Abstract
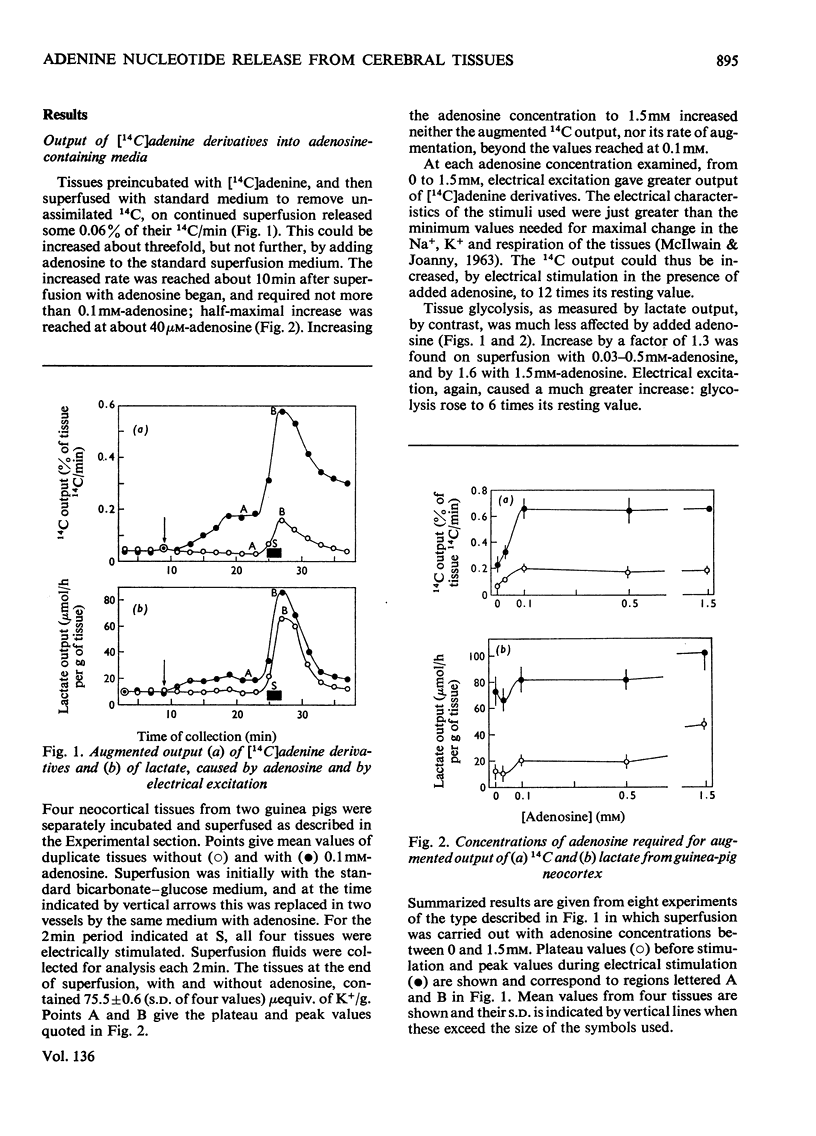
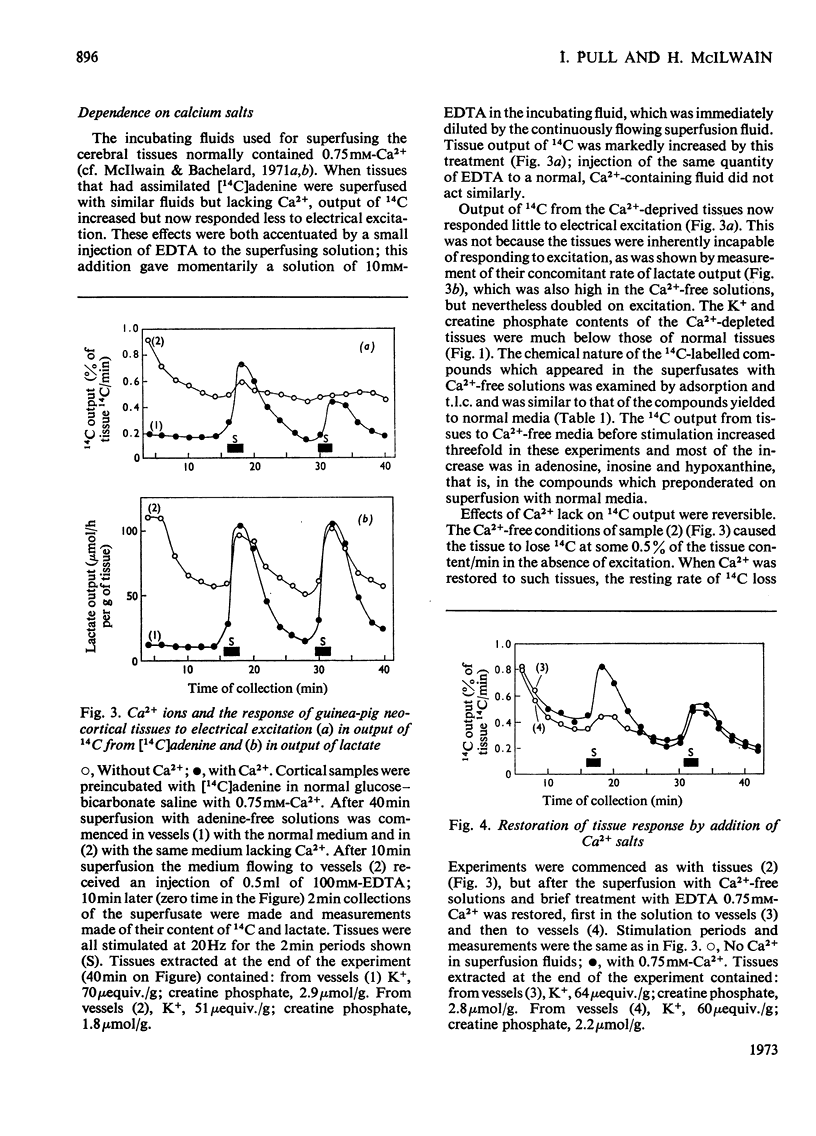
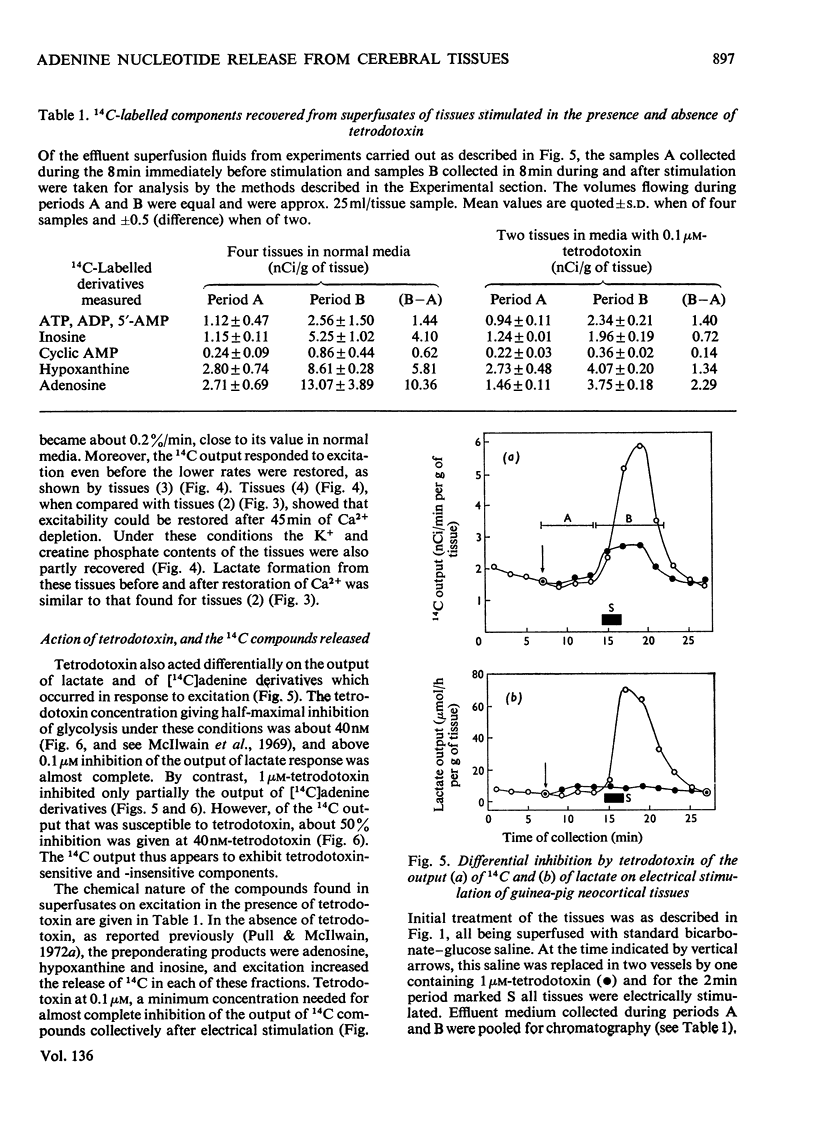
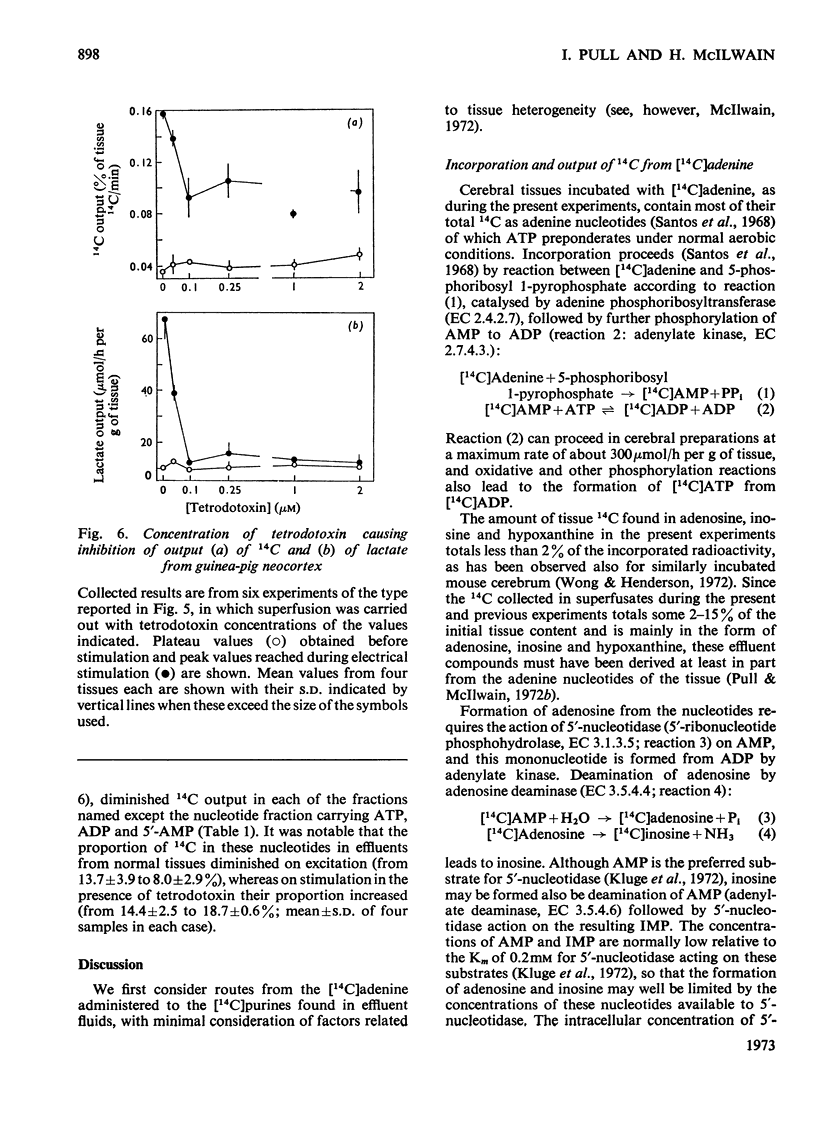
1. Neocortical tissues, exposed briefly to [14C]adenine and containing over 98% of their 14C as adenine nucleotides, when superfused with glucose–bicarbonate salines released about 0.1% of their 14C content/min to the superfusate. 2. Addition of unlabelled adenosine to the superfusing fluid increased the 14C output three- to four-fold; half-maximal increase was given by about 40μm-adenosine, and reasons are adduced for considering the activity of adenosine kinase to be a major factor in conditioning the 14C output. Adenosine similarly increased the enhanced 14C output caused by electrical excitation of the superfused tissue; it brought about only a small increase in tissue glycolysis. 3. Output of 14C from the [14C]adenine-labelled tissues was increased when Ca2+ was omitted from the superfusing fluids, but electrical stimulation did not then liberate more 14C. Nevertheless, such tissues still responded to electrical stimulation by increased glycolysis, and their 14C output again became susceptible to increase by electrical stimulation when Ca2+ was restored. 