ABSTRACT
Nivolumab plus ipilimumab (aCTLA-4/aPD-1) combination therapy has significantly improved clinical outcomes in patients with metastatic melanoma, with 50%-60% of patients responding to treatment, but predictors of response are poorly characterized. We hypothesized that circulating cytokines and peripheral white blood cells may predict response to therapy and evaluated 15 cytokines and complete blood counts (CBC with differentials) from 89 patients with advanced melanoma treated with combination therapy from three points in time: pre-treatment, one month and approximately three months after starting therapy. Clinical endpoints evaluated included durable clinical benefit (DCB), progression-free survival (PFS), and overall survival (OS). A parsimonious predictive model was developed to identify cytokines predictors of response to combination therapy. In this study, we found that pre-treatment, patients with DCB had higher IL-23, lower CXCL6, and lower IL-10 levels. Lower NLR one month after starting therapy predicted better PFS and OS, primarily driven by an increase in absolute lymphocytes. A multivariate model demonstrated that baseline CXCL6, IL-10, IL-23 were independent predictors of therapy response, and the combined model has reached an area under the curve (AUC) of 0.79 in prediction of response to combination therapy. Our study identified baseline CXCL6, IL-23, and IL-10 as predictors of response to aCTLA4/aPD1 combination therapy among patients with metastatic melanoma. This study also provides a framework for identifying patients who are likely to respond to combination ICB, as well as a subset of patients with high risk of developing resistance and are thus in need of alternative therapeutic options, such as clinical trials.
KEYWORDS: Checkpoint blockade, biomarkers for immunotherapy, combination immunotherapy, melanoma, translational research, cytokines
Introduction
Immunotherapy has led to significant improvement in clinical outcomes for patients with advanced melanoma. Approximately 50% of patients receive long term benefit to nivolumab (anti-PD1) plus ipilimumab (anti-CTLA4),1 but biomarkers predicting response to combination therapy are not well characterized. Improved understanding of the mechanisms involved with improved outcomes help predict which patients will likely benefit from combination immune checkpoint therapy as well as gain insight into novel therapeutic strategies.
To date, several soluble biomarkers have been studied, including microRNAs, cfDNA, exosomes, circulating tumor cells and proteins.2,3 Given their critical roles in host immune responses, pro- and anti-inflammatory cytokines and chemokines have been examined as potential biomarkers in multiple disease settings including ovarian cancer, gliomas, genitourinary, and head and neck cancers.4–7 In the clinical context of metastatic melanoma, multiple cytokines in circulation, such as IL-10, IFN-γ, IL-6, IL-8, were found to be associated with response to anti-PD1 treatment,8,9 but whether they are predictors for ipilimumab/nivolumab combination therapy outcome is unknown.
Immune-based biomarkers from peripheral blood, such as absolute lymphocyte counts, absolute neutrophils count, and the neutrophil to lymphocyte ratio (NLR), are considered to reflect systemic inflammation and found to be predictive of survival of immunotherapy-treated patients in multiple cancer settings including melanoma.10–14 However, most patients from these examined cohorts received immune checkpoint blockade (ICB) monotherapy, and whether NLR or the change in NLR with treatment15 is a predictive marker for combination therapy has not been clearly demonstrated.
Overall, only a few studies to date have investigated circulating cytokines as predictive markers specifically for combination ICB16 with a limited panel while most were done in single or mixed ICB settings.8,17,18 One of the largest cytokines studies investigated response and immune related toxicity in a mixed cohort of patients treated with anti-PD1 or combination therapy and found different predictors between single and combination ICB, highlighting the need for specific predictors for combination therapy.19
In our study, cytokines and clinical laboratory values from pre and on treatment peripheral blood samples collected from patients with metastatic melanoma treated with anti-PD1 and anti-CTLA4 combination therapy were evaluated. A panel was designed to include circulating cytokines and chemokines with neutrophil biology relevance or with pro-/anti-inflammatory properties, which we hypothesized to be associated with immunotherapy effectiveness, including some markers previously associated with response to single-agent immunotherapy regimens. Using statistical and machine learning approaches, the potential role of inflammatory mediators as predictors of clinical response to combination checkpoint inhibition was explored.
Materials and methods
Sample collection and cytokine quantification
All samples were collected and processed on Dana-Farber/Harvard Cancer Center approved protocols. An observational study of 89 melanoma patients with unresectable stage III or stage IV disease was conducted. Patients received ipi/nivolumab at the standard dosing of ipilimumab 3 mg/kg and nivolumab 1 mg/kg x 4 cycles (each cycle is 3 weeks), with maintenance nivolumab either 3 mg/kg (or flat dose 240 mg) every 2 weeks or 480 mg flat dose every 4 weeks. Some patients received ipilimumab/nivolumab at a “flip dose” (ipilimumab 1 mg/kg, nivolumab 3 mg/kg) followed by maintenance nivolumab. Clinically, not all patients received 4 cycles of ipilimumab/nivolumab due to toxicity. All patients received treatment at the Dana-Farber Cancer Institute between March 2015 and April 2019 for cohort 1, and April 2019 and January 2020 for cohort 2. Median follow-up was 35 months in cohort 1 (range 12–47) and 13 months in cohort 2 (range 10–14). This dataset includes 60 patients who had cutaneous (N = 57) or acral (N = 3) melanoma in cohort 1 and 29 patients in cohort 2 (cutaneous melanoma only).
For translational analyses, durable clinical benefit (hereon termed DCB) was defined as a best response of complete or partial response or stable disease lasting at least 6 months after starting treatment according to RECIST version 1.1 (Eisenhauer et al., 2009). Patients who derived minimal or no clinical benefit (hereon termed NCB) had progressive disease within 6 months of starting therapy that was not preceded by complete or partial response. High dose steroids use was defined as having glucocorticoids (GCC) above 40 mg once a day.
Blood samples were collected at three timepoints: pre-treatment (Pre), 3 weeks (Post 1) and 2–4 months after the first combination dose (Post 2). Clinical laboratory values were collected for 95.5% of patients (85 out of 89) for pre-treatment timepoint and 82% for timepoints Post 1 and Post 2 respectively.
Whole blood samples were collected at three timepoints: pre-treatment (Pre), 3 weeks (Post 1) and 2–4 months after the first combination dose (Post 2) from all patients using 10 mL EDTA tubes and processed following Standard Operating Procedures. Briefly, the tubes were centrifuged at 1500 rpm for 10 minutes using the Sorvall Legend XTR centrifuge. Subsequently, 2 ml of plasma per tube was aspirated and aliquoted into four microcentrifuge tubes (Fisherbrand, 05-408-138). The plasma was then subjected to another centrifugation step at 3000 RPM for 5 minutes using the Sorvall Legend Micro 21 R centrifuge. After centrifugation, the plasma was aspirated into Cryogenic tubes at a volume of 2 ml per tube (Corning 430,488) and stored at −80°C, respectively. At the start of the experiment, plasma samples were retrieved from the −80°C storage and thawed on ice for approximately 60 minutes. Once completely thawed, plasma samples were centrifuged at 10,000 × g for 10 minutes using a Sorvall Legend XTR centrifuge. All samples were diluted 2-fold following the manufacturer’s guidelines. Subsequently, 50 uL of each diluted sample was distributed into a 96-well U-bottom plate in duplicates.The lyophilized proteins were reconstituted and subjected to a 4-fold serial dilution to generate a total of 7 standard controls with one blank. Duplicates of 50 uL of standards were added to the remaining wells of the sample plate. Furthermore, 50 uL of bead mix was added to all wells and incubated 2 hours at room temperature. After incubation, the plate was washed twice and a detection mix was added to each well. The sealed plate was incubated for 30 minutes at room temperature. Following incubation, the wash steps were repeated three times. After the final wash, 50 uL of Streptavidin-PE was added to each well of the plate and incubated on the for 30 minutes at room temperature. Following a final round of wash steps, 120 uL of reading buffer was added to each well. The plate was incubated for an additional 5 minutes at room temperature. After the wash, the samples were ready to run on the FLEXMAP-3D instrument (Luminex Inc, TX).The xPONENT software was used to extrapolate Median Fluorescent Intensity (MFI) to known standards. All markers were expressed as “pg/ml”.
