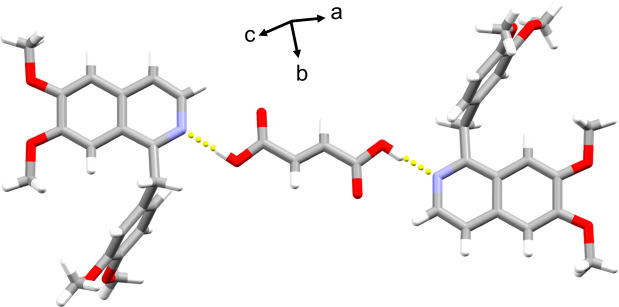
Two different multi-component crystals consisting of papaverine [1-(3,4-dimethoxybenzyl)-6,7-dimethoxyisoquinoline, C20H21NO4] and fumaric acid [C4H4O4] were obtained.
Keywords: crystal structure, fumaric acid, papaverine, multi-component crystal
Abstract
Two different multi-component crystals consisting of papaverine [1-(3,4-dimethoxybenzyl)-6,7-dimethoxyisoquinoline, C20H21NO4] and fumaric acid [C4H4O4] were obtained. Single-crystal X-ray structure analysis revealed that one, C20H21NO4·1.5C4H4O4 (I), is a salt co-crystal composed of salt-forming and non-salt-forming molecules, and the other, C20H21NO4·0.5C4H4O4 (II), is a salt–co-crystal intermediate (i.e., in an intermediate state between a salt and a co-crystal). In this study, one state (crystal structure at 100 K) within the salt–co-crystal continuum is defined as the ‘intermediate’.
1. Chemical context
Papaverine (1-[3,4-dimethoxybenzyl]-6,7-dimethoxyisoquinoline) is an isoquinoline alkaloid compound extracted from the mature seed capsules of poppies (Kang et al., 2018 ▸). It is an antispasmodic and vasodilator, used primarily in the treatment of smooth muscle spasms and for vasodilation and improvement of symptoms in acute arterial embolism, acute pulmonary embolism, peripheral circulatory disturbance, and coronary circulatory disturbance. The active pharmaceutical ingredient papaverine has been developed as a hydrochloride salt whose crystal structure has been determined (Reynolds et al., 1974 ▸). In the pharmaceutical industry, studies on salt crystallization and co-crystallization are conducted for purposes such as improving the solid-state stability of the active pharmaceutical ingredient or improvement of its dissolution properties. Fumaric acid is a dicarboxylic acid and a cis–trans isomer of maleic acid and is used in the pharmaceutical industry as a counter-ion in salts and as a conformer of co-crystals. For example, among 1372 new drugs approved by the US Food and Drug Administration between 1939 and 2020, fumaric acid was used as a counter-ion in the salts of ten drugs (Bharate et al., 2021 ▸). The recently developed COVID-19 antiviral drug substance Ensitrelvir is crystallized as a co-crystal with fumaric acid (Kawajiri et al., 2023 ▸).
