Abstract
Objective
Anti-melanoma differentiation-associated gene 5-positive dermatomyositis-associated interstitial lung disease (MDA5+DM-ILD) often leads to acute respiratory failure and endangers lives. This study quantitatively analysed chest high-resolution computed tomography (HRCT) images to assess MDA5+DM-ILD and establish a risk prediction model for severe ILD within six months.
Methods
We developed a ‘Standardized Threshold Ratio Analysis & Distribution’ (STRAD) to analyse lung HRCT images. In this retrospective study, 51 patients with MDA5+DM-ILD were included and divided into severe-ILD and non-severe-ILD groups based on the occurrence of acute respiratory failure within six months post-diagnosis of MDA5+DM. The STRAD parameters, clinical indicators and treatments were compared between the two groups. Least absolute shrinkage and selection operator (LASSO) regression was used to select the optimal STRAD parameters. Multivariate analysis selected clinical factors to be further combined with STRAD to enhance the predictive performance of the final model (STRAD-Ro52 model).
Results
Significant differences were observed between the two groups in STRAD parameters, anti-Ro52 antibody titers, presence of anti-Ro52 antibodies, age, ESR, ALB, Pa/FiO2, IgM and IL-4 levels. The STRAD parameters were significantly correlated with demographic, inflammatory, organ function and immunological indicators. Lasso logistic regression analysis identified the −699 to −650 HU lung tissue proportion (%V7) as the optimal parameter for predicting severe ILD and S6·%V7, and the distribution of %V7 in the mid lungs was the optimal space parameter. Multifactorial regression of clinical indicators showed that the presence of anti-Ro52 antibodies was an independent risk factor for severe ILD, leading to the establishment of the STRAD-Ro52 model.
Conclusions
The STRAD-Ro52 model assists in identifying MDA5+DM patients at risk of developing severe ILD within six months, further optimizing precise disease management and clinical research design.
Keywords: Dermatomyositis, anti-melanoma differentiation-associated protein 5 antibody, severe interstitial lung disease, computed tomography, risk model, Standardized Threshold Ratio Analysis & Distribution
KEY MESSAGES
Standardized Threshold Ratio Analysis & Distribution (STRAD) was developed to analyse lung high-resolution computed tomography (HRCT) images.
Significant differences were observed between the severe-ILD and non-severe-ILD groups in STRAD parameters.
The STRAD-Ro52 model, utilizing HRCT images and anti-Ro52 antibody, accurately predicts severe-ILD risk in patients with MDA5+DM-ILD.
Introduction
Idiopathic inflammatory myopathy (IIM) is a group of autoimmune diseases primarily characterized by inflammatory muscle damage, often accompanied by extra-muscular manifestations such as rashes and significant organ involvement [1,2]. IIM-associated interstitial lung disease (IIM-ILD) notably affects the prognosis of adults with IIM [3], with a reported global prevalence rate of 41% [4]. Anti-melanoma differentiation-associated gene 5 (anti-MDA5) antibodies have been identified as crucial risk factors for exacerbation and mortality in IIM patients [5]. Adults diagnosed with anti-MDA5 positive dermatomyositis (MDA5+DM) frequently exhibit prominent clinical features of dermatomyositis rashes and are prone to rapidly progressing interstitial lung disease (RP-ILD), with the incidence rate of MDA5+DM-associated RP-ILD reported to be between 38% and 87.5% [5,6]. Once RP-ILD occurs, the mortality rate can reach 50% [7].
The clinical characteristics of MDA5+DM-ILD display high heterogeneity [8]: patients with mild/stable ILD often maintain stability and respond well to treatment, whereas RP-ILD can rapidly progress to severe ILD, leading to patient mortality [1]. Recent studies have demonstrated that compared to traditional ‘step-up’ treatment strategies, ‘early aggressive treatment’ can significantly enhance the survival rates of patients with MDA5+DM-ILD [9]. However, the potential risk associated with this early intensified immunosuppressive strategy is overtreatment of patients with chronic or stable ILD [10]. Therefore, precise risk stratification during the early stages of this disease is essential. Nevertheless, critical biomarkers for early identification of potentially worsening cases are still lacking [11].
RP-ILD is defined as a measurable progression of interstitial lung changes within a short period from the onset of ILD, yet without a unified standard definition [5,12,13]. Typically, RP-ILD is characterized by acute and progressive respiratory distress secondary to ILD within three months of diagnosis, necessitating hospitalization, oxygen therapy or intubation due to respiratory failure [5]. However, this definition is broad and fails to differentiate between disease severity levels. Severe ILD is a subtype of RP-ILD, referring specifically to RP-ILD that results in acute respiratory failure [12]. To clarify the standards, this study employed physiological indicators, including arterial oxygen tension (PaO2), oxygen saturation (SpO2) and oxygenation index (PaO2/FiO2 or SpO2/FiO2 ratio), to quantify severe-ILD, thereby grading its severity and covering the majority of clinical scenarios encountered.
High-resolution computed tomography (HRCT) remains the primary tool used for ILD research today [14]. The types and distribution characteristics of HRCT imaging have been confirmed as predictors of disease prognosis in MDA5+DM-ILD using visual assessment and semi-quantitative scoring methods [13,15]. However, the disease’s high heterogeneity in imaging features makes assessment challenging [14]. In recent years, several quantitative HRCT analyses based on commercial software and radiomics have been conducted to investigate the prognosis of MDA5+DM-ILD [16–20]. However, no study has evaluated the risk of developing severe ILD in patients with MDA5+DM-ILD. In this study, we developed a quantitative analysis method named ‘Standardized Threshold Ratio Analysis & Distribution (STRAD)’ for HRCT image data in conjunction with clinical parameters to assess the risk of severe ILD in patients with MDA5+DM-ILD.
Methods
Patients
This study retrospectively reviewed 76 adult cases of MDA5+DM-ILD treated at the Department of Rheumatology and Immunology of the Southern Medical University Nanfang Hospital from January 2018 to April 2022. The diagnosis of Polymyositis/Dermatomyositis/Amyopathic Dermatomyositis (PM/DM/ADM) was based on the criteria of Bohan and Peter [21,22] or Sontheimer [23]. All patients were reclassified according to the 2017 European League Against Rheumatism/American College of Rheumatology (EULAR/ACR) classification criteria [24]. Patients who developed severe-ILD within 6 months after the diagnosis of MDA5+DM were categorized into the severe-ILD group, whereas those who did not develop severe-ILD were placed into the non-severe-ILD group. Baseline chest HRCT scans were used as the subject of the study. Healthy controls were selected from individuals undergoing routine health checkups with chest HRCT scans during the same period as the case group. The healthy control group is matched with the patient group in terms of gender and age.
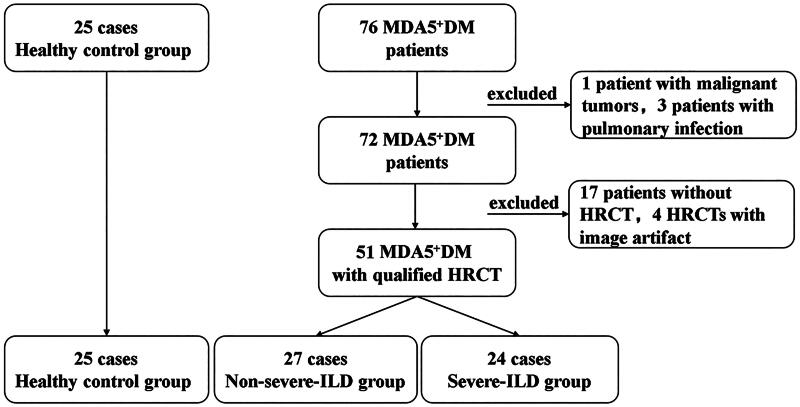
This study received ethical approval from the Medical Ethics Committee of Nanfang Hospital, Southern Medical University (NFEC-2023-192). It adhered strictly to the principles of the Declaration of Helsinki. All HRCT scans, pulmonary function tests (PFTs) and laboratory examinations conducted for this study served clinical purposes. Given the retrospective nature of the study, the Medical Ethics Committee of Nanfang Hospital, Southern Medical University granted an exemption from informed consent. However, written consent was obtained from the participants to publish images of their individual scan reports included in the paper. The process for case selection is illustrated in Figure 1.
Figure 1.
Overview of patient selection. DM, Dermatomyositis; HRCT, high-resolution computed tomography; ILD, interstitial lung disease; MDA5+,Anti–Melanoma Differentiation associated gene 5 positive.