Quality control of luminex data
Analytes that showed concentrations at LLOQ across all samples were excluded from the analysis. Fifteen cytokines available in both cohorts were included in downstream analysis (Supp Table S5). Values of cytokines below the lower limit of quantifications were substituted with the LLOQ or the minimum value of that cytokines across samples if the LLOQ is not specified. In total, 11 of 15 cytokines analyzed had at least one sample with a substitution. As a sensitivity analysis, we also tested the substitution with zeros rather than the LLOQ, which did not affect our conclusions.
H&E staining and neutrophil quantification
Standard clinical histology techniques were used to formalin fix and embed tumor biopsies into Formalin-Fixed Paraffin-Embedded (FFPE) blocks. Thin (4 um) microtome sections were affixed to glass microscope slides and stained for hematoxylin & eosin (H&E). Samples were de-identified and reviewed by a pathologist blinded to other aspects of study findings (e.g., patient outcome). Neutrophil abundance within the tumor area was visually scored by a board-certified pathologist (S.J.R.) using a scale of 0–3, with a score of 0 indicating that no neutrophils were seen in the tumor; 1 indicating rare neutrophils within the tumor, 2 indicating moderate numbers of neutrophils, and 3 indicating the highest number of neutrophils detected in the tumor.
Statistical method
The Wilcoxon rank-sum test was utilized to compare protein levels between patients with and without DCB. For all analyses, two-tailed p ≤ 0.05 was considered statistically significant and no corrections for multiple comparisons were made unless stated otherwise. Changes in cytokines over time between patients with/without DCB were evaluated using longitudinal mixed models, with log2 (marker) fit as a function of DCB/no DCB, time, and the interaction of DCB and time. A first-order autoregressive covariance structure was used within patient for the repeated measurements. Models allow for stratification according to cohort. Comparisons of fixed effects are based on F-tests; pairwise comparisons are based on contrasts. There are no corrections for multiple comparisons. Spearman’s correlation was used to assess associations among cytokines, chemokines, clinical labs values, and immune cells proportion. In the analyses where cytokines were categorized into high/low, they were divided at the median of the respective distribution values. The effect of prior IO on cytokines was evaluated using van Elteren test, with subgroups stratified by ranked propensity scores. Propensity scores were calculated using the baseline characteristics of age, gender, M stage, and ECOG, to predict the probabilities of patients with these characteristics would receive prior IO as estimated by a logistic regression. Outcomes (response to therapy, survival) were determined separately and prior to assessments of potential predictors. Unless specified otherwise, only patient samples with available data on the variables of interest were included in the analyses.
Survival analysis
The distributions of overall and progression-free survival are summarized using the method of Kaplan-Meier with comparisons based on the log-rank test. Cox proportional hazards regression was used to estimate hazard ratios and 95% confidence intervals. To investigate the stratifying ability of different combinations of circulating cytokines and chemokines that are associated with DCB, pairwise comparison results were evaluated and generated combined features that best stratified patients in both overall and progression-free survival. The survival analysis was implemented with R package survival,surminer.
Prediction of response to combination ICB
To develop a predictive model, we used a logistic regression due to its simplicity and interpretability given our cohort size. The initial feature set included all analyzed cytokines and clinical laboratory values. To develop a parsimonious model and reduce overfitting, we employed a forwards stepwise search and a backward stepwise search as feature selection techniques using BIC (Bayesian Information Criteria) as our scoring criteria to penalize model complexity and search the space for the best combination of predictive features. Forward selection begins with a model without predictors but just includes the intercept. At each step, this method tests adding a feature to the current model and chooses the model (with the added feature) that results in the lowest BIC. The search stops when no better model is found by testing addition of another feature. During backward selection, this method starts out with all features included in the model, and then it evaluates models after dropping out features to find improved models (by BIC criteria), and uses the best model as the new model for consideration in the next step. We then did tenfold cross validation to estimate the performance of our model on unseen data, implemented with R package caret. We performed a permutation test to generate an empirical p value for the cross-validation AUC, where we permutated the outcome labels (DCB vs. nDCB) randomly for 1,000 times and generated cross-validation AUC for the three-feature predictive model (CXCL6, IL-10, IL-23) to produce a null distribution of cross-validation AUC, under the null hypothesis that these features are not associated with outcome. The proportion of simulations with cross-validation AUC greater than or equal to our initial tenfold cross-validation AUC was considered to be a permutation-based empirical p value.
Results
Patients and treatment characteristics
Blood samples were collected at three timepoints: pre-treatment (pre), 3 weeks (post 1) and 2 to 4 months after the first combination dose (post 2) according to Dana-Farber/Harvard Cancer Center approved protocols. 89 melanoma patients with unresectable stage III or stage IV disease treated with combination ipilimumab and nivolumab were identified.
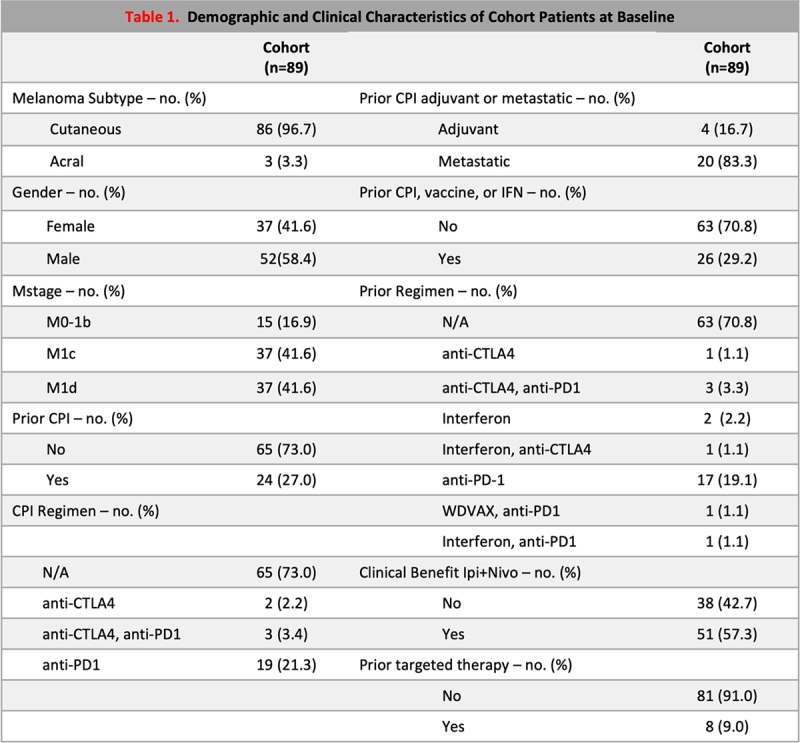
Among 89 patients, 86 patients had cutaneous melanoma and 3 had acral melanoma. 41.6% were females, 58.4% were males. Median age was 59 (range 27–83). 64.7% patients received prior treatment with ICB, vaccine, and/or Interferon. 57.3% patients had durable clinical benefit (DCB), defined as having a RECIST best response of complete or partial response or stable disease and at least 6 months PFS, with the reminder annotated as no durable clinical benefit (NCB). Additional patient demographics and clinical annotations are summarized in Table 1.
Table 1.