In this work, we synthesized two multicomponent crystals — a salt co-crystal (I) and a salt–co-crystal intermediate (II) — consisting of papaverine 1 and fumaric acid 2, and we determined their crystal structures. In this study, one state (crystal structure at 100 K) within the salt–co-crystal continuum is defined as the ‘intermediate’.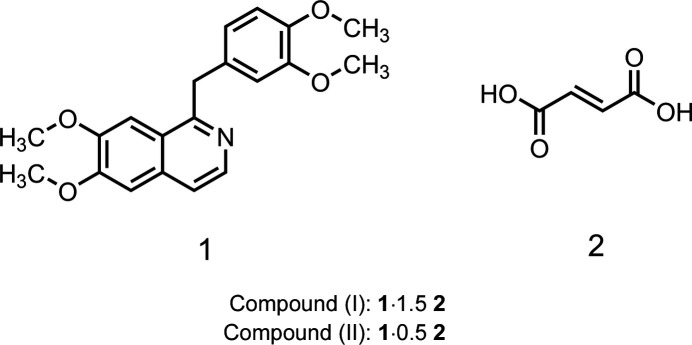
2. Structural commentary
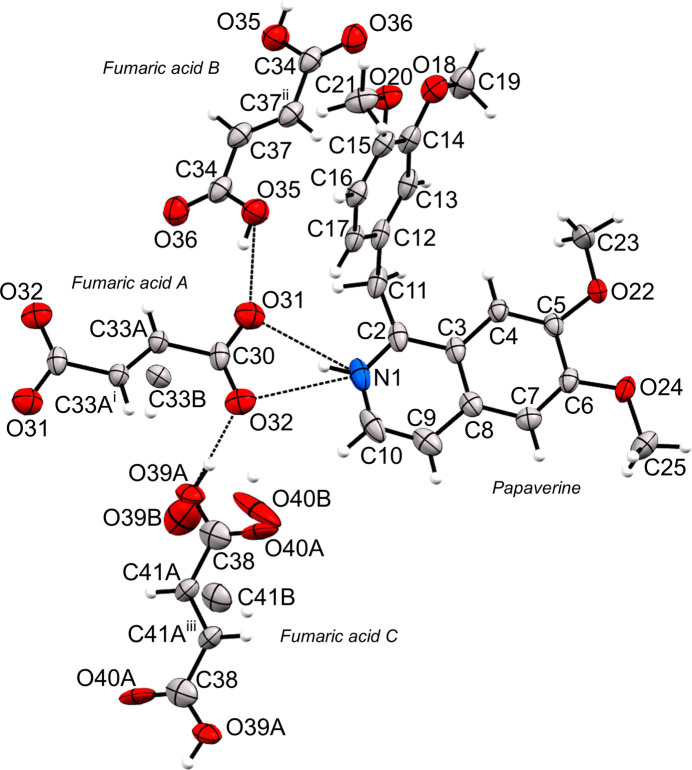
The crystal structure of (I) is shown in Fig. 1 ▸. It crystallized with a 1:1.5 papaverine:fumaric acid stoichiometric ratio in the space group P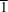 with Z = 2, with one full molecule of papaverine and three half molecules of fumaric acid (fumaric acids A, B, and C) in the asymmetric unit. The three fumaric acid molecules were positioned on a center of symmetry, with molecules A and C being disordered over two positions (O39/O40/C41 and C33).
with Z = 2, with one full molecule of papaverine and three half molecules of fumaric acid (fumaric acids A, B, and C) in the asymmetric unit. The three fumaric acid molecules were positioned on a center of symmetry, with molecules A and C being disordered over two positions (O39/O40/C41 and C33).
Figure 1.
The molecular structure of (I). Hydrogen bonds are shown as dashed lines and displacement ellipsoids are drawn at the 50% probability level. [Symmetry codes: (i) −x + 2, −y + 2, −z + 1; (ii) −x + 1, −y + 2, −z; (iii) −x + 2, −y + 2, −z + 2.]
Since the C30—O31 and C30—O32 distances of fumaric acid molecule A are 1.248 (2) Å and 1.246 (3) Å, respectively, the carboxy group of molecule A is dissociated (Childs et al., 2007 ▸; Chen et al., 2012 ▸). In addition, N1 of the papaverine molecule is protonated and is engaged in N—H⋯O hydrogen bonding (Table 1 ▸). Therefore, it was determined that fumaric acid molecule A and the papaverine molecule form a salt. The fumaric acid molecules B and C are hydrogen-bonded to fumaric acid molecule A. The C34—O35 and C34—O36 distances in fumaric acid molecule B are 1.324 (3) Å and 1.211 (2) Å, respectively, thus the carboxy group of molecule B is not dissociated. The C38—O39A and C38—O40A distances in fumaric acid molecule C are 1.280 (5) Å and 1.231 (6) Å, respectively, thus the carboxy group of molecule C is not dissociated (Childs et al., 2007 ▸; Chen et al., 2012 ▸). Therefore, this multicomponent crystal includes both salt-forming and non–salt-forming molecules and was thus concluded to be a salt co-crystal, (I).