The inclusion criteria were as follows: (1) age over 18 years; (2) positive for anti-MDA5; and (3) underwent at least one chest HRCT examination. The exclusion criteria were as follows: (1) concurrent lung infection; (2) pulmonary oedema or known moderate-to-severe pulmonary arterial hypertension with a mean pulmonary artery pressure ≥30 mmHg; (3) barotrauma including pneumothorax, pneumomediastinum, pneumopericardium and subcutaneous emphysema; (4) overlap with other connective tissue diseases (CTDs); (5) coexisting malignancy; and (6) drug-related lung injury and environmental and occupational exposures.
Classification of severe-ILD
The diagnosis of ILD is based on respiratory symptoms, PFT results and HRCT imaging. Depending on clinical presentations and disease progression, IIM-ILD often manifests in one of three clinical patterns [8]: (1) acute onset/rapid progression; (2) chronic slow progression; (3) no respiratory symptoms, with only radiological or PFT abnormalities. In this study, severe ILD was defined as ‘RP-ILD leading to acute respiratory failure.’ According to the definition of PM/DM-severe interstitial lung disease proposed by Furuya et al. [25], and referring to the new global definition of ARDS [26], the diagnostic criteria for severe-ILD are defined as follows: presence of ILD on HRCT, accompanied by any of the following conditions: (1) PaO2 < 60 mmHg; (2) PaO2/FiO2 ≤300 mmHg or SpO2/FiO2 ≤315 mmHg (if SpO2 ≤97%); and (3) invasive mechanical ventilation.
Clinical data collection
Clinical data for all cases were obtained from the hospital information system (HIS), including information on disease progression, clinical features, laboratory data, physiological indicators, lung involvement, autoantibodies and treatment information. PFTs were conducted in accordance with the standards set by the American Thoracic Society/European Respiratory Society [27], collecting measured/predicted ratios of forced vital capacity (FVC), forced expiratory volume in 1 s (FEV1) and carbon monoxide diffusion capacity of the lung (DLCO). Myositis-specific autoantibodies (MSAs), such as anti-MDA5 antibodies, and myositis-associated autoantibodies (MAAs), such as anti-Ro52 antibodies, were detected in immunoblot testing based on the manufacturer’s instructions, as described in our previous studies [28]. The results were defined based on the signal intensity: no signal (0): negative; very weak band ((+)): borderline; medium to strong band (+ or ++): positive; very strong band (+++) with an intensity comparable to the control band: strong positive, as indicated by the manufacturer.
These data were based on the closest time point within one week after or before undergoing HRCT. If there were no relevant clinical indicators within the week before or after, they were recorded as missing. Visual assessment of HRCT was independently completed by two radiologists, both of whom were blinded to the patients’ clinical information. According to the American Thoracic Society/European Respiratory Society guidelines [27], images were classified into the following patterns: (1) nonspecific interstitial pneumonia (NSIP), (2) organizing pneumonia (OP) and (3) NSIP combined with OP. Based on HRCT findings, parenchymal abnormalities were divided into consolidative (OP, OP + NSIP) and non-consolidative (NSIP) types [13]. Consolidation was defined as a uniform increase in pulmonary parenchymal opacification that obscured vessels and airway walls [29].
Measurement
HRCT scans
A review was conducted on patients with MDA5+DM-ILD and 25 healthy controls from the same period. The CT brands and models used were uCT960+ (United Imaging Healthcare, Shanghai, China), iCT256 (Philips Healthcare, Amsterdam, Netherlands) and SOMATOM Definition (Siemens Healthineers, Forchheim, Germany). The patients were positioned supine, and axial HRCT images were acquired at full inspiration. The scanning parameters were 100–120 kV and 120–300 mA, with a CT slice thickness of 0.6–1.5 mm, a spacing of 10 mm and images were reconstructed using a thin-slice algorithm. The matrix size was 512 pixels × 512 pixels. HRCT images of each case were exported in Digital Imaging and Communications in Medicine (DICOM) format from the picture archiving and communication system (PACS) for further analysis.
Images selection and segmentation
In each axial HRCT image, nine images evenly distributed across the vertical plane were selected for analysis. The digitization and processing of HRCT images were performed using ImageJ software (version 1.53q, developed by the National Institutes of Health (Bethesda, MD), Java 1.8.0_172 (64-bit)) for lung segmentation. The entire lung area was identified as the region of interest (ROI) [20], following the method reported in the literature [30]. Sampling of the whole lung volume was performed on axial HRCT images, and trachea, bronchi and large blood vessels were manually excluded as previously described. Consolidation lesions under the pleura were carefully preserved.
STRAD parameters calculation
The histogram tool in ImageJ (National Institutes of Health, Bethesda, MD) was used to extract the Hu histogram data from the HRCT images after segmentation. A threshold range of −1000 to +100 HU was selected (a total of 1100 HU) [31]. %V1–%V22 represent the voxel percentage in 22 threshold intervals with a step size of 50 HU within the total lung tissue range of −1000 to +100 HU [32], respectively. The percentage of V1–V22 was calculated using the formula %Vn = (number of voxels between Vn/number of total voxels) × 100%.
We then summarized the V1–V22 of nine HRCT images in each case, calculated the whole lung %Vn, and obtained the STRA parameters (%V1–%V22). Calculate the %Vn of nine HRCT images for each case separately and arrange them in vertical spatial order to obtain the STRAD parameters, which are (S1–S9)·(%V1–%V22). S1–S9 represents nine selected axial HRCT layers, and %V1–%V22 represents the voxel percentage in 22 threshold intervals with a step size of 50 HU within the total lung tissue range of −1000 to +100 HU, respectively. A histogram was used to represent the summarized threshold ratio characteristics of each case, and a heatmap representing the threshold ratio and vertical spatial distribution characteristics of each case.
Outcomes
Severe-ILD occurrences within 6 months in MDA5+DM-ILD cases.
Determination of optimal STRAD parameters
The STRAD parameters were analysed using R 4.2.3 (2023-03-15) and R package ‘glmnet’ 4.1-7 (R Foundation for Statistical Computing, Vienna, Austria). The least absolute shrinkage and selection operator (LASSO) regression was used to select the optimal variables and estimate the model parameters. All patient information is included in the model.
Statistical analysis
Statistical analyses were performed using the SPSS software (IBM SPSS Statistics, version 25.0, 2018, Armonk, NY). For the comparison of demographic indicators, clinical parameters and imaging parameters between healthy control groups, IIM-ILD groups, severe-ILD groups and non-severe-ILD groups, continuous variables following a normal distribution were described using mean and standard deviation, and tested using two independent samples t-tests; continuous variables not following a normal distribution were described using median (interquartile range) and tested using the Mann–Whitney U-test; categorical variables were tested using Chi-square and Fisher’s exact tests. Spearman’s correlation analysis was also performed. Univariate logistic regression models were used to select meaningful clinical parameter variables, and multivariate logistic regression models were used to identify predictors of severe-ILD risk in MDA5+DM-ILD cases within 6 months, further integrating the STRAD parameters to establish a predictive model. Receiver operating characteristic curve (ROC) analysis was used to evaluate the diagnostic efficacy of statistically significant indicators. The Kaplan–Meier method was employed to plot the survival curves of MDA5+DM-ILD patients at risk of severe ILD within six months. p < .05 was considered to be statistically significant.
Results
Comparative analysis of early clinical features between severe-ILD and non-severe-ILD groups
The baseline characteristics at the first visit of the 51 adult patients with MDA5+DM-ILD are summarized in Table 1. The average age of the patients was 45 years, with a median disease duration of 4.2 months, and the majority were female (68.63%). All patients received glucocorticoid treatment, with 17.64% receiving doses greater than 1 mg/kg/day, and 15.69% undergoing steroid pulse therapy. Additionally, 76.46% were treated with immunosuppressants (including cyclophosphamide, cyclosporine A, tacrolimus and mycophenolate mofetil), 25.49% with Jak-inhibitors, 35.29% with intravenous immunoglobulin (IVIG) and 33.33% received anti-fibrosis therapy. A total of 19.61% of patients were treated with glucocorticoid, cyclophosphamide and a calcineurin inhibitor, while another 19.61% received a combination of glucocorticoid, immunosuppressant drugs and a JAK inhibitor. There is no statistically significant difference in treatment between the severe-ILD and non-severe-ILD groups.
Table 1.