4. The six-fold increase in tissue glycolysis caused by electrical excitation was almost completely inhibited by tetrodotoxin at 0.1μm and above, but this was associated with about 50% inhibition only in the output of 14C from tissues preincubated with [14C]adenine. The 14C-labelled compounds of which output was most inhibited by tetrodotoxin were adenosine, inosine and hypoxanthine whereas output in a nucleotide fraction was little affected.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banik N. L., Davison A. N. Enzyme activity and composition of myelin and subcellular fractions in the developing rat brain. Biochem J. 1969 Dec;115(5):1051–1062. doi: 10.1042/bj1151051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G. Purinergic nerves. Pharmacol Rev. 1972 Sep;24(3):509–581. [PubMed] [Google Scholar]
- Douglas W. W., Poisner A. M. On the relation between ATP splitting and secretion in the adrenal chromaffin cell: extrusion of ATP (unhydrolysed) during release of catecholamines. J Physiol. 1966 Mar;183(1):249–256. doi: 10.1113/jphysiol.1966.sp007864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmqvist D., Feldman D. S. Spontaneous activity at a mammalian neuromuscular junction in tetrodotoxin. Acta Physiol Scand. 1965 Aug;64(4):475–476. doi: 10.1111/j.1748-1716.1965.tb04206.x. [DOI] [PubMed] [Google Scholar]
- Geffen L. B., Livett B. G. Synaptic vesicles in sympathetic neurons. Physiol Rev. 1971 Jan;51(1):98–157. doi: 10.1152/physrev.1971.51.1.98. [DOI] [PubMed] [Google Scholar]