Table 1. Hydrogen-bond geometry (Å, °) for (I).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| N1—H1⋯O31 | 0.88 | 2.20 | 3.034 (3) | 159 |
| N1—H1⋯O32 | 0.88 | 2.18 | 2.919 (2) | 141 |
| O35—H35⋯O31 | 0.84 | 1.83 | 2.617 (2) | 156 |
| O39A—H39A⋯O32 | 0.84 | 1.67 | 2.502 (4) | 169 |
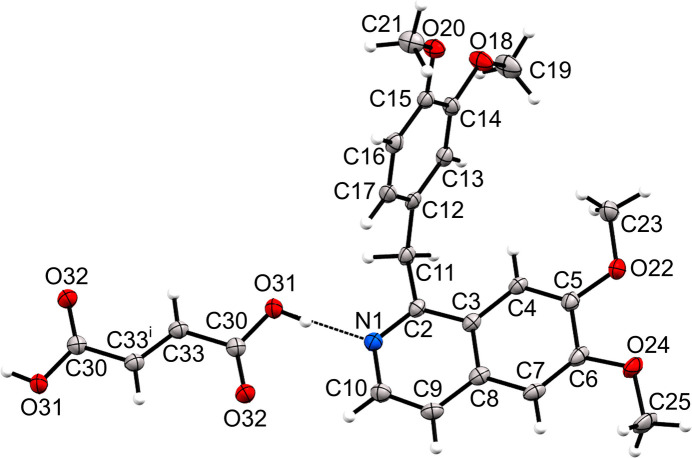
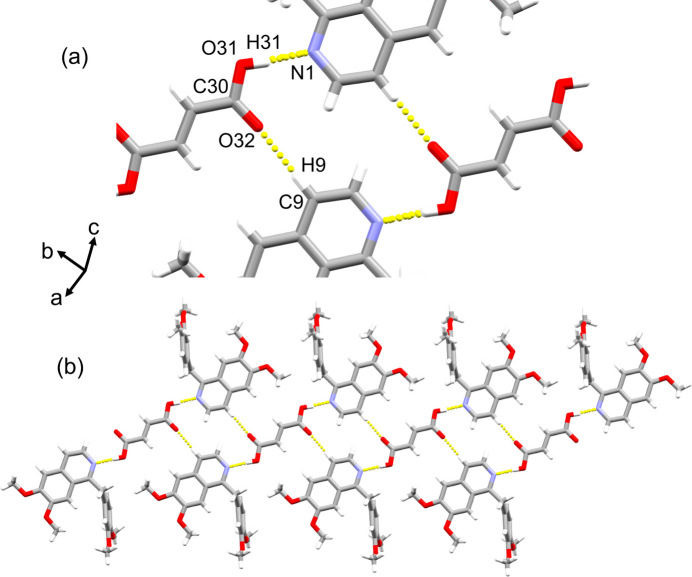
The crystal structure of (II) is given in Fig. 2 ▸. It crystallized with a 1:0.5 papaverine:fumaric acid stoichiometric ratio in space group P21/n with Z = 4, with one full molecule of papaverine and half a molecule of fumaric acid in the asymmetric unit. The fumaric acid molecule is positioned on the center of symmetry. The C30–O31 and C30–O32 distances in the fumaric acid molecule are 1.306 (1) and 1.223 (1) Å, respectively, indicating that the carboxylic acid of the fumaric acid molecule is not dissociated (Childs et al., 2007 ▸; Chen et al., 2012 ▸). Therefore, the fumaric acid and papaverine molecules were determined to form a co-crystal. However, the O—H⋯N hydrogen bond [D⋯A = 2.5687 (12) Å, Table 2 ▸) is shorter than that in neutral or ionic synthons, which indicates an intermediate state between a salt and a co-crystal (Childs et al., 2007 ▸; Thipparaboina et al., 2015 ▸; Stevens et al., 2020 ▸; Tothadi et al., 2021 ▸; Kotte et al., 2023 ▸). It was thus concluded that this multicomponent crystal, (II), is a salt–co-crystal intermediate.
Figure 2.
The molecular structure of (II). The hydrogen bond is shown as a dashed line and displacement ellipsoids are drawn at the 50% probability level. [Symmetry code: (i) −x, −y + 1, −z + 1.]
Table 2. Hydrogen-bond geometry (Å, °) for (II).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| O31—H31⋯N1 | 0.84 (1) | 1.73 (1) | 2.5687 (12) | 175 (1) |
| C9—H9⋯O32i | 0.95 (1) | 2.27 (1) | 3.2191 (14) | 176 (1) |
Symmetry code: (i) 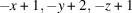 .