Comparison of clinical characteristics between severe-ILD group and non-severe ILD group in MDA5+DM-ILD.
| Characteristic | Overall cohort (N = 51) | Severe-ILD group (n = 24) | Non-severe-ILD group (n = 27) | p Value |
|---|---|---|---|---|
| Demographic | ||||
| Age of onset, years | 45.45 ± 13.03 | 50.17 ± 2.42 | 41.26 ± 2.46 | .013* |
| Female, n (%) | 35 (68.63) | 15 (62.50) | 20 (74.07) | .374 |
| Height, cm | 160.02 ± 6.91 | 160.71 ± 6.84 | 159.10 ± 7.38 | .569 |
| Weight, kg | 54.67 ± 11.21 | 57.09 ± 11.04 | 53.21 ± 10.90 | .216 |
| Duration from onset to treatment, months | 4.24 ± 4.56 | 3.46 ± 3.48 | 4.93 ± 5.32 | .245 |
| Duration of ILD, months | 1.57 ± 2.83 | 1.625 ± 2.86 | 1.519 ± 2.86 | .895 |
| Smoker ever, n (%) | 10 (19.61) | 5 (20.83) | 5 (18.52) | 1.000 |
| Clinical features | ||||
| Fever, n (%) | 19 (37.25) | 10 (41.67) | 9 (33.33) | .539 |
| Cough, n (%) | 22 (41.14) | 12 (50.00) | 10 (37.04) | .351 |
| Dyspnoea, n (%) | 24 (47.06) | 14 (58.33) | 10 (37.04) | .128 |
| Myalgia, n (%) | 10 (19.61) | 5 (20.83) | 5 (18.52) | .714 |
| Hoarseness or sore throat, n (%) | 7 (13.73) | 5 (20.83) | 2 (7.41) | .232 |
| Mouth ulcers, n (%) | 5 (9.80) | 3 (12.50) | 2 (7.41) | .656 |
| Muscle weakness, n (%) | 19 (37.25) | 10 (41.67) | 9 (33.33) | .539 |
| Arthralgia, n (%) | 27 (51.94) | 10 (41.67) | 17 (62.96) | .128 |
| Raynaud phenomenon, n (%) | 1 (1.96) | 1 (4.17) | 0 (0.00) | .471 |
| Finger swollen, n (%) | 8 (15.69) | 5 (20.83) | 3 (11.11) | .451 |
| Mechanics hand, n (%) | 18 (35.29) | 9 (37.5) | 9 (33.33) | .756 |
| Skin ulcer, n (%) | 13 (25.49) | 9 (37.5) | 4 (14.81) | .107 |
| Periorbital swelling, n (%) | 17 (33.33) | 11 (45.83) | 6 (22.22) | .074 |
| Heliotrope rash, n (%) | 29 (56.86) | 13 (54.17) | 16 (59.26) | .782 |
| Gottron’s sign, n (%) | 33 (64.71) | 15 (62.5) | 18 (66.67) | .756 |
| Laboratory data | ||||
| Lymphocyte count, ×109/L | 0.93 ± 0.48 | 0.87 ± 0.41 | 0.99 ± 0.54 | .396 |
| Lymphocyte ration, (%) | 16.65 ± 8.61 | 17.80 ± 9.39 | 15.35 ± 7.62 | .316 |
| ESR, mm/1 h | 42.07 ± 28.15 | 53.22 ± 32.20 | 33.35 ± 21.41 | .032* |
| CRP, mg/L | 6.29 [1.30, 16.28] | 8.87 [3.07, 26.57] | 2.890 [0.76, 7.88] | .015[U] |
| CK, IU/L | 68.50 [41.00, 230.00] | 130.00 [51.50, 527.75] | 48.500 [34.00, 163.75] | .023[U] |
| LDH, IU/L | 327.00 [249.50, 516.00] | 400.00 [331.00, 527.00] | 281.00 [232.00, 352.50] | .001[U] |
| Ferritin, ng/mL | 1171.70 [326.96, 2076.16] | 1954.50 [1308.45, 2225.48] | 471.86 [141.09, 1664.86] | .006[U] |
| PCT, ng/mL | 0.080 [0.05, 0.15] | 0.11 [0.07, 0.19] | 0.08 [0.03, 0.09] | .022[U] |
| Albumin, g/L | 32.60 ± 4.92 | 31.11 ± 3.83 | 33.98 ± 5.47 | .039* |
| ALT, U/L | 33.00 [22.00, 85.00] | 51.000 [28.00, 99.50] | 30.00 [16.00, 57.00] | .027[U] |
| AST, U/L | 43.00 [25.00, 94.00] | 76.50 [43.00, 128.25] | 28.00 [22.00, 73.00] | .001[U] |
| Cr, μmol/L | 55.92 ± 15.28 | 55.83 ± 17.70 | 56.00 ± 13.22 | .968 |
| PRO-BNP, pg/mL | 186.05 [91.95, 186.05] | 146.70 [76.93, 254.13] | 271.05 [152.75, 325.15] | .111[U] |
| P/F ration, mmHg | 336.60 ± 94.75 | 288.20 ± 77.38 | 402.28 ± 75.74 | .000** |
| D-dimer, μg/mL | 1.40 [0.78, 1.99] | 1.675 [0.82, 2.09] | 1.03 [0.73, 1.67] | .118[U] |
| IgG, g/L | 15.92 ± 6.73 | 17.97 ± 8.72 | 13.97 ± 3.18 | .066 |
| IgM, g/L | 1.46 ± 0.53 | 1.26 ± 0.51 | 1.65 ± 0.49 | .017* |
| TNF-α, pg/mL | 1.47 ± 1.20 | 1.81 ± 1.48 | 1.12 ± 0.76 | .183 |
| IFN-γ, pg/mL | 1.48 [0.78, 1.99] | 0.90 [0.68, 2.07] | 1.65 [0.40, 2.32] | .818[U] |
| IL-4, pg/mL | 1.11 ± 0.70 | 1.52 ± 0.60 | 0.71 ± 0.56 | .004** |
| IL-6, pg/mL | 6.50 [3.40, 23.21] | 9.49 [5.44, 91.90] | 4.15 [1.93, 14.02] | .073[U] |
| MDA5 titer | .872 | |||
| (+) | 16 (31.4) | 8 (33.3) | 8 (29.6) | |
| (++) | 10 (19.6) | 4 (16.7) | 6 (22.2) | |
| (+++) | 25 (49.0) | 12 (50) | 13 (48.1) | |
| Presence of Ro52, n (%) | 35 (68.63) | 22 (91.67) | 13 (48.15) | .001** |
| Ro52 titer | .004** | |||
| (+) | 7 (13.73) | 5 (20.83) | 2 (7.41) | |
| (++) | 5 (9.80) | 4 (16.67) | 1 (3.70) | |
| (+++) | 23 (45.10) | 13 (54.17) | 10 (37.04) | |
| Pulmonary function test | ||||
| FVC, %pred | 75.48 ± 16.48 | 71.10 ± 18.58 | 77.91 ± 15.20 | .304 |
| DLCO, %pred | 57.23 ± 11.32 | 51.59 ± 11.37 | 60.27 ± 10.48 | .103 |
| FEV1, %pred | 74.35 ± 15.02 | 72.51 ± 19.88 | 75.37 ± 12.10 | .685 |
| Type of ILD at diagnosis | ||||
| OP/OP + NSIP, n (%) | 34 (66.67) | 15 (62.50) | 19 (70.37) | .552 |
| NSIP, n (%) | 17 (33.33) | 9 (37.50) | 8 (29.63) | .552 |
| UIP, n (%) | 0 (0.00) | 0 (0.00) | 0 (0.00) | – |
| Initial treatment | ||||
| Glucocorticoid dose | ||||
| ≤1 mg/kg/d, n (%) | 42 (82.35) | 18 (75.00) | 24 (88.89) | .276 |
| >1 mg/kg/d, n (%) | 9 (17.64) | 6 (25.00) | 3 (11.11) | .276 |
| Pulse therapy, n (%) | 8 (15.69) | 5 (20.83) | 3 (11.11) | .451 |
| CXT, n (%) | 17 (33.33) | 11 (45.83) | 6 (22.22) | .074 |
| CsA, n (%) | 5 (9.80) | 4 (16.67) | 1 (3.70) | .175 |
| Tacrolimus, n (%) | 13 (25.49) | 5 (20.83) | 8 (29.63) | .472 |
| MMF, n (%) | 4 (7.84) | 0 (0.00) | 4 (14.81) | .113 |
| JAK, n (%) | 13 (25.49) | 6 (25.00) | 7 (25.93) | 1.000 |
| IVIG, n (%) | 18 (35.29) | 11 (45.83) | 7 (25.93) | .138 |
| Anti-fibrosis therapy, n (%) | 17 (33.33) | 7 (29.17) | 10 (37.04) | .552 |
| Combination therapy | ||||
| Glucocorticoid + CTX + CNI, n (%) | 9 (17.65) | 5 (20.83) | 4 (14.81) | .718 |
| Glucocorticoid + IS + JAK, n (%) | 9 (17.65) | 4 (16.67) | 5 (18.52) | 1.000 |
[U]: results of Mann–Whitney’s U-test; ALB: albumin; ALT: alanine transaminase; AST: aspartate transaminase; CK: creatine kinase; Cr: creatinine; CRP: C-reactive protein; CNI: calcineurin inhibitor (cyclosporine or tacrolimus); CsA: cyclosporine A; CTX: cyclophosphamide; DLCO: carbon monoxide diffusion capacity of the lung; ESR: erythrocyte sedimentation rate; FEV1: forced expiratory volume in 1 s; FVC: forced vital capacity ; IFN-γ: interferon-γ; IL-4/6: interleukin-4/6; IS: immunosuppressant drugs, including cyclophosphamide, cyclosporine, tacrolimus, mycophenolate mofetil; IVIG: intravenous immunoglobulin; JAK inhibitor: Janus kinase inhibitor; LDH: lactic dehydrogenase; MDA5: anti-melanoma differentiation-associated gene 5 antibody; MMF: mycophenolate mofetil; MSAs: myositis-specific autoantibodies; NSIP: nonspecific interstitial pneumonia; OP: organic pneumonia; P/F ration: arterial oxygen/fraction of inspiration oxygen; PCT: procalcitonin; pro-BNP: pro-brain natriuretic peptide; Ro52: anti-Ro52 antibody; TNF-α: tumour necrosis factor-α; UIP: usual interstitial pneumonia.