- Hagiwara S., Nakajima S. Differences in Na and Ca spikes as examined by application of tetrodotoxin, procaine, and manganese ions. J Gen Physiol. 1966 Mar;49(4):793–806. doi: 10.1085/jgp.49.4.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEESEY J. C., WALLGREN H. MOVEMENTS OF RADIOACTIVE SODIUM IN CEREBRAL-CORTEX SLICES IN RESPONSE TO ELECTRICAL STIMULATION. Biochem J. 1965 May;95:301–310. doi: 10.1042/bj0950301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao C. Y. Tetrodotoxin, saxitoxin and their significance in the study of excitation phenomena. Pharmacol Rev. 1966 Jun;18(2):997–1049. [PubMed] [Google Scholar]
- Kluge H., Hartmann W., Wieczorek V., Zahlten W. Kinetic properties of cerebral 5'-nucleotidase. J Neurochem. 1972 May;19(5):1409–1411. doi: 10.1111/j.1471-4159.1972.tb01468.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., PASSONNEAU J. V., HASSELBERGER F. X., SCHULZ D. W. EFFECT OF ISCHEMIA ON KNOWN SUBSTRATES AND COFACTORS OF THE GLYCOLYTIC PATHWAY IN BRAIN. J Biol Chem. 1964 Jan;239:18–30. [PubMed] [Google Scholar]
- Lindberg B., Klenow H., Hansen K. Some properties of partially purified mammalian adenosine kinase. J Biol Chem. 1967 Feb 10;242(3):350–356. [PubMed] [Google Scholar]
- Mandel P., Edel-Harth S. Free nucleotides in the rat brain during post-natal development. J Neurochem. 1966 Jul;13(7):591–595. doi: 10.1111/j.1471-4159.1966.tb11955.x. [DOI] [PubMed] [Google Scholar]
- McIlwain H., Harvery J. A., Rodriguez G. Tetrodotoxin on the sodium and other ions of cerebral tissues, excited electrically and with glutamate. J Neurochem. 1969 Mar;16(3):363–370. doi: 10.1111/j.1471-4159.1969.tb10375.x. [DOI] [PubMed] [Google Scholar]