.
3. Supramolecular features
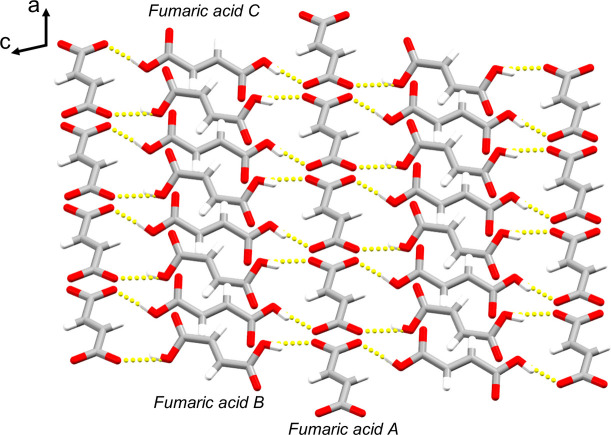
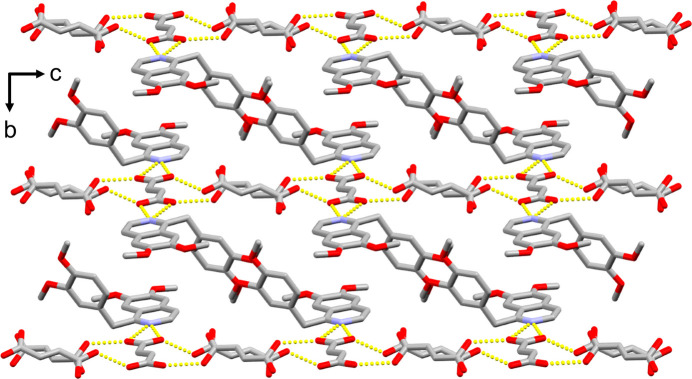
The combination of the same two components – papaverine and fumaric acid – led to two different multicomponent crystals each with a different stoichiometric ratio and packing. The fumaric acid molecules in (I) form a systematic two-dimensional sheet structure parallel to the ac plane with hydrogen bonds linking fumaric acid molecules A, B, and C (Fig. 3 ▸). The space between the fumaric acid sheets is filled with a two-dimensional layer of papaverine molecules hydrogen-bonded to fumaric acid molecules A, resulting in (I) having a layered structure (Fig. 4 ▸).
Figure 3.
Systematic two-dimensional sheet structure of fumaric acid in (I) viewed along the ac plane. Intermolecular O—H⋯O hydrogen bonds are shown as dashed lines. One of the two disorder components has been omitted for clarity.
Figure 4.
The layered structure of (I) viewed along the a axis. Intermolecular O—H⋯O and N—H⋯O hydrogen bonds are shown as dashed lines. All hydrogen atoms and one of the two disordered components of the fumaric acid molecules have been omitted for clarity.
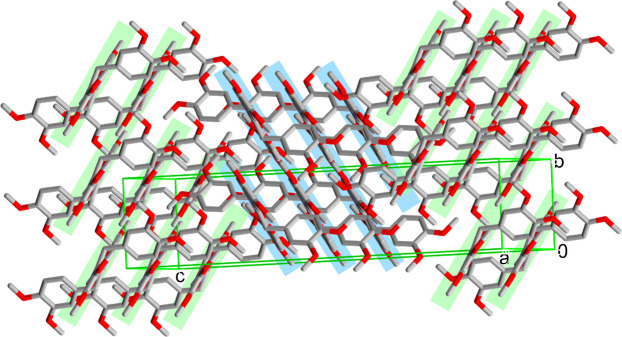
Compound (II) exhibits a three-molecule unit structure with hydrogen bonds between two papaverine molecules and one fumaric acid molecule (Fig. 5 ▸). The H9⋯O32 and C9⋯O32 distances between two of these three-molecule units are 2.2706 (14) and 3.2191 (14) Å, respectively, with a C9—H9⋯O32 angle of 176.13 (11)° (Fig. 6 ▸a, Table 2 ▸); thus, it was concluded that there is a C—H⋯O hydrogen bond (Steiner, 1997 ▸). A ring structure consisting of two O—H⋯N hydrogen bonds and two C—H⋯O hydrogen bonds between two papaverine molecules and two fumaric acid molecules is observed (Fig. 6 ▸a). As a result, a one-dimensional ribbon structure is formed by the combination of O—H⋯N and C—H⋯O hydrogen bonds (Fig. 6 ▸b). The final crystal structure is formed by the repeated overlapping of these ribbon structures (Fig. 7 ▸).