Values are expressed as mean ± standard deviation, median (IQR) or n (%).
p < .05.
p < .01.
Single-factor analysis and multifactor Logistics regression analysis
The results of the univariate and multivariate analyses are presented in Table 2 and Supplementary Table S1. All clinical variables were included in a univariate regression analysis to preliminarily identify significant clinical parameters. Variables with more than 20% missing data were excluded. This analysis found that age, LDH, ALB, Ro52 titers, and the presence of Ro52 were significantly related to the risk of severe ILD. Subsequent multifactor logistic regression analysis identified the presence of Ro52 as an independent risk factor for developing severe ILD early in MDA5+DM-ILD.
Table 2.
Univariate and multivariate logistics regression analysis of severe risk factors in MDA5+DM-ILD patients.
| Variable | Univariate logistics regression |
Multivariate logistics regression |
|||||
|---|---|---|---|---|---|---|---|
| Coefficients | SE | p Value | Coefficients | SE | p Value | ||
| 1 | Ro52 titer | 0.552 | 0.232 | .017 | −0.587 | 0.555 | .290 |
| 2 | Presence of Ro52 | 2.472 | 0.833 | .003 | 3.343 | 1.596 ** | .036 * |
| 3 | AGE | 0.061 | 0.026 | .020 | 0.053 | 0.034 | .115 |
| 4 | LDH | 0.005 | 0.002 | .027 | 0.004 | 0.003 | .146 |
| 5 | ALB | −0.131 | 0.065 | .046 | −0.006 | 0.092 | .946 |
ALB: albumin; LDH: lactic dehydrogenase; Ro52: anti-Ro52 antibody.
p < .05.
p < .01.
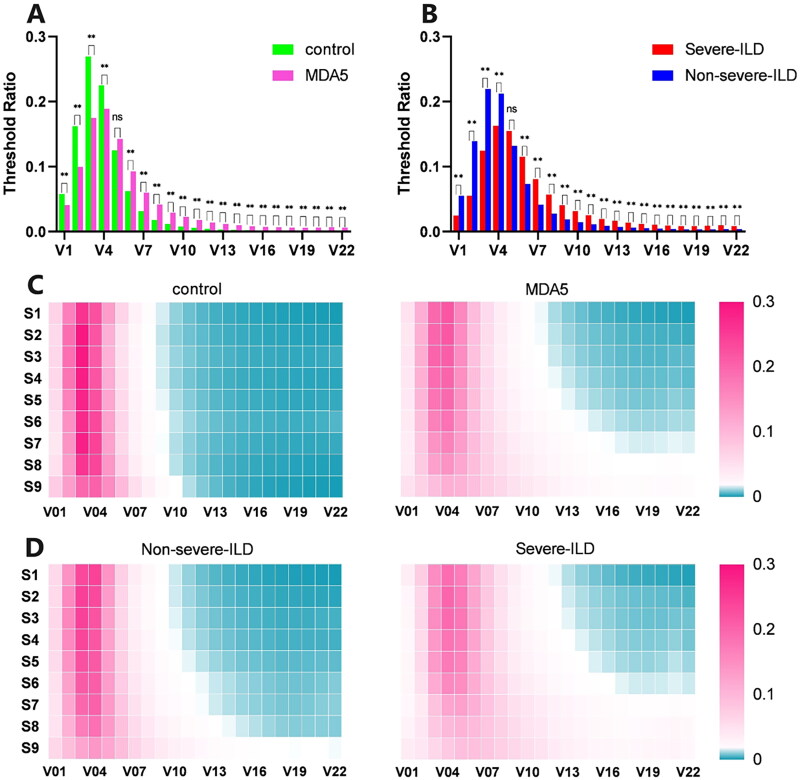
Comparison of STRA histograms between control and MDA5+DM-ILD groups, severe-ILD and non-severe-ILD groups
The histograms show shifts in the threshold ratio characteristics. Compared to healthy controls, the histogram of the MDA5+DM-ILD group shifted right, with decreased peak values; the ratio of well-ventilated lung tissue (V2–V4) decreased, while poorly ventilated (V7–V18) and non-ventilated (V19–V22) lung tissue ratios increased (Figure 2(A)). In MDA5+DM-ILD patients, compared to the non-severe-ILD group, the severe-ILD group’s histogram further shifted to the right with further decreased ratios of well-ventilated lung tissue and increased ratios of poorly and non-ventilated lung tissues (Figure 2(B)).
Figure 2.
Histogram and heat map comparison of healthy control group and patients. (A)histogram of healthy control group and MDA5 +DM group; (B)histogram of severe-ILD group and non-severe-ILD group in all MDA5 +DM patients. (C)heat map comparison of STRAD for healthy control and MDA5 +DM group; (D)heat map comparison of STRAD for severe-ILD group and non-severe ILD group. S1-S9 represent nine selected axial HRCT layers and V1-V22 represent the voxel percentage in 22 threshold intervals with a step size of 50 HU within the total lung tissue range of -1000 to 100 HU, respectively. HU, Hounsfield unit; ILD, interstitial lung disease. *p<0.05; **p<0.01.
Comparison of STRAD heatmaps between control and MDA5+ groups, severe-ILD and non-severe-ILD groups
STRAD heatmap comparisons revealed that compared to healthy controls, MDA5+DM-ILD patients had a significantly increased proportion of denser lung tissue (V10–V22), especially in the (S8–S9)·(V16–V22) area, showing an increasing trend from the upper to the lower lungs (Figure 2(C)). Comparing severe-ILD and non-severe-ILD patients, both showed increased proportions of denser lung tissue, which was more pronounced in the severe-ILD group, and the spatial distribution trend of increasing from the upper to the lower lungs was more evident in the severe-ILD group (Figure 2(D)), correlating with more severe lower lung consolidation in severe-ILD, aligning with reports of a ‘gravity gradient’ trend in lower lung consolidation [7].