Patients, treatment, and clinical characteristics.
 |
Baseline cytokines CXCL6, CXCL5, IL-10 and IL-23 associate with durable clinical benefit
We evaluated the association of expression levels of 15 cytokines at the 3 time points with response (Figure 1(a), Supplemental Figure S1). At baseline, CXCL6, IL-10, and CXCL5 were significantly higher in patients with NCB (MWW, p < 0.05) while IL-23 was more abundant in patients with DCB (MWW, p < 0.05)(Figure 1(b)). When characterizing patients by RECIST criteria (42 patients with CR/PR, 9 patients with SD, and 37 patients with PD as best response), we found that there was no statistically significant difference in CXCL6, IL23, or IL10 levels between CR/PR and SD, but substantial differences in patients with PD (Supplemental Figure S1). Patients with NCB had higher IL-10 at all three time points (MWW, p < 0.05, Figure 1(a)). Patients with NCB also showed higher IL-1RA on treatment (Figure 1(a)).
Figure 1.
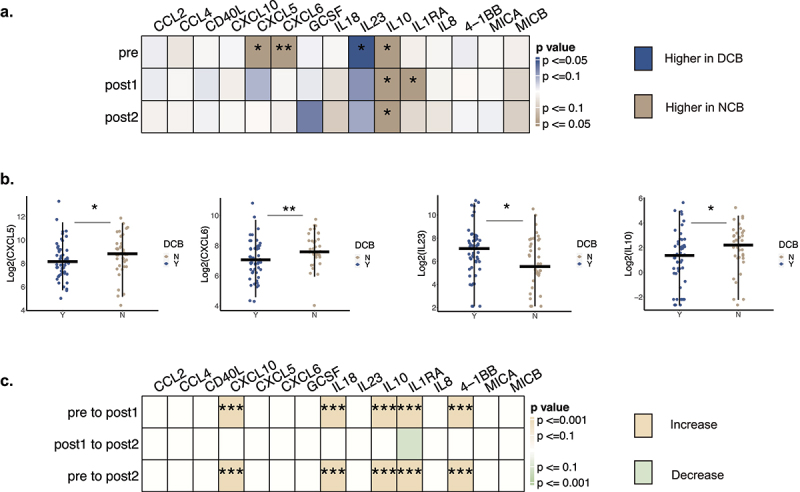
Association between cytokines levels and response to combination immune checkpoint blockade. A. Differential cytokines and chemokines abundance in responders (DCB, patients with durable clinical benefit) versus non-responders (NCB, patients without durable clinical benefit) at three different time points (pre, post1, post2). The comparisons were done using Wilcoxon rank-sum test. Cytokines concentration higher in responders are shown in blue while those higher in non-responders are shown in sand. Significance test results are marked with asterisks in heatmap (*p <0.05, **:p<0.01, ***:p <0.001). (Pre: DCB =51 NCB = 38 ntotal= 89; post1: DCB =43 NCB = 34 total= 77; post2: DCB =47 NCB = 29 total= 76). B. Baseline CXCL5, CXCL6, IL-23, IL-10 in responders versus non-responders. (DCB Y: Patients with durable clinical benefit. DCB N: Patients without durable clinical benefits). Responders had higher CXCL5 (MWW, p < 0.05), CXCL6 (MWW, p <0.01), higher IL-10 (MWW, p <0.05), and lower IL-23 (MWW, P<0.05) (DCB =51, NCB = 38). C. Longitudinal changes of cytokines and chemokines following treatment. Cytokines changes were evaluated with a longitudinal mixed model combining both DCB and NCB (see methods), with yellow for increasing concentration and green for decreasing concentration (*:p <0.05, **:p<0.01, ***:p <0.001).
There were clear patterns of longitudinal cytokine change with treatment, with increases in CXCL10, IL-18, IL-10, IL1RA, 4-1BB (Wald p < 0.001, Figure 1(c), Supplemental Figure S1) from pre to post 1 time points, which persisted at post 2. High dose steroids use (GCC >40 mg/day) between pre and post 1 was associated with decreased log fold changes of CXCL5, CXCL6, CXCL10, and 4-1BB (MWW, p < 0.05) at the post 1 time point (Supplemental Figure S2), but the effects of immunotherapy remained consistent after adjusting for high dose steroid administration. Interestingly, patients with DCB and NCB displayed different longitudinal changes in CXCL5, IL8, and MICB (Supplemental Figure S3). More specifically, CXCL5 (associated with neutrophil trafficking) and MICB (associated with natural killer cells activation), were decreased and increased, respectively, uniquely in patients with NCB (Supplemental Figure S3). Among patients with NCB, the plasma concentration of CXCL5 significantly decreased from pre treatment timepoint to post 1 while MICB displayed an increase from pre-treatment to post 2 (Supplemental Figure S3). The fold change of IL-8 from pre to post 2 time point was higher in patients with NCB (Supplemental Figure S3).
Neutrophils to lymphocytes ratio (NLR), absolute lymphocytes count, and fold change of lymphocytes are associated with durable clinical benefit
Neutrophil-to-lymphocyte ratio (NLR) has been posited to represent systemic inflammation and the balance between pro- and anti-tumor factors.14,20,21 Higher NLR prior to treatment has previously been shown to be associated with resistance to single-agent immune checkpoint blockade.10,22
In our cohort, baseline NLR was not predictive or prognostic of clinical outcome, but NLR specifically at post 1 timepoint was associated with response to treatment, with lower NLR favoring durable clinical outcome (Figure 2(a)). Patients with lower NLR (stratified by median = 2.76) also exhibited reduced rate of disease progression or death (Figure 2(b)).
Figure 2.
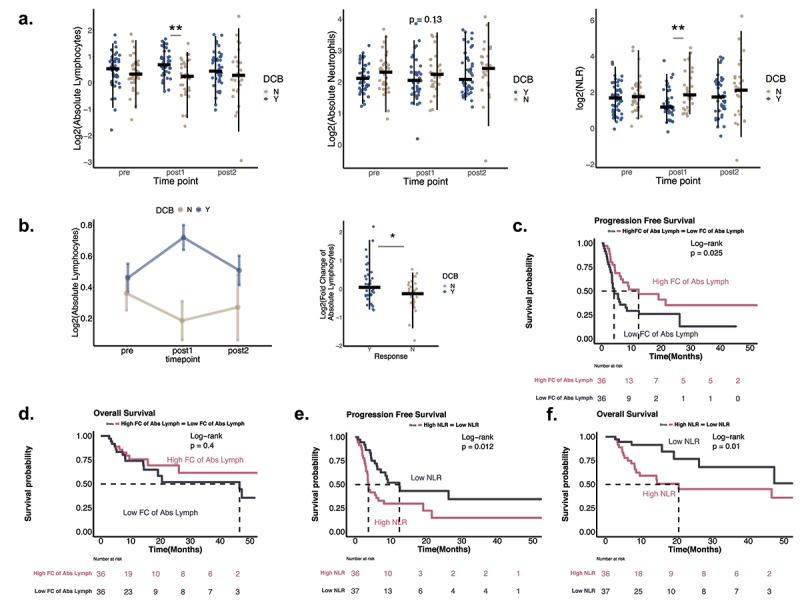
Association of peripheral neutrophil and lymphocyte counts with response to therapy and survival. A. Association of peripheral neutrophil and lymphocyte counts at three time points with response to therapy. Wilcoxon rank-sum test was performed, with asterisks indicating significance level: p- value<=0.05*, p-value<=0.01**. Counts in DCB are shown in blue and in NCB in sand. NLR: Neutrophils to Lymphocytes ratio. (Pre: n = 85 samples, post1: n = 73 samples, post2: n = 73 samples) B. Change in peripheral lymphocyte counts by response to therapy. Left, change in mean lymphocyte count at three time points in DCB and NCB patients. Whiskers indicate mean standard error. Right, fold change of lymphocyte counts from pre to post1 between responders and non-responders (Wilcoxon rank-sum, p <0.05). (Pre: n = 85 samples, post1: n = 73 samples, post2: n = 73 samples; patients with matched pre and post1: n = 72 samples, DCB = 39, NCB = 33) C.D. Survival in patients stratified by fold changes of ALC from pre to post1 time point. PFS and OS stratified patients by the median of ALC. E.F. Survival in patients stratified by high and low NLR at the post1 time point. PFS and OS, stratifying by median level of NLR at post1 time point (2.67).