Figure 5.
Structural unit in the crystal of (II). Intermolecular O—H⋯N hydrogen bonds are shown as dashed lines.
Figure 6.
One-dimensional ribbon structure of (II). Intermolecular O—H⋯N and C—H⋯O hydrogen bonds are shown as dashed lines. (a) Enlarged view of hydrogen-bonded ring. (b) Overview of the one-dimensional ribbon structure.
Figure 7.
The packing of (II). Each blue and green line represents a one-dimensional ribbon structure. All hydrogen atoms have been removed for clarity.
4. Database survey
A survey of the Cambridge Structural Database (WebCSD, v5.44, April 2023; Groom et al., 2016 ▸) for structures with papaverine resulted in four hits. Two crystal structures were free-base, single-component crystals [refcodes MVERIQ (Baggio & Baggio, 1973 ▸) and MVERIQ01 (Marek et al., 1997 ▸)]. The other two crystals were salts: one was a hydrochloride salt (refcode PAPAVC; Reynolds et al., 1974 ▸) and the other was a hydrobromide salt (refcode ZZZGYK; Van Hulle et al., 1953 ▸). There were no reports of multi-component crystals of papaverine.
5. Synthesis and crystallization
Compound (I) was prepared as follows. About 3 mg (0.009 mmol) of papaverine and 2 mg (0.018 mmol) of fumaric acid were dissolved in 0.025 mL of ethanol. The prepared solution was shaken at room temperature at 100 r.p.m. overnight, and clear light colorless, block-shaped crystals were obtained. Compound (II) was prepared as follows. About 20 mg (0.06 mmol) of papaverine and 10 mg (0.09 mmol) of fumaric acid were dissolved in 0.28 mL of a mixture of acetone and water (6:1) with heating at 368K. The prepared solution was shaken at room temperature at 100 r.p.m. overnight, and clear, light, colorless, block-shaped crystals was obtained.
6. Refinement
Crystal data, data collection, and structure refinement details are summarized in Table 3 ▸. The N-bound H atom in (I) was positioned geometrically and refined using a riding model with isotropic displacement parameter Uiso(H) = 1.2Ueq(N). The O-bound H atoms in (I) were located in difference-Fourier maps and refined with O—H = 0.84 Å and with isotropic displacement parameters Uiso(H) = 1.5Ueq(O). The C-bound H atoms in (I) were positioned geometrically (C—H = 0.95, 0.98, and 0.99 Å for sp2-hybridized, methyl, and methylene hydrogen atoms, respectively) and refined using a riding model, with isotropic displacement parameters Uiso(H) = 1.5Ueq(C) for methyl and Uiso(H) = 1.2Ueq(C) for all other H atoms. The fumaric acid was disordered over two positions (O39/O40/C41 and C33), for which occupancies were refined, converging to 0.598/0.402 and 0.742/0.258, respectively. Restraints by DFIX were applied for C38/O39/O40, O39/O40, O40/H40, O32/H40, and C38/H40. For compound (II), there were no N-bound H atoms or disorders, and the refinement conditions for O-bound H atoms and C-bound H atoms were the same as those for compound (I).