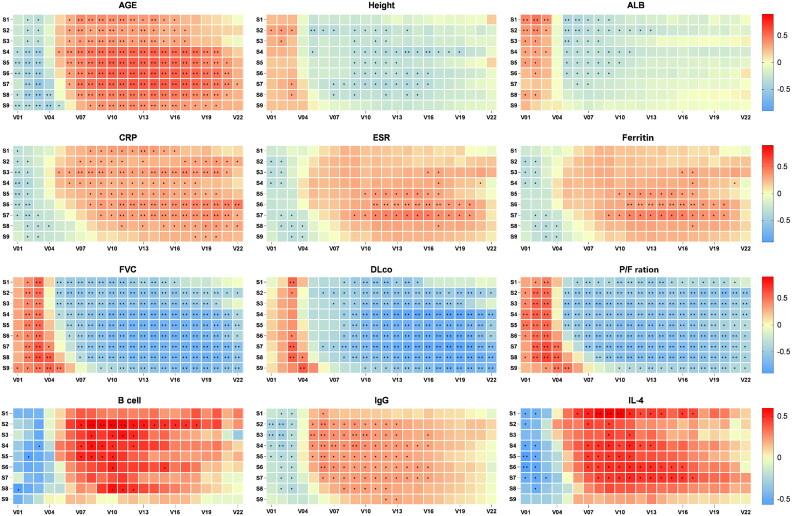
Correlation of demographic and dermatomyositis-related characteristics with STRAD parameters
Spearman’s correlation analysis showed that STRAD parameters were correlated with demographic, inflammatory, organ function and immune indicators (Figure 3). Notably, heatmap analysis indicated that these correlations were not uniform across different threshold intervals and spatial distributions within the STRAD parameters, highlighting the unique value of STRAD parameters in ILD assessment.
Figure 3.
Correlation heatmap between STRAD and clinical parameters. S1-S9 represents nine selected axial HRCT layers. V1 to V22 represent the voxel percentage in 22 threshold intervals with a step size of 50 HU within the total lung tissue range of -1000 to 100 HU, respectively. ALB, albumin; CRP, C reactive protein; DLCO, carbon monoxide diffusion capacity of the lung; ESR, erythrocyte sedimentation rate; FVC, forced vital capacity; IL-4, interleukin-4; P/F ration, arterial oxygen/fraction of inspiration oxygen. *p<0.05; **p<0.01.
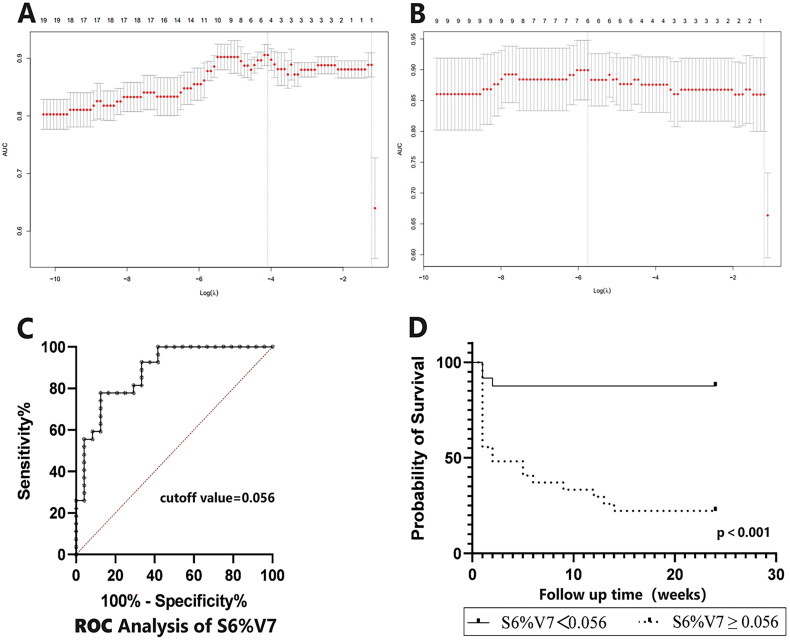
Predictive value of optimal STRAD parameters for 6-month severe-ILD risk in MDA5+DM-ILD
Given the high correlation between adjacent V and S parameters in the STRAD metrics (%V1–%V22)·(S1–S9), we obtained the optimal STRAD parameter through two rounds of Lasso regression analysis. Lasso logistic regression results indicated that V7 (−699Hu to −650Hu threshold range) was the optimal predictive parameter for the 6-month severe-ILD risk in MDA5+DM-ILD (Supplementary Table S2, Figure 4(A)). Further selection among S1–S9 for the layer most affecting %V7 found S6 to be the most significant vertical spatial distribution parameter, making S6%V7 the optimal STRAD predictive parameter for 6-month severe-ILD risk in MDA5+DM-ILD (Supplementary Table S3, Figure 4(B)). ROC analysis identified the optimal diagnostic cutoff value for S6%V7 as 0.056 (Figure 4(C)), with an AUROC of 0.883 [95% CI: 0.7911–0.9744, p < .0001], sensitivity of 77.78% [95% CI: 59.24–89.39%], specificity of 87.5% [95% CI: 69.00–95.66%] and a likelihood ratio of 6.222. Survival data further explored the prognostic impact of risk stratification, with high-risk group patients having significantly shorter survival times than low-risk group patients (p < .001, Figure 4(D)).
Figure 4.
Selection of the optimal penalization coefficient in the LASSO regression and clinical significance of value of the optimal STRAD parameter. The cross-validation curve was used to select variables. The bottom x-axis was the log scale of λ and the number of related variables in the model were shown on the top x-axis. The AUC was chosen to be the loss function, and y-axis shows the different cross-validation AUC under different choice of λ. The left dashed line was λ min which provides the highest cross-validation AUC for the model. The right dashed line is λ1se which is the largest λ value within 1 standard error of λmin. To avoid overfitting, λ1se was used to determine final lasso model for optimal threshold interval (A) and slicer (B). (C) ROC analysis of S6%V7 and best cutoff value was 0.056; (D) Comparison of 6-month severe risk curve between higher and lower S6%V7 in MDA5 +DM patients. Kaplan-Meier follow-up survival curves for the different risk stratification groups according to the optimal STRAD(S6%V7). The survival time in patients in the high-risk group (n=27) was significantly shorter compared with the low-risk (n=24).
Predictive value of the STRAD-Ro52 model for predicting 6-month severe-ILD risk in MDA5+DM-ILD
Further integration of S6%V7 with Ro52 through multifactor logistic regression yielded the STRAD-Ro52 model, a predictive framework for 6-month severe-ILD risk in MDA5+DM-ILD (Table 3). We conducted ROC analysis on six statistically significant indicators from Table 1 – age of onset, albumin, ESR, IgM, IL-4 and P/F ratio – along with STRAD (S6%V7) and STRAD (S6%V7)-Ro52. The results demonstrated that IL-4, P/F ratio, STRAD (S6%V7) and STRAD (S6%V7)-Ro52 have predictive value for the risk of severe MDA5+DM-ILD at 6 months. Among these, STRAD (S6%V7)-Ro52 showed the highest predictive value, with an area under the curve (AUC) of 0.899, a sensitivity of 0.846 and a specificity of 0.864 (Table 4). Subgroup analysis within all datasets showed significantly higher 6-month severe-ILD incidence in the Ro52(+) S6%V7 ≥ 0.056 group than in other subgroups, suggesting a higher risk in older patients and those with early respiratory symptoms, cough, dyspnoea, sore throat, elevated ESR and IgG (Supplementary Table S4), indicating that patients with older age, early respiratory symptoms and a high inflammatory state are at increased risk for severe-ILD.
Table 3.
Results of multivariate logistics regression based on STRAD parameters.
| Variable | Multivariate logistics regression |
|||
|---|---|---|---|---|
| Coefficients | SE | p Value | ||
| 1 | Presence of Ro52 | 3.070 | 1.145 | .007* |
| 2 | S6%V7 | 68.010 | 19.703 | <.001** |
Ro52: anti-Ro52 antibody; S6%V7 represents the percentage of lung tissue voxels from −699 to −650 HU in the total lung tissue voxels on the 6th layer HRCT image.
p < .05.
p < .01.
Table 4.