- McIlwain H. Regulatory significance of the release and action of adenine derivatives in cerebral systems. Biochem Soc Symp. 1972;(36):69–85. [PubMed] [Google Scholar]
- Ozeki M., Freeman A. R., Grundfest H. The membrane components of crustacean neuromuscular systems. I. Immunity of different electrogenic components to tetrodotoxin and saxitoxin. J Gen Physiol. 1966 Jul;49(6):1319–1334. doi: 10.1085/jgp.0491319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pull I., Jones D. A., McIlwain H. Superfused cerebral tissues in hypoxia: neurotransmitter and amino acid retention; labile constituents and response to excitation. J Neurobiol. 1972;3(4):311–323. doi: 10.1002/neu.480030405. [DOI] [PubMed] [Google Scholar]
- Pull I., McIlwain H. Adenine derivatives as neurohumoral agents in the brain. The quantities liberated on excitation of superfused cerebral tissues. Biochem J. 1972 Dec;130(4):975–981. doi: 10.1042/bj1300975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pull I., McIlwain H. Metabolism of ( 14 C)adenine and derivatives by cerebral tissues, superfused and electrically stimulated. Biochem J. 1972 Feb;126(4):965–973. doi: 10.1042/bj1260965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen H. Cell communication, calcium ion, and cyclic adenosine monophosphate. Science. 1970 Oct 23;170(3956):404–412. doi: 10.1126/science.170.3956.404. [DOI] [PubMed] [Google Scholar]
