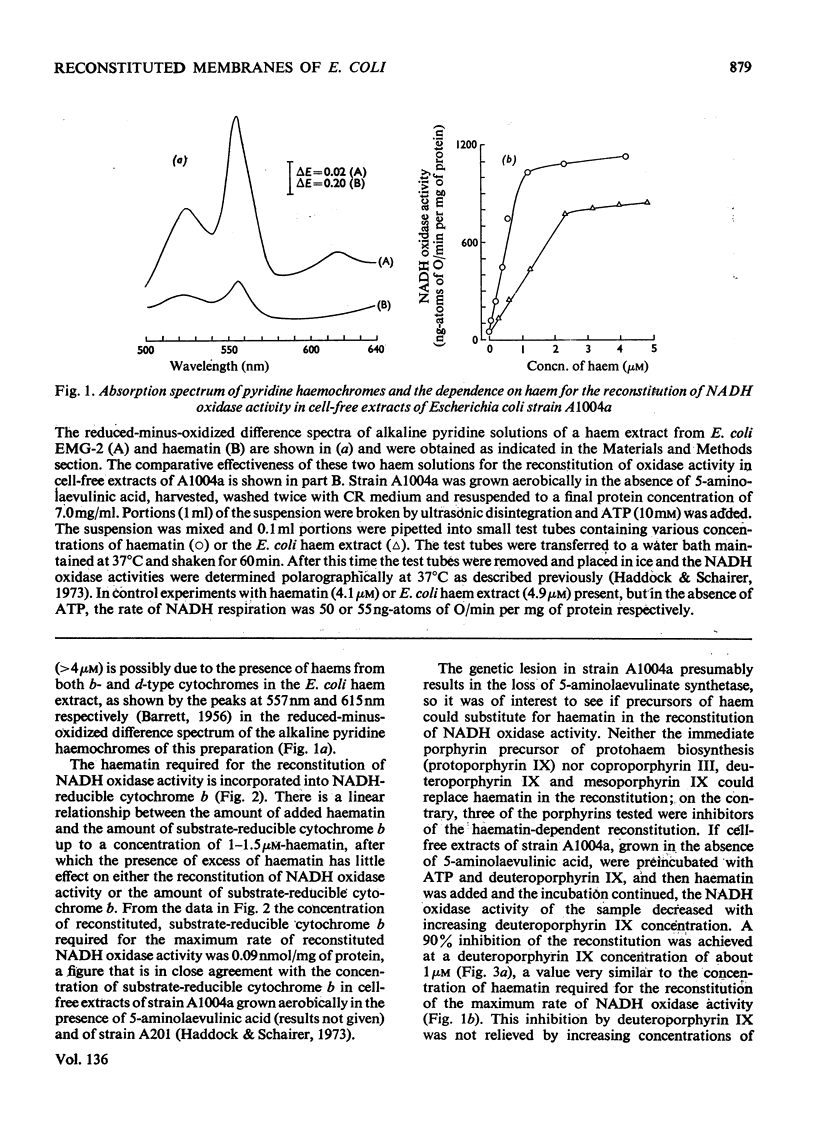
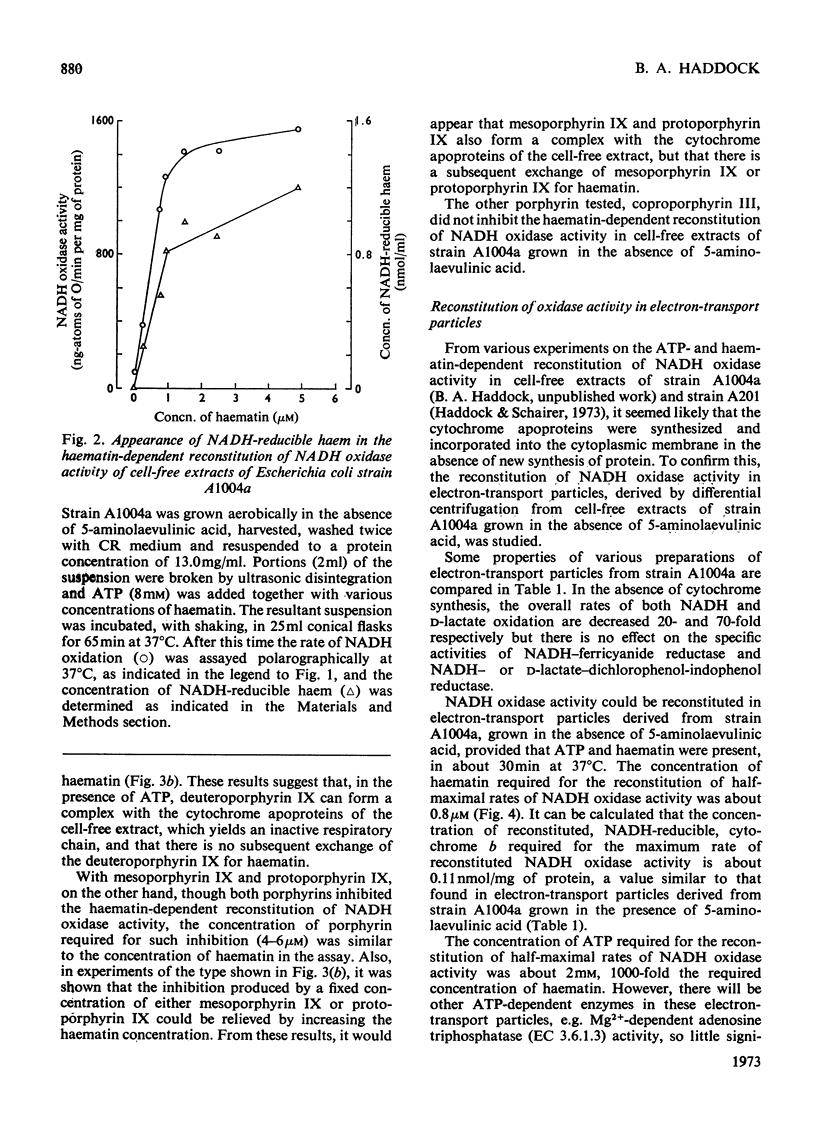
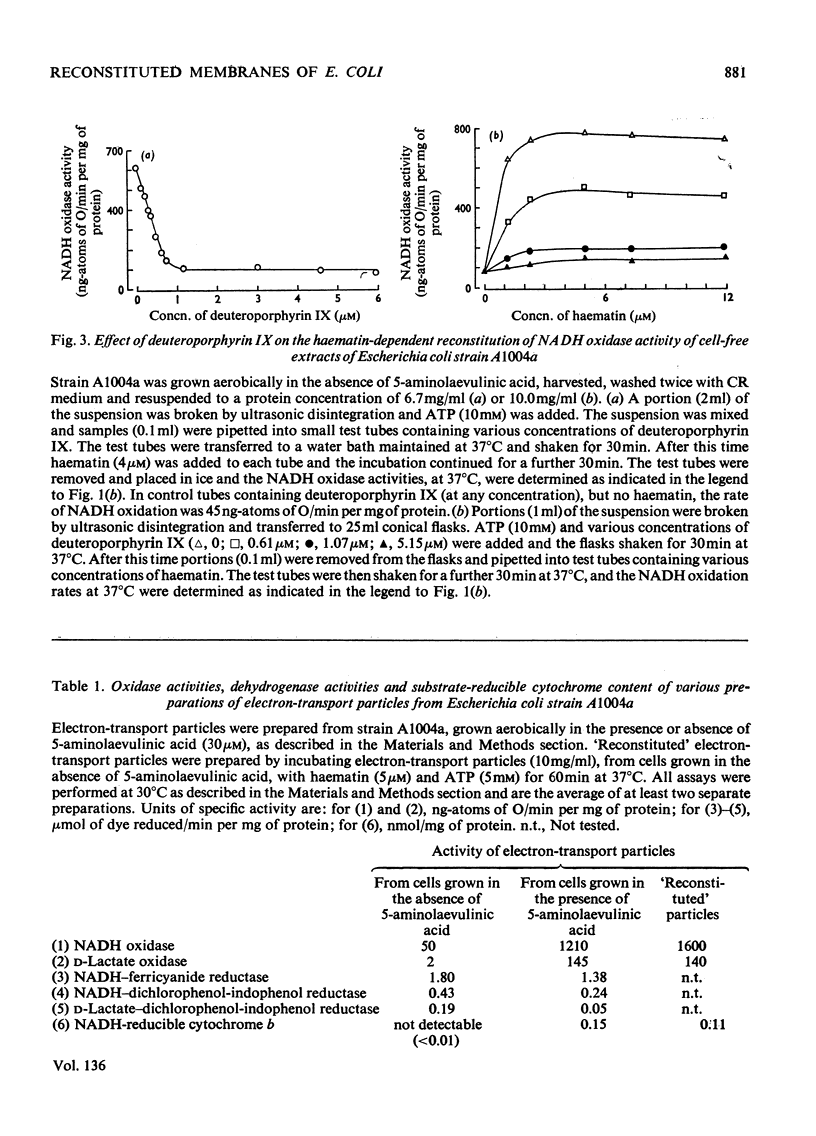
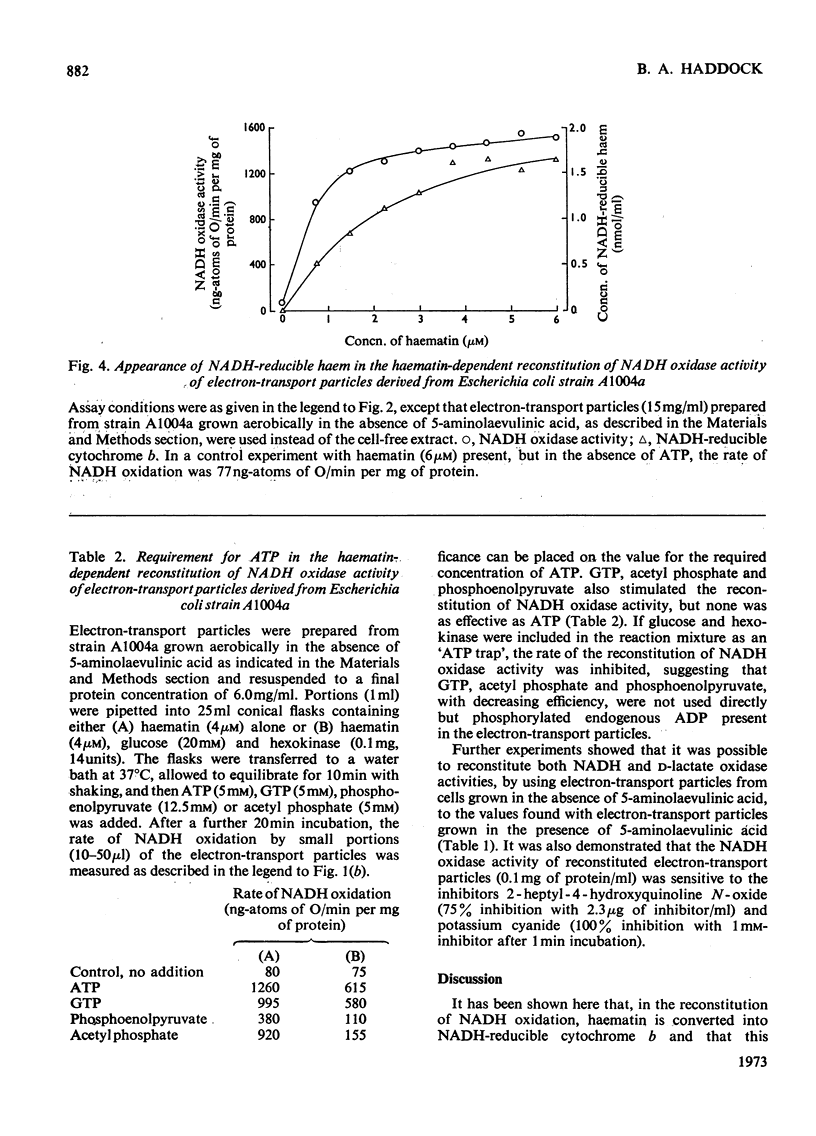
1. The reconstitution of oxidase activity in cell-free extracts of a mutant of Escherichia coli K12Ymel, that require 5-aminolaevulinic acid for growth on non-fermentable carbon sources, is described. 2. The reconstitution is dependent on haematin or a haem extract from a prototrophic strain of E. coli, and the product of the reaction has been identified as NADH-reducible cytochrome b. 3. The requirement for haematin cannot be replaced by four other porphyrins. Coproporphyrin III does not inhibit the haematin-dependent reconstitution, mesoporphyrin IX and protoporphyrin IX apparently compete with haematin for a binding site on the cytochrome apoprotein(s) and deuteroporphyrin IX binds to cytochrome apoprotein(s) and cannot be subsequently replaced by haematin. 4. The properties of electron-transport particles from cell-free extracts of the mutant strain, grown aerobically in the presence or absence of 5-aminolaevulinic acid, are described. In the absence of 5-aminolaevulinic acid no detectable cytochromes are produced, and oxidase activities are lowered but there is no apparent effect on the activities of the NADH dehydrogenase and d-lactate dehydrogenase. 