Analysis of ROC by significantly different indices between severe ILD group and non-severe ILD group.
| Analysis of ROC |
||||||
|---|---|---|---|---|---|---|
| AUC | 95% CI | Value of cut-off | Sensitivity (%) (CI) | Specificity (%) (CI) | p Value | |
| Age of onset | 0.691 | 0.546–0.837 | 52.50 | 0.458 (0.279, 0.649) | 0.852 (0.675, 0.941) | .019* |
| ALB | 0.669 | 0.517–0.822 | 35.90 | 0.917 (0.742, 0.985) | 0.462 (0.288, 0.645) | .041* |
| ESR | 0.690 | 0.523–0.856 | 21.50 | 0.944 (0.742, 0.997) | 0.478 (0.292, 0.670) | .039* |
| IgM | 0.706 | 0.547–0.865 | 1.825 | 0.900 (0.699, 0.982) | 0.429 (0.245, 0.635) | .024* |
| IL-4 | 0.855 | 0.696–1.000 | 0.895 | 0.909 (0.623, 0.995) | 0.727 (0.434, 0.903) | .005** |
| P/F ratio | 0.865 | 0.729–1.000 | 342.2 | 0.842 (0.624, 0.945) | 0.857 (0.601, 0.975) | .000** |
| STRAD(S6%V7) | 0.883 | 0.791–0.974 | 0.056 | 0.833 (0.642, 0.932) | 0.778 (0.592, 0.894) | .000** |
| STRAD(S6%V7)-Ro52 | 0.899 | 0.795–1.000 | – | 0.846 (0.578, 0.973) | 0.864 (0.667, 0.953) | .000** |
ALB: albumin; ESR: erythrocyte sedimentation rate; IgM: immunoglobulin M; IL-4: interleukin-4; P/F ratio: arterial oxygen/fraction of inspiration oxygen.
p < .05.
p < .01.
Discussion
In patients with MDA5+DM-ILD, those who progress to severe ILD are more likely to benefit from early aggressive treatment than those who remain chronic/stable ILD [9]. However, currently, patients with severe ILD risk lack typical clinical manifestations and biomarkers for early diagnosis [11]. Studies have reported that ‘ground-glass opacity (GGO) scores in the right middle lobes’ and ‘lower lung zone consolidation’ are closely associated with RP-ILD/poor prognosis [13,15,33], suggesting that the imaging types of HRCT (including GGO, reticular shadow, consolidation, honeycombing, etc.) combined with distribution characteristics (upper/middle/lower lung distribution) may be predictive factors for the prognosis of MDA5+DM-ILD. Based on the differences in HU values among various ILD imaging types in HRCT and their vertical distribution, we propose a quantitative analysis method named ‘STRAD’ for HRCT evaluation of MDA5+DM-ILD patients. This study reviews the situation of severe-ILD risk occurrence in MDA5+DM-ILD patients at our medical institution over the past five years, using ‘acute respiratory failure’ as a quantifiable clinical outcome to clarify the predictive factors of imaging quantitative analysis indicators and clinical indicators, and establishes a simple predictive model: the STRAD-Ro52 model. Preliminary results showed that STRAD parameters have a wide correlation with demographics, inflammation, immunity, organ function and other indicators. The STRAD-Ro52 model had a good predictive ability for the risk of severe ILD in MDA5+DM-ILD patients within six months. Therefore, it may become an effective tool for clinicians to assess the risk of severe ILD in patients with MDA5+DM-ILD.
In our cohort, 47.05% (24/51) of MDA5+DM-ILD patients progressed to severe ILD, while about half had chronic or stable ILD, which is close to the data reported previously [34,35]. Our research found that in the early stage of the disease, as the percentage of V7 (−699 to −650 HU voxels out of the total lung threshold voxels) increased, the risk of severe-ILD within six months increases. The analysis of vertical space distribution characteristics suggests that S6 is the most meaningful level (V7 corresponds to the HU threshold range of GGO [36]; S6 is the location of the lower part of the middle lung). Reports on PM/DM-ILD confirm that HRCT presents consolidation and GGO, corresponding to OP and diffuse alveolar damage (DAD) pathological types, often complicated by rapid progressive respiratory failure, leading to fatal outcomes [37]. Kobayashi et al.’s study [38] confirmed that in patients with JDM-ILD combined with respiratory failure, GGO on chest HRCT scans was consistent with DAD found in autopsies or biopsies. A study on serial changes on HRCT in MDA5+DM-ILD [39] found that RP-ILD at the early onset of the disease presents a predominance of diffuse GGO, which is closely related to DAD. These findings are consistent with ours: V7 may be an imaging biomarker for early prediction of severe ILD. In terms of the distribution characteristics of lung lesions, data show that the ‘right middle lobe GGO score’ in MDA5+DM-ILD patients is related to the six-month survival rate [15,40]. ‘Lower lung zone consolidation’ is closely related to the 90-day mortality rate [33] and the occurrence of RP-ILD [13]. Our research found that S6%V7 is the most meaningful indicator for predicting the risk of severe ILD in MDA5+DM-ILD patients’ early chest HRCT, which is close to the research results of the ‘right middle lobe GGO score’ [15,40]. These conclusions may help clinicians to make more aggressive treatment decisions in the early stages of the disease in such patients. Our research conclusion does not support ‘lower lung consolidation’ as the best indicator for predicting severe ILD within six months, possibly because: (i) both the severe ILD group and the non-severe ILD group show ‘lower lung consolidation’ characteristics in the early STRAD parameters; (ii) our research cohort (MDA5+DM-ILD vs. PM/DM-ILD), clinical outcomes (severe ILD vs. RP-ILD/death risk) and time limitation (six months vs. three months) are not consistent with the above studies.
Our data showed that among all clinical parameters, the presence of anti-Ro52 antibody was the only independent risk factor for the progression of MDA5+DM-ILD to severe ILD in the early stage. Anti-Ro52 antibody is the most common MAA in IIM [41], and it has been reported that 74.7% of MDA5+DM-ILD are positive for the anti-Ro52 antibody [35]; our data show an anti-Ro52 antibody positivity rate of 68.63% (35/51), which is close to it. Among the 24 patients who eventually developed severe ILD, 91.67% (22/24) were positive for anti-Ro52 antibody, while this indicator was 48.14% (13/27) in the non-severe ILD group. In a study of a population with anti-Jo-1 (+) combined with anti-Ro52 (+), the presence of anti-Ro52 antibody implied acute severe ILD and non-response to conventional immunosuppressive drugs, indicating that anti-Ro52 (+) serves as a marker for the severity of ILD and responsiveness to treatment [42]. Recent studies have shown that the anti-Ro52 antibody is a risk factor for ILD in JDM and DM patients [43,44] and is related to the occurrence of RP-ILD [35]. The co-expression of anti-MDA5 and anti-Ro52 antibodies is related to a lower survival rate [35,45] and a higher HRCT score [46], suggesting its potential predictive value for severe ILD. It is worth noting that our research results are not entirely consistent with those of some studies [20,47], which may be related to the following reasons: (i) retrospective study, incomplete laboratory examination data, especially prominent in early cases; additionally, some patients in the severe-ILD group were unable to tolerate and did not undergo PFTs. (ii) The research endpoint of severe-ILD differs from that of RP-ILD/death, and some known risk factors come from a broader ILD cohort. (iii) Some parameters may be important predictors in late-stage/short-term ILD cases but may not be as important in early cases.
We established the STRAD-Ro52 model using the presence of anti-Ro52 antibody and S6%V7, which can predict the risk of severe ILD in MDA5+DM-ILD within six months. Several studies have proposed risk-prediction models for the prognosis of MDA5+DM-ILD. The FLAIR model [47] includes ferritin, lactate dehydrogenase, MDA5 antibody, RP-ILD and HRCT imaging semi-quantitative scores as five indicators, which can predict the poor prognosis of CADM-ILD. However, the positivity rate of the anti-Ro-52 antibody in the study cohort was 31%, which was not related to prognosis. The Wang et al.’s study [19] used the effective lung ventilation area ratio (ELVAR) as a biomarker for the prognosis of MDA5+DM-ILD; surprisingly, ELVAR was not related to PFT parameters, such as FVC and DLCO. Xu et al.’s study [17] used ‘CT pneumonia analysis’ (syngo) software for quantitative analysis to predict the six-month mortality risk of MDA5+DM-ILD patients, which has a stronger recognition ability for specific imaging types (GGO and consolidation), but may miss contributions on other imaging types. Radiomics research [20] confirmed that the Rad-score plus model is an independent predictor of the six-month mortality risk in MDA5+DM-ILD patients. These studies emphasize the important role of quantitative HRCT analysis in the prognosis assessment of MDA5+DM-ILD. Additionally, some studies [20,47] have indicated that clinical indicators can enhance the predictive power of imaging biomarkers for disease prognosis, which is consistent with the findings of our study. Considering the potentially rapid deterioration of the disease course in MDA5+DM-ILD, we recommend evaluating the STRAD-Ro52 when patients are initially diagnosed with MDA5+DM. This model can help in the early stratification of the risk of severe disease, allowing these cases with severe risk to have the opportunity for more careful monitoring and more aggressive early intervention in clinical practice.