Further evaluation reveals that the prognostic value of NLR at post 1 timepoint was primarily driven by an elevation of absolute lymphocytes counts from pre to post 1 (Figure 2(b), Supplemental Figure S4a). Patients with DCB had overall larger increases of lymphocytes compared with NCB (Figure 2(b)), and increased log-fold change of absolute lymphocytes counts (ALC) was associated with longer progression free survival (Figure 2(c,d)). However, stronger associations with survival were found with NLR, suggesting that neutrophils contribute additional prognostic value (Figure 2(e,f)). Interestingly, logfold changes in NLR were associated with response but not PFS or OS (Supplemental Figure S4). Adjusting for the effects of high dose steroids (Supplemental Figure S2) did not impact these associations.
An integrative analysis revealed the association between CXCL6 and a neutrophils-related coordinated program; IL-10 and IL-23 correlate with other circulating cytokines
To identify coordinated programs of cytokine expression, we performed a hierarchical clustering of correlation between all baseline cytokines and chemokines (Figure 3(a), Supplemental Figure S5). Among the identified features associated with clinical outcome, CXCL5 and CXCL6 were strongly correlated (Spearman <rho ≥0.77, p < 2.2e-16), but IL-10 and IL-23 were uncorrelated. CXCL6, CXCL5, IL-8 and CCL4 formed a strong cluster (minimum pairwise Spearman correlation rho = 0.52, p < 2.1e-07)(Figure 3(a)), suggesting coordinated activities of these neutrophil-related cytokines.
Figure 3.
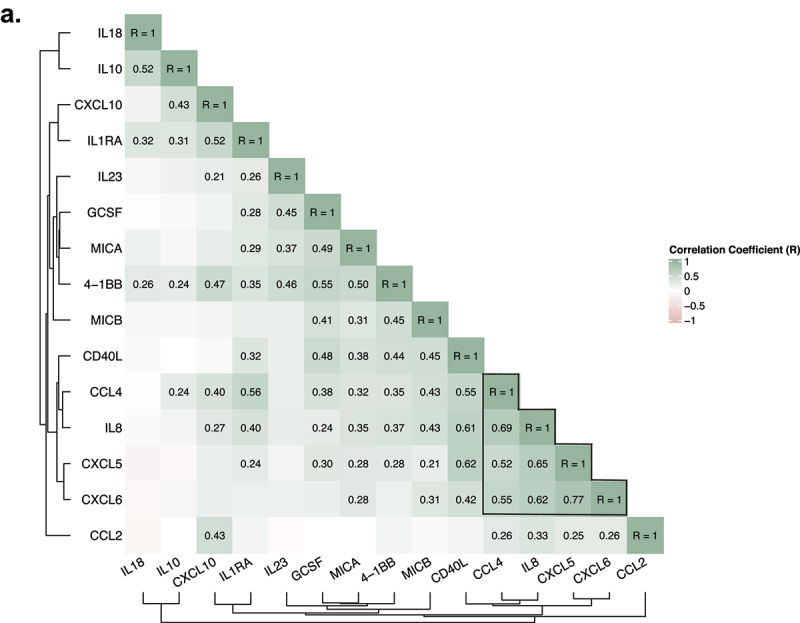
An integrative analysis of the association between baseline chemokine CXCL6 and neutrophils related chemokines and cytokines; IL-10 and IL-23 correlated with other circulating cytokines. A. Correlation matrix among all baseline cytokines and chemokines. (Spearman correlation, only statistically significant coefficient values displayed; n = 89 samples).
In a subset of samples (n = 44), we characterized the level of neutrophils in the pre-treatment tumor microenvironment as high or low (n = 11 high, 33 low, Methods). In this size cohort, the level of neutrophils in the tumor microenvironment was not significantly associated with response (OR = 0.39, p = 0.30 Fisher’s Exact, Supplemental Figure S6), and further was not associated with circulating absolute neutrophils counts (ANC) or CXCL6 levels (Supplemental Figure S6).
Baseline IL-23 formed a cluster of positive correlates with G-CSF, MICA, 4-1BB, and pretreatment IL-10 positively correlated with 4-1BB, CCL4, IL1RA, CXCL10, respectively (Figure 3(a)).
Evaluating joint predictive performance of CXCL6, IL-10, and IL-23
We hypothesized that a multivariate model using features derived from peripheral blood may improve our ability to predict response and outcomes to combination immune checkpoint blockade using less invasive and readily accessible assays. Considering all cytokines and clinical lab values as potential features (Supp Table S1), we developed a multivariate logistic regression model to predict DCB vs NCB and performed feature selection using Bayesian Information Criteria (BIC) as criteria to develop parsimonious models (details in Methods). Forward and backwards selection converged on the same model (Figure 4(b)) with three independent features: CXCL6, IL-10, and IL-23 (Figure 4(a,b), Supplemental Figure S7). The multivariate logistic regression model with CXCL6, IL-10, and IL-23 as binary high and low (split by median value) had an AUC of 0.79 (tenfold cross-validation mean AUC = 0.77, empirical p < 0.001), and patients predicted to be DCB by this model had an OR of 8.97 [95% CI 3.36–23.99] (Figure 4(b)). High CXCL6 (OR = 0.14 [95% CI 0.05–0.40], p = 2.39e-4) and high IL10 (OR = 0.27 [95% CI 0.10–0.74], p = 0.011) predicted NCB, while high IL23 (OR = 3.47 [95% CI 1.22–9.85], p = 0.019) predicted DCB to therapy.
Figure 4.
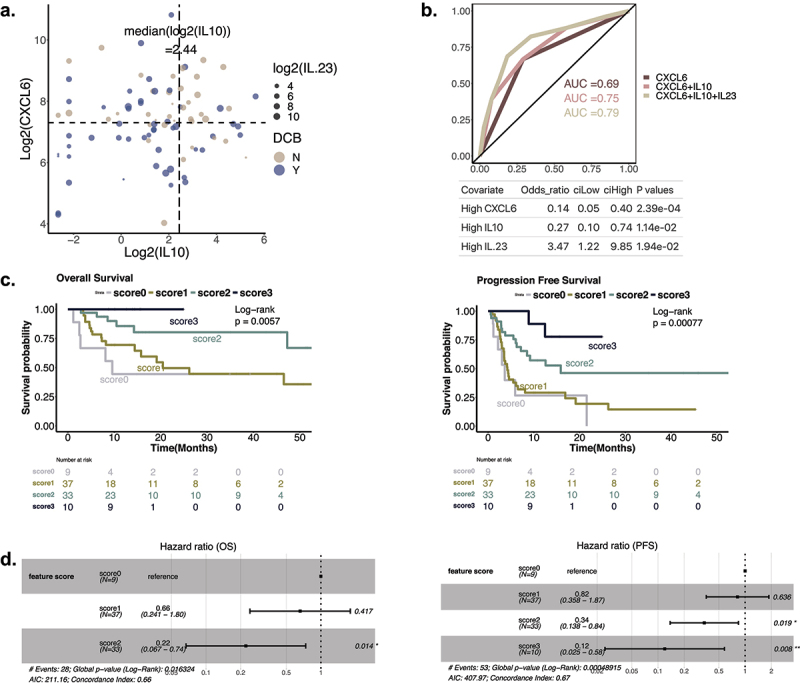
Predicting response and survival with CXCL6, IL-10, andIL-23. A. Joint association of DCB with CXCL6, IL-10, and IL-23. x and y axes correspond with the levels of IL-10 and CXCL6, and the size of the dots represent the IL-23 level. Responders are colored with blue and non-responders with sand. The dotted lines represent the median value of CXCL6 and IL-10. B. ROC of the multivariate logistic regression models implemented with different features. Odds ratio of patients predicted to be DCB by the three-feature model = 8.97 (95% CI: 3.36-24.00, p = 0.0002827). C. Effect of feature scores on patients’ survival outcome. Patients were stratified into four groups using a feature score defined by the number of positive features (high IL23, low CXCL6, low IL10). D. Cox model on feature scores. (left) OS model showing hazard ratios of feature score 1 and 2, with 0 as reference. Feature score 3 was excluded from the model as the patient group with score 3 did not have any deaths. (right) PFS model showing hazard ratios of feature scores 1, 2, and 3, with 0 as reference.