5. The reconstitution of oxidase activity by electron-transport particles from cells grown in the absence of 5-aminolaevulinic acid requires ATP and haematin, and the product of the reaction was identified as NADH-reducible cytochrome b. 6. It is concluded that the cytochrome apoproteins are synthesized and incorporated into the cytoplasmic membrane of E. coli in the absence of haem synthesis. The subsequent reconstitution of functional cytochrome(s) requires protohaem, but the nature of the side chain on the 2 and 4 positions of the porphyrin appears to be important.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARRETT J. The prosthetic group of cytochrome a2. Biochem J. 1956 Dec;64(4):626–639. doi: 10.1042/bj0640626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BASFORD R. E., TISDALE H. D., GLENN J. L., GREEN D. E. Studies on the terminal electron transport system. VII. Further studies on the succinic dehydrogenase complex. Biochim Biophys Acta. 1957 Apr;24(1):107–115. doi: 10.1016/0006-3002(57)90152-x. [DOI] [PubMed] [Google Scholar]
- BELJANSKI M. Sur la formation d'enzymes respiratoires chez un mutant d'Escherichia coli streptomycino-résistant et auxotrophe pour l'hémine. Ann Inst Pasteur (Paris) 1957 Mar;92(3):396–412. [PubMed] [Google Scholar]
- Bragg P. D., Hou C. Reduced nicotinamide adenine dinucleotide oxidation in Escherichia coli particles. I. Properties and cleavage of the electron transport chain. Arch Biochem Biophys. 1967 Mar;119(1):194–201. doi: 10.1016/0003-9861(67)90446-8. [DOI] [PubMed] [Google Scholar]