To date, studies evaluating MDA5+DM-ILD using HU threshold values combined with spatial distribution in HRCT are rare. Our study has several advantages. First, it achieves a comprehensive quantification of ILD based on the basic principles of CT, incorporating highly heterogeneous lesions into a single analysis system (STRAD) for comparative analysis. Compared to other methods, STRAD heatmaps are more intuitive and comprehensive, showing a certain degree of innovation. Second, we chose ‘severe-ILD’ as the research endpoint and set strict quantitative standards, which can cover most clinical scenarios, helping in the precise classification of the disease. Third, the wide correlation of STRAD parameters with clinical data and their internal imbalance features lays the foundation for further stratified localization research. Finally, the STRAD-Ro52 predictive model combines quantitative imaging parameters with clinical parameters, allowing for a more comprehensive early prediction of the risk of severe ILD in MDA5+DM-ILD.
Our study also has limitations: (1) our data come from a retrospective review of the past five years. Owing to the inability to perform quality control in advance, missing data cannot be completely avoided. (2) The initial treatment regimens were not entirely consistent with subsequent treatments, making it impossible to balance treatment responsiveness. (3) The absence of bronchoalveolar lavage fluid analysis, lung biopsy and dynamic monitoring of anti-MDA5 antibody levels represents a significant limitation. (4) This is an exploratory study. MDA5+DM-ILD patients are relatively rare, and the number of cases included and HRCT data were relatively small, which may affect the stability of statistical analysis results, necessitating further verification through multicentre prospective large cohort studies.
Conclusions
We performed a quantitative analysis of chest HRCT in MDA5+DM-ILD using STRAD parameters and established the STRAD-Ro52 model. The increase in S6%V7 and the presence of anti-Ro52 antibodies have been identified as independent predictive factors for the occurrence of severe ILD in MDA5+DM-ILD within six months. The STRAD-Ro52 model can help identify these patients, which may positively impact clinical treatment strategies and help optimize the clinical research design and precise management of this challenging disease.
Supplementary Material
Funding Statement
This study was supported by the Real World Research Project Grant Fund from the Hainan Institute of Real World Data (HNLC2022RWS016).
Author contributions
FX, SC and JL contributed to the project design. FX, FC, XL, SZ, DL and JW collected clinical data of the patients. JZho and XT conducted the visual HRCT assessment. FX and FC performed the quantitative analysis of the HRCT. FX, RC and JZ contributed to data analysis. FX, FC, SC and JL wrote and revised the manuscript accordingly. JL and SC supervised the project and helped revise the manuscript. All authors read and agreed to the final version of the manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
References
- 1.Selva-O’Callaghan A, Pinal-Fernandez I, Trallero-Araguas E, et al. Classification and management of adult inflammatory myopathies. Lancet Neurol. 2018;17(9):816–828. doi: 10.1016/S1474-4422(18)30254-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lundberg IE, de Visser M, Werth VP.. Classification of myositis. Nat Rev Rheumatol. 2018;14(5):269–278. doi: 10.1038/nrrheum.2018.41. [DOI] [PubMed] [Google Scholar]
- 3.Fathi M, Lundberg IE.. Interstitial lung disease in polymyositis and dermatomyositis. Curr Opin Rheumatol. 2005;17(6):701–706. doi: 10.1097/01.bor.0000179949.65895.53. [DOI] [PubMed] [Google Scholar]
- 4.Sun KY, Fan Y, Wang YX, et al. Prevalence of interstitial lung disease in polymyositis and dermatomyositis: a meta-analysis from 2000 to 2020. Semin Arthritis Rheum. 2021;51(1):175–191. doi: 10.1016/j.semarthrit.2020.11.009. [DOI] [PubMed] [Google Scholar]
- 5.Moghadam-Kia S, Oddis CV, Sato S, et al. Anti-melanoma differentiation-associated gene 5 is associated with rapidly progressive lung disease and poor survival in US patients with amyopathic and myopathic dermatomyositis. Arthritis Care Res. 2016;68(5):689–694. doi: 10.1002/acr.22728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jiang L, Wang Y, Peng Q, et al. Serum YKL-40 level is associated with severity of interstitial lung disease and poor prognosis in dermatomyositis with anti-MDA5 antibody. Clin Rheumatol. 2019;38(6):1655–1663. doi: 10.1007/s10067-019-04457-w. [DOI] [PubMed] [Google Scholar]
- 7.Wu W, Guo L, Fu Y, et al. Interstitial lung disease in anti-MDA5 positive dermatomyositis. Clin Rev Allergy Immunol. 2021;60(2):293–304. doi: 10.1007/s12016-020-08822-5. [DOI] [PubMed] [Google Scholar]
- 8.Fathi M, Lundberg IE, Tornling G.. Pulmonary complications of polymyositis and dermatomyositis. Semin Respir Crit Care Med. 2007;28(4):451–458. doi: 10.1055/s-2007-985666. [DOI] [PubMed] [Google Scholar]
- 9.Tsuji H, Nakashima R, Hosono Y, et al. Multicenter prospective study of the efficacy and safety of combined immunosuppressive therapy with high-dose glucocorticoid, tacrolimus, and cyclophosphamide in interstitial lung diseases accompanied by anti-melanoma differentiation-associated gene 5-positive dermatomyositis. Arthritis Rheumatol. 2020;72(3):488–498. doi: 10.1002/art.41105. [DOI] [PubMed] [Google Scholar]
- 10.Huang K, Vinik O, Shojania K, et al. Clinical spectrum and therapeutics in Canadian patients with anti-melanoma differentiation-associated gene 5 (MDA5)-positive dermatomyositis: a case-based review. Rheumatol Int. 2019;39(11):1971–1981. doi: 10.1007/s00296-019-04398-2. [DOI] [PubMed] [Google Scholar]
- 11.Ye Y, Fu Q, Wang R, et al. Serum KL-6 level is a prognostic marker in patients with anti-MDA5 antibody-positive dermatomyositis associated with interstitial lung disease. J Clin Lab Anal. 2019;33(8):e22978. doi: 10.1002/jcla.22978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Motegi SI, Sekiguchi A, Toki S, et al. Clinical features and poor prognostic factors of anti-melanoma differentiation-associated gene 5 antibody-positive dermatomyositis with rapid progressive interstitial lung disease. Eur J Dermatol. 2019;29(5):511–517. doi: 10.1684/ejd.2019.3634. [DOI] [PubMed] [Google Scholar]
- 13.Zuo Y, Ye L, Liu M, et al. Clinical significance of radiological patterns of HRCT and their association with macrophage activation in dermatomyositis. Rheumatology. 2020;59(10):2829–2837. doi: 10.1093/rheumatology/keaa034. [DOI] [PubMed] [Google Scholar]
- 14.Mehta P, Aggarwal R, Porter JC, et al. Management of interstitial lung disease (ILD) in myositis syndromes: a practical guide for clinicians. Best Pract Res Clin Rheumatol. 2022;36(2):101769. doi: 10.1016/j.berh.2022.101769. [DOI] [PubMed] [Google Scholar]
- 15.Fujiki Y, Kotani T, Isoda K, et al. Evaluation of clinical prognostic factors for interstitial pneumonia in anti-MDA5 antibody-positive dermatomyositis patients. Mod Rheumatol. 2018;28(1):133–140. doi: 10.1080/14397595.2017.1318468. [DOI] [PubMed] [Google Scholar]
- 16.Shen N, Zhou X, Jin X, et al. MDA5 expression is associated with TGF-beta-induced fibrosis: potential mechanism of interstitial lung disease in anti-MDA5 dermatomyositis. Rheumatology. 2022;62(1):373–383. doi: 10.1093/rheumatology/keac234. [DOI] [PubMed] [Google Scholar]
- 17.Xu W, Wu W, Zhang D, et al. A novel CT scoring method predicts the prognosis of interstitial lung disease associated with anti-MDA5 positive dermatomyositis. Sci Rep. 2021;11(1):17070. doi: 10.1038/s41598-021-96292-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yamaguchi K, Nakajima T, Yamaguchi A, et al. Quantitative CT analysis of interstitial pneumonia in anti-melanoma differentiation-associated gene 5 antibody-positive dermatomyositis: a single center, retrospective study. Clin Rheumatol. 2022;41(5):1473–1481. doi: 10.1007/s10067-021-06033-7. [DOI] [PubMed] [Google Scholar]