Using a multivariate cox regression model, we found that high CXCL6 and high IL-10 was associated with worse PFS and OS, while high IL-23 was related to improved PFS and OS (Supplemental Figure S8a, b). After correcting for clinical features including age, Mstage, previous IO treatment, ECOG, and high dose steroids use, higher CXCL6, IL10, and lower IL-23 still robustly associated with increased risk of progression and death (Supplemental Figure S8c,d).
We then calculated a composite score for each patient based on the number of favorable CXCL6, IL-10, and IL-23 features (i.e., a score from 0–3 positive features of low CXCL6, low IL-10, and high IL-23), and found improved survival as the score increased (Figure 4(c), Supplemental Figure S9c). Each additional positive feature in the samples was associated with 51% improvement of progression free survival and 42% improvement in overall survival (Cox PFS HRR (hazard ratio for progression): 0.51, CI: 0.36–0.71, p < 0.001; Cox OS HRR (hazard ratio for death): 0.42, CI: 0.25–0.69, p < 0.001) (Supplemental Figure S9c). Specifically, a score of 3 identified an excellent prognosis group with 89% PFS (95% CI: 0.706–1.000) and 100% OS (95% CI: 1–1) at 1 year, while a score of 2 favorable features was associated with 57.2% PFS and 85.6% OS at 1 year, with a 66% decreased rate of progression (HRR: 0.34, CI: 0.138–0.84, p = 0.019) and 78% decreased rate of death (HRR: 0.22, CI: 0.067–0.74, p = 0.014)(Figure 4(d)) compared to patients with no favorable features.
Prior immunotherapy affects cytokine levels and association of cytokines with DCB
As a subset of our patients had immunotherapy (IO) prior to combination ICB (n = 24), and past studies have shown differential predictors of response to IO based on prior treatment,23,24 we wanted to perform exploratory analyses of the effect of prior IO therapy on prediction of response to combination ICB. First, patients with prior IO in our cohort had a numerically lower response rate to combination therapy (50% vs 60%, Fisher’s exact test, p = 0.4, OR = 0.67)(Figure 5(a)). Adjusting for propensity to have had prior IO (Methods), baseline pre-combination therapy GCSF, 4-1BB, MICA, and MICB levels were higher in prior IO treated patients (Figure 5(b)). The predictive model with CXCL6, IL-23, and IL-10 developed in the entire cohort had similar performance when applied to patients with and without prior IO separately (AUC = 0.73 and 0.8, respectively, Figure 5(c)). Higher levels of CXCL6 and IL-10 levels in NCB vs DCB were similarly found in both subgroups though only statistically significant in one (CXCL6: IO-naive subgroup; IL10: prior IO subgroup, Figure 5(d,e))) potentially due to differences in sample and effect size. Interestingly, the finding that IL23 was higher in patients with DCB appears to be driven primarily by the IO-naive subgroup (Figure 5(f), p = 0.0006 for the IO-naive subgroup). Formally, there was no statistical evidence of interaction with prior IO for the relationship of CXCL6 and IL-10 levels with DCB status (Supplemental Figure S10, Supp Table S2), but strong evidence of interaction with IL-23 (Supplemental Figure S10, Supp Table S2, p = 0.009 for the interaction of prior IO and IL-23)). When we looked at the significant baseline features in IO treated and untreated patients separately, we observed that among IO treated patients, higher IL23, lower CXCL5, and lower CXCL6 associated with durable clinical benefit (MWW, IL23 p = 0.000562, CXCL5 p = 0.0411, CXCL6 p = 0.0216, patients n = 65)(Supplemental Figure S10c), while for patients without prior IO, lower IL10 associated with response (MWW, p = 0.00352, patients n = 24) (Supplemental Figure S10d).
Figure 5.
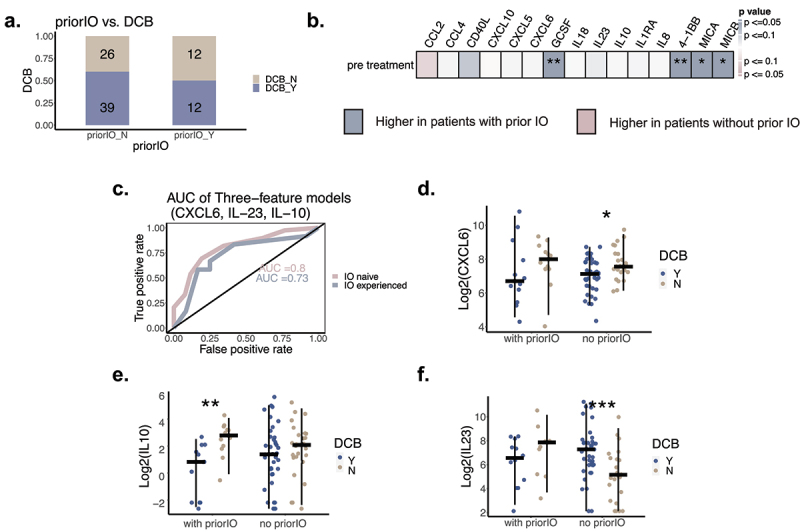
The effect of prior IO treatment on cytokines and their associations with response. A. Association between prior IO and DCB (Fisher’s exact test, p = 0.4, OR = 0.67). B. Systemic effect of prior IO on circulating cytokines from pre treatment time point. Van elteren tests were performed to evaluate baseline cytokines association with priorIO, adjusted by propensity scores for receiving priorIO. Significance test results are marked with asterisks in heatmap (*:p <0.05, **:p<0.01, ***:p <0.001) (patients with priorIO n = 24; patients without priorIO n= 65). C. AUC of applying three- feature model on IO treated and untreated patients subsets. (patients without priorIO: AUC = 0.8, patients with priorIO: AUC = 0.73). D. Association between response to therapy and CXCL6 levels stratified by prior IO. MWW tests were performed, with asterisks showing statistical significance (*:p <0.05, **:p<0.01, ***:p <0.001). E. Association between response to therapy and IL10 levels stratified by prior IO. MWW tests were performed, with asterisks showing statistical significance (*:p <0.05, **:p<0.01, ***:p <0.001). F. Association between response to therapy and IL23 levels stratified by prior IO. MWW tests were performed, with asterisks showing statistical significance (*:p <0.05, **:p<0.01, ***:p <0.001).
Discussion
In this study, a cohort of 89 patients with metastatic melanoma treated with nivolumab and ipilimumab (aCTLA4/aPD1) combination therapy was analyzed. Pre-treatment circulating chemokines and cytokines, specifically lower CXCL6, IL-10, and higher IL-23, predicted response to combination ICB treatment. Coordinated immune programs suggest the roles of these cytokines in regulation of response. In addition, lower NLR measured approximately one month after combination treatment started was associated with better survival outcome, mainly driven by an increase in absolute lymphocytes in responders.
IL-23 can exhibit both pro- and anti-tumor effects.25 Despite its importance in host immunity regulation and tumor immunity, IL-23 has not previously been reported as associated with durable clinical benefits in ICB in metastatic melanoma. To our knowledge, only one study of combo aPD-1/aCTLA-4 treated previously IO naive metastatic melanoma patients evaluated peripheral IL23 and found it was not statistically associated with response, but in a limited cohort with only 11 non-responders.19 In our cohort, higher baseline IL-23 was associated with response to treatment. When acting as a pro-inflammatory cytokine, IL-23 can prime T cell-mediated tumor control, but whether the fulfillment of an inflammatory function can explain the enhanced response with higher IL-23 in our cohort requires future study.