- CHANG J. P., LASCELLES J. NITRATE REDUCTASE IN CELL-FREE EXTRACTS OF A HAEMIN-REQUIRING STRAIN OF STAPHYLOCOCCUS AUREUS. Biochem J. 1963 Dec;89:503–510. doi: 10.1042/bj0890503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHEN G. N., RICKENBERG H. V. Concentration spécifique réversible des amino acides chez Escherichia coli. Ann Inst Pasteur (Paris) 1956 Nov;91(5):693–720. [PubMed] [Google Scholar]
- Chappell J. B. The oxidation of citrate, isocitrate and cis-aconitate by isolated mitochondria. Biochem J. 1964 Feb;90(2):225–237. doi: 10.1042/bj0900225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R., Charles H. P. Porphyrin-accumulating mutants of Escherichia coli. J Bacteriol. 1973 Jan;113(1):122–132. doi: 10.1128/jb.113.1.122-132.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrard W. T. Synthesis, assembly, and localization of periplasmic cytochrome c. J Biol Chem. 1972 Sep 25;247(18):5935–5943. [PubMed] [Google Scholar]
- Gross M., Rabinovitz M. Control of globin synthesis in cell-free preparations of reticulocytes by formation of a translational repressor that is inactivated by hemin. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1565–1568. doi: 10.1073/pnas.69.6.