Table 3. Experimental details.
| (I) | (II) | |
|---|---|---|
| Crystal data | ||
| Chemical formula | C20H22NO4·1.5C4H4O4 | C20H21NO4·0.5C4H4O4 |
| M r | 513.48 | 397.43 |
| Crystal system, space group | Triclinic, P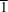
|
Monoclinic, P21/n |
| Temperature (K) | 100 | 100 |
| a, b, c (Å) | 9.5290 (2), 10.5445 (3), 12.6509 (4) | 9.05718 (12), 6.71363 (11), 32.8419 (4) |
| α, β, γ (°) | 91.606 (2), 104.980 (2), 97.823 (2) | 90, 95.9308 (12), 90 |
| V (Å3) | 1213.87 (6) | 1986.31 (5) |
| Z | 2 | 4 |
| Radiation type | Cu Kα | Cu Kα |
| μ (mm−1) | 0.92 | 0.80 |
| Crystal size (mm) | 0.17 × 0.08 × 0.03 | 0.22 × 0.11 × 0.11 |
| Data collection | ||
| Diffractometer | XtaLAB Synergy, Single source at home/near, HyPix3000 | XtaLAB Synergy, Single source at home/near, HyPix3000 |
| Absorption correction | Multi-scan (CrysAlis PRO; Rigaku OD, 2022 ▸) | Multi-scan (CrysAlis PRO; Rigaku OD, 2022 ▸) |
| Tmin, Tmax | 0.862, 1.000 | 0.908, 1.000 |
| No. of measured, independent and observed reflections | 10089, 4366, 3457 [I > 2σ(I)] | 8710, 3589, 3325 [I ≥ 2u(I)] |
| R int | 0.026 | 0.018 |
| (sin θ/λ)max (Å−1) | 0.601 | 0.601 |
| Refinement | ||
| R[F2 > 2σ(F2)], wR(F2), S | 0.047, 0.133, 1.06 | 0.033, 0.087, 1.04 |
| No. of reflections | 4366 | 3589 |
| No. of parameters | 382 | 268 |
| No. of restraints | 282 | 0 |
| H-atom treatment | H atoms treated by a mixture of independent and constrained refinement | H atoms treated by a mixture of independent and constrained refinement |
| Δρmax, Δρmin (e Å−3) | 0.38, −0.39 | 0.27, −0.21 |
Supplementary Material
Crystal structure: contains datablock(s) II, I. DOI: 10.1107/S2056989024009794/ox2007sup1.cif
Structure factors: contains datablock(s) II. DOI: 10.1107/S2056989024009794/ox2007IIsup3.hkl
Structure factors: contains datablock(s) I. DOI: 10.1107/S2056989024009794/ox2007Isup2.hkl
Supporting information file. DOI: 10.1107/S2056989024009794/ox2007Isup4.mol
Supporting information file. DOI: 10.1107/S2056989024009794/ox2007IIsup5.mol
Supporting information file. DOI: 10.1107/S2056989024009794/ox2007Isup6.cml
Supporting information file. DOI: 10.1107/S2056989024009794/ox2007IIsup7.cml
Additional supporting information: crystallographic information; 3D view; checkCIF report
Acknowledgments
The authors thank Ayano Horikawa (Chugai Pharmaceutical Co., Ltd.) for helpful discussions of the single-crystal structure.
supplementary crystallographic information
1-(3,4-Dimethoxybenzyl)-6,7-dimethoxyisoquinoline–fumaric acid (2/1) (II). Crystal data
| C20H21NO4·0.5C4H4O4 | F(000) = 843.079 |
| Mr = 397.43 | Dx = 1.329 Mg m−3 |
| Monoclinic, P21/n | Cu Kα radiation, λ = 1.54184 Å |
| a = 9.05718 (12) Å | Cell parameters from 6957 reflections |
| b = 6.71363 (11) Å | θ = 2.7–67.9° |
| c = 32.8419 (4) Å | µ = 0.80 mm−1 |
| β = 95.9308 (12)° | T = 100 K |
| V = 1986.31 (5) Å3 | Block, clear light colourless |
| Z = 4 | 0.22 × 0.11 × 0.11 mm |