CXCL6 can function as an angiogenic cytokine that promotes angiogenesis,26 and is also known for its ability to recruit neutrophils to target sites.27 However, CXCL6 has not been reported to associate with clinical outcome in melanoma patients previously. In the current study, higher CXCL6 predicts a worse response, and is additionally part of a coordinated immune program consisting of CXCL5, IL-8, and CCL4. The function and sources of cytokines present in this program are all known to connect with neutrophils. CXCL5 and IL-8 are important mediators of neutrophils trafficking28 and CCL4 is mainly produced by neutrophils, monocytes, and macrophages and attracts other immune cells.29 This cytokine program suggests a potential link between CXCL6 and neutrophil activities in regulation of response to therapy. Interestingly, a correlation between CXCL6 and absolute neutrophil counts in peripheral blood or tumor microenvironment was not detected in our data, suggesting that neutrophils interaction is a complex process. Whether peripheral CXCL6 level is associated with cytokine levels and neutrophil activity in the tumor microenvironment is an interesting future direction of investigation.
IL-10 may exert both pro and anti-tumor effects.30 Previous studies have shown that melanoma cells can secrete IL-10 to reduce immune surveillance in advanced melanoma patients.31 Higher IL-10 levels may therefore be an indicator of an immunosuppressive state orchestrated by both tumor cells and tumor-associated inflammatory cells that can be reflected in circulating peripheral blood. However, some studies also report that IL-10 is closely correlated with other immune factors, suggesting that the elevation of IL-10 can represent a state of initiation of immune responses and subsequent suppression of those responses.8 Reflecting these different potential roles, IL-10 in clinical studies have been associated with conflicting phenotypes with treatment of ICB. Consistent with our current study, IL-10 has been implicated as prognostic of worse survival in stage IV melanoma patients.31 Conversely, one recent study on ipilimumab plus nivolumab treated melanoma patients did not find pre treatment IL-10 associated with response to treatment,16 different from our cohort which finds a significant association with durable clinical benefit with lower IL-10 levels at baseline with a larger sample size. Instead, they found that IL-10 fold change was enriched in a subgroup of patients with better progression free survival, and in our cohort, although we did not see statistical significance between IL-10 increment and PFS, our data suggested a separation of patients survival outcomes (Supplemental Figure S11a). Further in that study, all the patients were treatment naive, whereas a subset of the patients in our cohort were IO-experienced. Indeed, when we stratified by prior IO-therapy, we found a stronger association in IO-experienced patients (Figure 5(e)), though the trends in both groups were similar and the same three-feature model predicted well in both subgroups (Figure 5(c)).
As patients with earlier stage disease increasingly receive IO as adjuvant therapy, predicting response to combination IO in the Stage IV setting in this IO-experienced subgroup will become increasingly important.32 In addition, several studies have shown evidence for differential predictors of response based on exposure to IO treatment.23,24 Some cytokines are sensitive to prior IO9 and we showed in our study that prior IO can exert different impacts on the association between specific, individual cytokines and response – e.g. association between IL23 and response is stronger in prior IO naïve patients, while prior IO did not affect IL10 or CXCL6 with response. This emphasizes the need to take prior IO into account to more accurately identify predictors and interpret their roles in clinical outcome.
As a peripheral blood marker accessible from routine clinical testing, NLR has been widely investigated for its predictive and prognostic utilities, although a definitive conclusion about the mechanistic basis for NLR’s prognostic importance is still unclear. Neutrophils may facilitate the implantation of circulating tumor cells in the bloodstream33 by NETosis, the process of neutrophils releasing NETs (neutrophil extracellular traps, web-like structures composed of DNA-histone complexes and proteins), and contribute to tumor angiogenesis by producing vascular endothelial growth factor.34 Neutrophils may also exhibit anti-tumor activities possibly due to their functional plasticity.34 Lymphocytes are considered the major effector cells involved in anti-tumor responses.35 NLR, the ratio of neutrophils and lymphocytes counts, was therefore considered as a potential indicator of the balance between tumor promoting inflammation and anti-tumor activities, with elevated NLR reflecting a dysfunctional state of host immune response.10,36
NLR has been shown to associate with higher risk of death broadly as a general disease marker in ICB-treated metastatic melanoma. Most studies have looked at single agent ICI, and found that higher NLR associated with resistance. Among stage III-IV melanoma patients treated with aCTLA-4, higher NLR at baseline has been shown to associate with higher risk of progression and death.12,22,37,38 Among metastatic melanoma patients treated with aPD-1, similarly, higher NLR has been reported consistently to associate with increased risk of overall survival.39,40 Contrary to the extensive studies in single-agent ICB settings, NLR’s prognostic value in ipi+nivo combination therapy is less well-known. In a prior study that evaluated NLR in ipi+nivo, pre treatment higher NLR (defined as ≥ 4.73) is reported to associate with higher risk of death.13 Differently from our cohort, after applying the reported thresholds of NLR (≥4.73), we did not find higher pre-treatment NLR significantly associated with survival (log rank test, p > 0.05), although the trends are similar (higher NLR trended toward worse outcome) (Supplemental Figure S11d). We reasoned that NLR was observed as predictive of response in monotherapy but not in our combination treatment setting potentially due to different response rates of these treatment regimens. NLR may be associated with resistance to aPD1, however, since response rates are much higher in combination therapy, combination treatment may overcome the resistance associated with aPD1 and therefore NLR is no longer a predictive biomarker in combination therapy setting. Different from prior investigations that only assessed pretreatment NLR, our study additionally profiled NLR dynamics during treatment. We found that early on-treatment (post 1 timepoint), higher NLR not only associated with increased risk of progression and death, but also associated with lack of durable clinical benefit. We further revealed that the elevation of NLR was primarily driven by lack of increase of lymphocytes among non-responders. Overall, our observation augments existing prognostic NLR findings while highlighting the value of early on treatment NLR assessment. Our results underscore the potential of longitudinally monitoring clinical labs values including NLR, particularly in the context of nivolumab plus ipilimumab combination ICB treatment.
Our data provides strong candidates and a potential biological explanation for response and resistance to combination checkpoint blockade, but further studies are necessary to functionally validate the biological mechanisms behind these cytokines. Our samples were collected from a single institution, which limits the variation in patient geography and history. Additional studies from independent cohorts will provide a broader scope.
Overall, our study, using immune features from peripheral blood, provides an approach for identifying patients who are likely to respond to nivolumab plus ipilimumab combination ICB, as well as a subset of patients with high risk of developing resistance and are thus in need of alternative therapeutic options, such as clinical trials.
Supplementary Material
Funding Statement
This publication is based on research supported by The G. Harold and Leila Y. Mathers Charitable Foundation, Sharon Crowley Martin Memorial Fund for Melanoma Research, Malcolm and Emily MacNaught Fund for Melanoma Research, E. Michael Egan Melanoma Research Fund, Doris Duke Charitable Foundation, Melanoma Research Alliance and the National Institutes of Health [NIH K08CA234458, David Liu]. GT work was supported by the American-Italian Cancer Foundation Post-Doctoral research fellowship.