- Rolleston F. S., Newsholme E. A. Control of glycolysis in cerebral cortex slices. Biochem J. 1967 Aug;104(2):524–533. doi: 10.1042/bj1040524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHWARTZ A., BACHELARD H. S., McIL WAIN H. The sodium-stimulated adenosine-triphosphatase activity and other properties of cerebral microsomal fractions and subfractions. Biochem J. 1962 Sep;84:626–637. doi: 10.1042/bj0840626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHANES A. M., BIANCHI C. P. The distribution and kinetics of release of radiocalcium in tendon and skeletal muscle. J Gen Physiol. 1959 May 20;42(5):1123–1137. doi: 10.1085/jgp.42.5.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos J. N., Hempstead K. W., Kopp L. E., Miech R. P. Nucleotide metabolism in rat brain. J Neurochem. 1968 May;15(5):367–376. doi: 10.1111/j.1471-4159.1968.tb11623.x. [DOI] [PubMed] [Google Scholar]
- Shimizu H., Tanaka S., Kodama T. Adenosine kinase of mammalian brain: partial purification and its role for the uptake of adenosine. J Neurochem. 1972 Mar;19(3):687–698. doi: 10.1111/j.1471-4159.1972.tb01384.x. [DOI] [PubMed] [Google Scholar]
- Wong P. C., Henderson J. F. Purine ribonucleotide biosynthesis, interconversion and catabolism in mouse brain in vitro. Biochem J. 1972 Oct;129(5):1085–1094. doi: 10.1042/bj1291085. [DOI] [PMC free article] [PubMed] [Google Scholar]