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddock B. A., Garland P. B. Effect of sulphate-limited growth on mitochondrial electron transfer and energy conservation between reduced nicotinamide-adenine dinucleotide and the cytochromes in Torulopsis utilis. Biochem J. 1971 Aug;124(1):155–170. doi: 10.1042/bj1240155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddock B. A., Schairer H. U. Electron-transport chains of Escherichia coli. Reconstitution of respiration in a 5-aminolaevulinic acid-requiring mutant. Eur J Biochem. 1973 May;35(1):34–45. doi: 10.1111/j.1432-1033.1973.tb02806.x. [DOI] [PubMed] [Google Scholar]
- Jones C. W., Redfearn E. R. Electron transport in Azotobacter vinelandii. Biochim Biophys Acta. 1966 Mar 7;113(3):467–481. doi: 10.1016/s0926-6593(66)80005-x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lodish H. F. Biosynthesis of reticulocyte membrane proteins by membrane-free polyribosomes. Proc Natl Acad Sci U S A. 1973 May;70(5):1526–1530. doi: 10.1073/pnas.70.5.1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PORRA R. J., JONES O. T. Studies on ferrochelatase. 1. Assay and properties of ferrochelatase from a pig-liver mitochondrial extract. Biochem J. 1963 Apr;87:181–185. doi: 10.1042/bj0870181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porra R. J., Langman L., Young I. G., Gibson F. The role of ferric enterochelin esterase in enterochelin-mediated iron transport and ferrochelatase activity in Escherichia coli. Arch Biochem Biophys. 1972 Nov;153(1):74–78. doi: 10.1016/0003-9861(72)90422-5. [DOI] [PubMed] [Google Scholar]