Disclosure statement
F. Stephen Hodi receives grants and personal fees from Bristol-Myers Squibb, personal fees from Merck, grants and personal fees from Novartis, personal fees from Surface, personal fees from Compass Therapeutics, personal fees from Apricity, personal fees from Bicara, personal fees from Checkpoint Therapeutics, personal fees from Genentech/Roche, personal fees from Bioentre, personal fees from Gossamer, personal fees from Iovance, personal fees from Catalym, personal fees from Immunocore, personal fees from Kairos, personal fees from Rheos, personal fees from Zumutor, personal fees from Corner Therapeutics, personal fees from Puretech, personal fees from Curis, personal fees from Astra Zeneca, outside the submitted work; In addition, Dr. Hodi has a patent Methods for Treating MICA-Related Disorders (#20100111973) with royalties paid, a patent Tumor antigens and uses thereof (#7250291) issued, a patent Angiopoietin-2 Biomarkers Predictive of Anti-immune checkpoint response (#20170248603) pending, a patent Compositions and Methods for Identification, Assessment, Prevention, and Treatment of Melanoma using PD-L1 Isoforms (#20160340407) pending, a patent Therapeutic peptides (#20160046716) pending, a patent Therapeutic Peptides (#20140004112) pending, a patent Therapeutic Peptides (#20170022275) pending, a patent Therapeutic Peptides (#20170008962) pending, a patent THERAPEUTIC PEPTIDES Therapeutic Peptides Patent number: 9402905 issued, a patent METHODS OF USING PEMBROLIZUMAB AND TREBANANIB pending, a patent Vaccine compositions and methods for restoring NKG2D pathway function against cancers Patent number: 10279021 issued, a patent Antibodies that bind to MHC class I polypeptide-related sequence A Patent number: 10106611 issued, and a patent ANTI-GALECTIN ANTIBODY BIOMARKERS PREDICTIVE OF ANTI-IMMUNE CHECKPOINT AND ANTI-ANGIOGENESIS RESPONSES.
David Liu serves on the scientific advisory board of Oncovalent Therapeutics.
Joanna Baginska declares personal fees from Compass Therapeutics.
Mariano Severgnini is employed by Curis Inc, Boudicca.
Scott J. Rodig receives research support from Bristol Myers Squibb and KITE/Gilead. He is a member of the SAB of Immunitas Therapeutics.
Authors participation
Conception, design, and supervision: FSH and DL. Project administration: FSH and SR. Data curation: MM, JDR. Experiments and data investigation: MB,EG,KP,SJR,RB,MN,EH,CM. Data analysis: JC,GT,MS,JB,AG,JLW,AYH. All authors interpreted the data, contributed to the writing, and provided final approval to submit for publication. FSH is responsible for the overall content.
Code availability
The code is available at https://github.com/davidliu-lab.
Data availability statement
Data are available in supplemental files or from the corresponding author upon reasonable request.
Ethics approval
This study involves human participants and was approved for clinical trial by Dana-Farber Cancer Institute IRB. Participants provided written informed consent to participate in the study before taking part.
Study approval
All patients were consented to Dana-Farber Cancer Institute approved protocol IRB-05-042 prior to the collection of specimens.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/2162402X.2024.2432723
References
- 1.Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, Schadendorf D, Dummer R, Smylie M, Rutkowski P, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373(1):23–12. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jones J, Nguyen H, Drummond K, Morokoff A.. Circulating biomarkers for Glioma: a review. Neurosurgery. 2021;88(3):E221–E230. doi: 10.1093/neuros/nyaa540. [DOI] [PubMed] [Google Scholar]
- 3.Castro-Giner F, Aceto N. Tracking cancer progression: from circulating tumor cells to metastasis. Genome Med. 2020;12(1):31. doi: 10.1186/s13073-020-00728-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fahmi MN, Pradjatmo H, Astuti I, Nindrea RD. Cytokines as prognostic biomarkers of epithelial ovarian cancer (EOC): a systematic review and meta-analysis. Asian Pac J Cancer Prev APJCP. 2021;22(2):315–323. doi: 10.31557/APJCP.2021.22.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peres LC, Townsend MK, Birmann BM, Conejo-Garcia JR, Kim Y, Kubzansky LD, Magpantay LI, Martinez-Maza O, Tworoger SS. Circulating biomarkers of inflammation and ovarian cancer risk in the nurses’ health studies. Cancer Epidemiol Biomark Prev Publ Am Assoc Cancer Res Cosponsored Am Soc Prev Oncol. 2021;30(4):710–718. doi: 10.1158/1055-9965.EPI-20-1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu B. Identification of blood protein biomarkers that aid in the clinical assessment of patients with malignant glioma. Int J Oncol. 2012. Feb 3. Published online. doi: 10.3892/ijo.2012.1355. [DOI] [PubMed] [Google Scholar]
- 7.Schalper KA, Carleton M, Zhou M, Chen T, Feng Y, Huang S-P, Walsh AM, Baxi V, Pandya D, Baradet T, et al. Elevated serum interleukin-8 is associated with enhanced intratumor neutrophils and reduced clinical benefit of immune-checkpoint inhibitors. Nat Med. 2020;26(5):688–692. doi: 10.1038/s41591-020-0856-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yamazaki N, Kiyohara Y, Uhara H, Iizuka H, Uehara J, Otsuka F, Fujisawa Y, Takenouchi T, Isei T, Iwatsuki K, et al. Cytokine biomarkers to predict antitumor responses to nivolumab suggested in a phase 2 study for advanced melanoma. Cancer Sci. 2017;108(5):1022–1031. doi: 10.1111/cas.13226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sanmamed MF, Perez-Gracia JL, Schalper KA, Fusco JP, Gonzalez A, Rodriguez-Ruiz ME, Oñate C, Perez G, Alfaro C, Martín-Algarra S, et al. Changes in serum interleukin-8 (IL-8) levels reflect and predict response to anti-PD-1 treatment in melanoma and non-small-cell lung cancer patients. Ann Oncol. 2017;28(8):1988–1995. doi: 10.1093/annonc/mdx190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bartlett EK, Flynn JR, Panageas KS, Ferraro RA, Sta Cruz JM, Postow MA, Coit DG, Ariyan CE. High neutrophil to lymphocyte ratio (NLR) is associated with treatment failure and death in melanoma patients treated with PD-1 inhibitor monotherapy. Cancer. 2020;126(1):76–85. doi: 10.1002/cncr.32506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gambichler T, Mansour R, Scheel CH, Said S, Abu Rached N, Susok L. Prognostic performance of the derived neutrophil-to-lymphocyte ratio in stage IV melanoma patients treated with immune checkpoint inhibitors. Dermato. 2022;2(2):14–20. doi: 10.3390/dermato2020003. [DOI] [Google Scholar]
- 12.Zaragoza J, Caille A, Beneton N, Bens G, Christiann F, Maillard H, Machet L. High neutrophil to lymphocyte ratio measured before starting ipilimumab treatment is associated with reduced overall survival in patients with melanoma. Br J Dermatol. 2016;174(1):146–151. doi: 10.1111/bjd.14155. [DOI] [PubMed] [Google Scholar]