- 19.Wang C, Du J, Mei X, et al. The value of effective lung ventilation area ratio based on CT image analysis is a new index to predict the shorter outcome of anti-melanoma differentiation-associated protein 5 positive dermatomyositis associated interstitial lung disease: a single-center retrospective study. Front Med. 2021;8:728487. doi: 10.3389/fmed.2021.728487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu W, Wu W, Zheng Y, et al. A computed tomography radiomics-based prediction model on interstitial lung disease in anti-MDA5-positive dermatomyositis. Front Med. 2021;8:768052. doi: 10.3389/fmed.2021.768052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bohan A, Peter JB.. Polymyositis and dermatomyositis (first of two parts). N Engl J Med. 1975;292(7):344–347. doi: 10.1056/NEJM197502132920706. [DOI] [PubMed] [Google Scholar]
- 22.Bohan A, Peter JB.. Polymyositis and dermatomyositis (second of two parts). N Engl J Med. 1975;292(8):403–407. doi: 10.1056/NEJM197502202920807. [DOI] [PubMed] [Google Scholar]
- 23.Sontheimer RD. Would a new name hasten the acceptance of amyopathic dermatomyositis (dermatomyositis sine myositis) as a distinctive subset within the idiopathic inflammatory dermatomyopathies spectrum of clinical illness? J Am Acad Dermatol. 2002;46(4):626–636. doi: 10.1067/mjd.2002.120621. [DOI] [PubMed] [Google Scholar]
- 24.Lundberg IE, Tjärnlund A, Bottai M, et al. 2017 European League Against Rheumatism/American College of Rheumatology classification criteria for adult and juvenile idiopathic inflammatory myopathies and their major subgroups. Ann Rheum Dis. 2017;76(12):1955–1964. doi: 10.1136/annrheumdis-2017-211468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Furuya H, Nakajima M, Ikeda K, et al. Prognosis and treatment of myositis-associated severe interstitial lung disease: a descriptive study using a nationwide inpatient database in Japan. Arthritis Care Res. 2022;74(3):478–483. doi: 10.1002/acr.24646. [DOI] [PubMed] [Google Scholar]
- 26.Matthay MA, Arabi Y, Arroliga AC, et al. A new global definition of acute respiratory distress syndrome. Am J Respir Crit Care Med. 2024;209(1):37–47. doi: 10.1164/rccm.202303-0558WS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias . This joint statement of the American Thoracic Society (ATS), and the European Respiratory Society (ERS) was adopted by the ATS board of directors, June 2001 and by the ERS Executive Committee, June 2001. Am J Respir Crit Care Med. 2002;165:277–304. [DOI] [PubMed] [Google Scholar]
- 28.Zhu J, Wu L, Zhou Y, et al. A retrospective cohort study in Chinese patients with adult polymyositis and dermatomyositis: risk of comorbidities and subclassification using machine learning. Clin Exp Rheumatol. 2022;40(2):224–236. doi: 10.55563/clinexprheumatol/i2xeao. [DOI] [PubMed] [Google Scholar]
- 29.Kishaba T, McGill R, Nei Y, et al. Clinical characteristics of dermatomyositis/polymyositis associated interstitial lung disease according to the autoantibody. J Med Invest. 2018;65(3.4):251–257. doi: 10.2152/jmi.65.251. [DOI] [PubMed] [Google Scholar]
- 30.Bocchino M, Bruzzese D, D’Alto M, et al. Performance of a new quantitative computed tomography index for interstitial lung disease assessment in systemic sclerosis. Sci Rep. 2019;9(1):9468. doi: 10.1038/s41598-019-45990-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cressoni M, Gallazzi E, Chiurazzi C, et al. Limits of normality of quantitative thoracic CT analysis. Crit Care. 2013;17(3):R93. doi: 10.1186/cc12738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang Y, Chen Y, Wei Y, et al. Quantitative analysis of chest CT imaging findings with the risk of ARDS in COVID-19 patients: a preliminary study. Ann Transl Med. 2020;8(9):594. doi: 10.21037/atm-20-3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tanizawa K, Handa T, Nakashima R, et al. The prognostic value of HRCT in myositis-associated interstitial lung disease. Respir Med. 2013;107(5):745–752. doi: 10.1016/j.rmed.2013.01.014. [DOI] [PubMed] [Google Scholar]
- 34.Chen Z, Cao M, Plana MN, et al. Utility of anti-melanoma differentiation-associated gene 5 antibody measurement in identifying patients with dermatomyositis and a high risk for developing rapidly progressive interstitial lung disease: a review of the literature and a meta-analysis. Arthritis Care Res. 2013;65(8):1316–1324. doi: 10.1002/acr.21985. [DOI] [PubMed] [Google Scholar]
- 35.Xu A, Ye Y, Fu Q, et al. Prognostic values of anti-Ro52 antibodies in anti-MDA5-positive clinically amyopathic dermatomyositis associated with interstitial lung disease. Rheumatology. 2021;60(7):3343–3351. doi: 10.1093/rheumatology/keaa786. [DOI] [PubMed] [Google Scholar]
- 36.Michiue T, Sakurai T, Ishikawa T, et al. Quantitative analysis of pulmonary pathophysiology using postmortem computed tomography with regard to the cause of death. Forensic Sci Int. 2012;220(1–3):232–238. doi: 10.1016/j.forsciint.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 37.Suda T, Fujisawa T, Enomoto N, et al. Interstitial lung diseases associated with amyopathic dermatomyositis. Eur Respir J. 2006;28(5):1005–1012. doi: 10.1183/09031936.06.00038806. [DOI] [PubMed] [Google Scholar]
- 38.Kobayashi N, Takezaki S, Kobayashi I, et al. Clinical and laboratory features of fatal rapidly progressive interstitial lung disease associated with juvenile dermatomyositis. Rheumatology. 2015;54(5):784–791. doi: 10.1093/rheumatology/keu385. [DOI] [PubMed] [Google Scholar]
- 39.Kim M, Harvey S, Danoff SK, et al. Rapidly progressive interstitial lung disease in patients with anti-melanoma differentiation-associated gene 5-positive dermatomyositis: serial changes on HRCT. Emerg Radiol. 2022;29(6):961–967. doi: 10.1007/s10140-022-02080-y. [DOI] [PubMed] [Google Scholar]
- 40.Kotani T, Takeuchi T, Yoshimatsu Y, et al. Initial limited three-level thin-section computed tomography scorings predict the prognosis of acute/subacute interstitial pneumonia in patients with dermatomyositis. Mod Rheumatol. 2016;26(5):738–743. doi: 10.3109/14397595.2015.1134392. [DOI] [PubMed] [Google Scholar]
- 41.Ohashi K, Sada KE, Nakai Y, et al. Cluster analysis using anti-aminoacyl-tRNA synthetases and SS-A/Ro52 antibodies in patients with polymyositis/dermatomyositis. J Clin Rheumatol. 2019;25(6):246–251. doi: 10.1097/RHU.0000000000000836. [DOI] [PubMed] [Google Scholar]
- 42.Bauhammer J, Blank N, Max R, et al. Rituximab in the treatment of Jo1 antibody-associated antisynthetase syndrome: anti-Ro52 positivity as a marker for severity and treatment response. J Rheumatol. 2016;43(8):1566–1574. doi: 10.3899/jrheum.150844. [DOI] [PubMed] [Google Scholar]
- 43.Sabbagh S, Pinal-Fernandez I, Kishi T, et al. Anti-Ro52 autoantibodies are associated with interstitial lung disease and more severe disease in patients with juvenile myositis. Ann Rheum Dis. 2019;78(7):988–995. doi: 10.1136/annrheumdis-2018-215004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weng C, Ding Z, Zhou Y, et al. Clinical characteristics of dermatomyositis with interstitial lung disease: a retrospective case-control study. Rheumatol Ther. 2023;10(3):635–648. doi: 10.1007/s40744-023-00540-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Temmoku J, Sato S, Fujita Y, et al. Clinical significance of myositis-specific autoantibody profiles in Japanese patients with polymyositis/dermatomyositis. Medicine. 2019;98(20):e15578. doi: 10.1097/MD.0000000000015578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xing X, Li A, Li C.. Anti-Ro52 antibody is an independent risk factor for interstitial lung disease in dermatomyositis. Respir Med. 2020;172:106134. doi: 10.1016/j.rmed.2020.106134. [DOI] [PubMed] [Google Scholar]
- 47.Lian X, Zou J, Guo Q, et al. Mortality risk prediction in amyopathic dermatomyositis associated with interstitial lung disease: the FLAIR model. Chest. 2020;158(4):1535–1545. doi: 10.1016/j.chest.2020.04.057. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting the findings of this study are available from the corresponding author upon reasonable request.