- Schairer H. U., Haddock B. A. -Galactoside accumulation in a Mg 2+ -,Ca 2+ -activated ATPase deficient mutant of E.coli. Biochem Biophys Res Commun. 1972 Aug 7;48(3):544–551. doi: 10.1016/0006-291x(72)90382-8. [DOI] [PubMed] [Google Scholar]
- Săsărman A., Surdeanu M., Horodniceanu T. Locus determining the synthesis of delta-aminolevulinic acid in Escherichia coli K-12. J Bacteriol. 1968 Nov;96(5):1882–1884. doi: 10.1128/jb.96.5.1882-1884.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Săsărman A., Surdeanu M., Szégli G., Horodniceanu T., Greceanu V., Dumitrescu A. Hemin-deficient mutants of Escherichia coli K-12. J Bacteriol. 1968 Aug;96(2):570–572. doi: 10.1128/jb.96.2.570-572.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemoto J., Lascelles J. Coupling between bacteriochlorophyll and membrane protein synthesis in Rhodopseudomonas spheroides. Proc Natl Acad Sci U S A. 1973 Mar;70(3):799–803. doi: 10.1073/pnas.70.3.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A. L., Trotter C. D. Linkage map of Escherichia coli strain K-12. Bacteriol Rev. 1972 Dec;36(4):504–524. doi: 10.1128/br.36.4.504-524.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wulff D. L. Delta-aminolevulinic acid-requiring mutant from Escherichia coli. J Bacteriol. 1967 Apr;93(4):1473–1474. doi: 10.1128/jb.93.4.1473-1474.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]