- 13.Rosner S, Kwong E, Shoushtari AN, Friedman CF, Betof AS, Brady MS, Coit DG, Callahan MK, Wolchok JD, Chapman PB, et al. Peripheral blood clinical laboratory variables associated with outcomes following combination nivolumab and ipilimumab immunotherapy in melanoma. Cancer Med. 2018;7(3):690–697. doi: 10.1002/cam4.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Capone M, Giannarelli D, Mallardo D, Madonna G, Festino L, Grimaldi AM, Vanella V, Simeone E, Paone M, Palmieri G, et al. Baseline neutrophil-to-lymphocyte ratio (NLR) and derived NLR could predict overall survival in patients with advanced melanoma treated with nivolumab. J Immunother Cancer. 2018;6(1):74. doi: 10.1186/s40425-018-0383-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li M, Spakowicz D, Burkart J, Patel S, Husain M, He K, Bertino EM, Shields PG, Carbone DP, Verschraegen CF, et al. Change in neutrophil to lymphocyte ratio during immunotherapy treatment is a non-linear predictor of patient outcomes in advanced cancers. J Cancer Res Clin Oncol. 2019;145(10):2541–2546. doi: 10.1007/s00432-019-02982-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pedersen JG, Sokac M, Sørensen BS, Luczak A, Aggerholm-Pedersen N, Birkbak N, Øllegaard T, Jakobsen M. Increased soluble PD-1 predicts response to nivolumab plus ipilimumab in melanoma. Cancers. 2022;14(14):3342. doi: 10.3390/cancers14143342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gérard A, Doyen J, Cremoni M, Bailly L, Zorzi K, Ruetsch-Chelli C, Brglez V, Picard-Gauci A, Troin L, Esnault VLM, et al. Baseline and early functional immune response is associated with subsequent clinical outcomes of PD-1 inhibition therapy in metastatic melanoma patients. J Immunother Cancer. 2021;9(6):e002512. doi: 10.1136/jitc-2021-002512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tarhini AA, Zahoor H, Lin Y, Malhotra U, Sander C, Butterfield LH, Kirkwood JM. Baseline circulating IL-17 predicts toxicity while tgf-β1 and IL-10 are prognostic of relapse in ipilimumab neoadjuvant therapy of melanoma. J Immunother Cancer. 2015;3(1):39. doi: 10.1186/s40425-015-0081-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lim SY, Lee JH, Gide TN, Menzies AM, Guminski A, Carlino MS, Breen EJ, Yang JYH, Ghazanfar S, Kefford RF, et al. Circulating cytokines predict immune-related toxicity in melanoma patients receiving anti-PD-1–Based immunotherapy. Clin Cancer Res. 2019;25(5):1557–1563. doi: 10.1158/1078-0432.CCR-18-2795. [DOI] [PubMed] [Google Scholar]
- 20.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140(6):883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zahorec R. Ratio of neutrophil to lymphocyte counts–rapid and simple parameter of systemic inflammation and stress in critically ill. Bratisl Lek Listy. 2001;102(1):5–14. [PubMed] [Google Scholar]
- 22.Ferrucci PF, Gandini S, Battaglia A, Alfieri S, Di Giacomo AM, Giannarelli D, Cappellini GCA, De Galitiis F, Marchetti P, Amato G, et al. Baseline neutrophil-to-lymphocyte ratio is associated with outcome of ipilimumab-treated metastatic melanoma patients. Br J Cancer. 2015;112(12):1904–1910. doi: 10.1038/bjc.2015.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu D, Schilling B, Liu D, Sucker A, Livingstone E, Jerby-Arnon L, Zimmer L, Gutzmer R, Satzger I, Loquai C, et al. Integrative molecular and clinical modeling of clinical outcomes to PD1 blockade in patients with metastatic melanoma. Nat Med. 2019;25(12):1916–1927. doi: 10.1038/s41591-019-0654-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Campbell KM, Amouzgar M, Pfeiffer SM, Howes TR, Medina E, Travers M, Steiner G, Weber JS, Wolchok JD, Larkin J, et al. Prior anti-CTLA-4 therapy impacts molecular characteristics associated with anti-PD-1 response in advanced melanoma. Cancer Cell. 2023;41(4):791–806.e4. doi: 10.1016/j.ccell.2023.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mirlekar B, Pylayeva-Gupta Y. IL-12 family cytokines in cancer and immunotherapy. Cancers. 2021;13(2):167. doi: 10.3390/cancers13020167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Monteagudo C, Pellín-Carcelén A, Martín JM, Ramos D. Role of chemokines in melanoma progression. Actas Dermosifiliogr. 2011;102(7):498–504. doi: 10.1016/j.ad.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 27.Proost P, De Wolf-Peeters C, Conings R, Opdenakker G, Billiau A, Van Damme J. Identification of a novel granulocyte chemotactic protein (GCP-2) from human tumor cells. In vitro and in vivo comparison with natural forms of GRO, IP-10, and IL-8. J Immunol. 1993;150(3):1000–1010. doi: 10.4049/jimmunol.150.3.1000. [DOI] [PubMed] [Google Scholar]
- 28.Bickel M. The role of interleukin-8 in inflammation and mechanisms of regulation. J Periodontol. 1993;64(5 Suppl):456–460. [PubMed] [Google Scholar]
- 29.Murphy K, Weaver C. Janeway’s immunobiology. 9th ed. LLC: Garland Science/Taylor & Francis Group; 2016. [Google Scholar]
- 30.Oft M. IL-10: master switch from tumor-promoting inflammation to antitumor immunity. Cancer Immunol Res. 2014;2(3):194–199. doi: 10.1158/2326-6066.CIR-13-0214. [DOI] [PubMed] [Google Scholar]
- 31.Torisu-Itakura H, Lee JH, Huynh Y, Ye X, Essner R, Morton DL. Monocyte-derived IL-10 expression predicts prognosis of stage IV melanoma patients. J Immunother Hagerstown Md 1997. 2007;30(8):831–838. doi: 10.1097/CJI.0b013e318158795b. [DOI] [PubMed] [Google Scholar]
- 32.Eggermont AMM, Blank CU, Mandala M, Long GV, Atkinson V, Dalle S, Haydon A, Lichinitser M, Khattak A, Carlino MS, et al. Adjuvant pembrolizumab versus placebo in resected stage III melanoma. N Engl J Med. 2018;378(19):1789–1801. doi: 10.1056/NEJMoa1802357. [DOI] [PubMed] [Google Scholar]
- 33.De Santis M, Mantovani A, Selmi C. The other side of the innate immune system: humoral arms favoring cancer. Cell Mol Immunol. 2020;17(10):1024–1025. doi: 10.1038/s41423-020-0512-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Masucci MT, Minopoli M, Del Vecchio S, Carriero MV. The emerging role of neutrophil extracellular traps (NETs) in tumor progression and metastasis. Front Immunol. 2020;11:1749. doi: 10.3389/fimmu.2020.01749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Restifo NP, Dudley ME, Rosenberg SA. Adoptive immunotherapy for cancer: harnessing the T cell response. Nat Rev Immunol. 2012;12(4):269–281. doi: 10.1038/nri3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dong C, Dang D, Zhao X, Wang Y, Wang Z, Zhang C. Integrative characterization of the role of IL27 in melanoma using bioinformatics analysis. Front Immunol. 2021;12:713001. doi: 10.3389/fimmu.2021.713001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cassidy MR, Wolchok RE, Zheng J, Panageas KS, Wolchok JD, Coit D, Postow MA, Ariyan C. Neutrophil to lymphocyte ratio is associated with outcome during ipilimumab treatment. EBioMedicine. 2017;18:56–61. doi: 10.1016/j.ebiom.2017.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ferrucci PF, Ascierto PA, Pigozzo J, Del Vecchio M, Maio M, Antonini Cappellini GC, Guidoboni M, Queirolo P, Savoia P, Mandalà M, et al. Baseline neutrophils and derived neutrophil-to-lymphocyte ratio: prognostic relevance in metastatic melanoma patients receiving ipilimumab. Ann Oncol Off J Eur Soc Med Oncol. 2016;27(4):732–738. doi: 10.1093/annonc/mdw016. [DOI] [PubMed] [Google Scholar]
- 39.Chasseuil E, Saint-Jean M, Chasseuil H, Peuvrel L, Quéreux G, Nguyen J, Gaultier A, Varey E, Khammari A, Dréno B. Blood predictive biomarkers for nivolumab in advanced melanoma. Acta Derm Venerol. 2018;98(4):406–410. doi: 10.2340/00015555-2872. [DOI] [PubMed] [Google Scholar]
- 40.Fujisawa Y, Yoshino K, Otsuka A, Funakoshi T, Fujimura T, Yamamoto Y, Hata H, Tanaka R, Yamaguchi K, Nonomura Y, et al. Baseline neutrophil to lymphocyte ratio combined with serum lactate dehydrogenase level associated with outcome of nivolumab immunotherapy in a Japanese advanced melanoma population. Br J Dermatol. 2018;179(1):213–215. doi: 10.1111/bjd.16427. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available in supplemental files or from the corresponding author upon reasonable request.