1-(3,4-Dimethoxybenzyl)-6,7-dimethoxyisoquinoline–fumaric acid (2/1) (II). Data collection
| XtaLAB Synergy, Single source at home/near, HyPix3000 diffractometer | 3589 independent reflections |
| Radiation source: micro-focus sealed X-ray tube, PhotonJet (Cu) X-ray Source | 3325 reflections with I≥ 2u(I) |
| Mirror monochromator | Rint = 0.018 |
| Detector resolution: 10.0000 pixels mm-1 | θmax = 68.0°, θmin = 2.7° |
| ω scans | h = −10→5 |
| Absorption correction: multi-scan (CrysAlisPro; Rigaku OD, 2022) | k = −8→8 |
| Tmin = 0.908, Tmax = 1.000 | l = −39→39 |
| 8710 measured reflections |
1-(3,4-Dimethoxybenzyl)-6,7-dimethoxyisoquinoline–fumaric acid (2/1) (II). Refinement
| Refinement on F2 | 37 constraints |
| Least-squares matrix: full | H atoms treated by a mixture of independent and constrained refinement |
| R[F2 > 2σ(F2)] = 0.033 | w = 1/[σ2(Fo2) + (0.0429P)2 + 0.6045P] where P = (Fo2 + 2Fc2)/3 |
| wR(F2) = 0.087 | (Δ/σ)max = −0.0003 |
| S = 1.04 | Δρmax = 0.27 e Å−3 |
| 3589 reflections | Δρmin = −0.21 e Å−3 |
| 268 parameters | Extinction correction: SHELXL2019/2 (Sheldrick, 2015b), Fc*=kFc[1+0.001xFc2λ3/sin(2θ)]-1/4 |
| 0 restraints | Extinction coefficient: 0.0012 (2) |
1-(3,4-Dimethoxybenzyl)-6,7-dimethoxyisoquinoline–fumaric acid (2/1) (II). Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| N1 | 0.51693 (10) | 0.60177 (14) | 0.44622 (3) | 0.0210 (2) | |
| C2 | 0.60617 (12) | 0.51334 (17) | 0.42229 (3) | 0.0187 (2) | |
| C3 | 0.74048 (11) | 0.60359 (17) | 0.41307 (3) | 0.0182 (2) | |
| C4 | 0.83390 (12) | 0.51426 (17) | 0.38613 (3) | 0.0197 (2) | |
| H4 | 0.80587 (12) | 0.39140 (17) | 0.37322 (3) | 0.0237 (3)* | |
| C5 | 0.96396 (12) | 0.60367 (18) | 0.37863 (3) | 0.0218 (2) | |
| C6 | 1.00943 (13) | 0.78635 (18) | 0.39912 (3) | 0.0238 (3) | |
| C7 | 0.92008 (13) | 0.87524 (17) | 0.42489 (3) | 0.0229 (3) | |
| H7 | 0.94998 (13) | 0.99708 (17) | 0.43796 (3) | 0.0274 (3)* | |
| C8 | 0.78282 (12) | 0.78679 (17) | 0.43229 (3) | 0.0200 (2) | |
| C9 | 0.68699 (13) | 0.87280 (17) | 0.45879 (3) | 0.0227 (2) | |
| H9 | 0.71313 (13) | 0.99387 (17) | 0.47268 (3) | 0.0272 (3)* | |
| C10 | 0.55693 (13) | 0.77991 (18) | 0.46414 (3) | 0.0233 (3) | |
| H10 | 0.49118 (13) | 0.84156 (18) | 0.48104 (3) | 0.0279 (3)* | |
| C11 | 0.56339 (12) | 0.30741 (17) | 0.40725 (3) | 0.0206 (2) | |
| H11a | 0.47821 (12) | 0.26165 (17) | 0.42142 (3) | 0.0247 (3)* | |
| H11b | 0.64743 (12) | 0.21623 (17) | 0.41512 (3) | 0.0247 (3)* | |
| C12 | 0.52185 (11) | 0.29044 (16) | 0.36140 (3) | 0.0192 (2) | |
| C13 | 0.58166 (12) | 0.13608 (17) | 0.33954 (3) | 0.0205 (2) | |
| H13 | 0.64718 (12) | 0.04275 (17) | 0.35364 (3) | 0.0246 (3)* | |
| C14 | 0.54649 (12) | 0.11766 (17) | 0.29756 (3) | 0.0208 (2) | |
| C15 | 0.44833 (11) | 0.25584 (17) | 0.27672 (3) | 0.0200 (2) | |
| C16 | 0.38820 (12) | 0.40628 (17) | 0.29850 (4) | 0.0223 (2) | |
| H16 | 0.32136 (12) | 0.49876 (17) | 0.28463 (4) | 0.0268 (3)* | |
| C17 | 0.42478 (12) | 0.42374 (17) | 0.34077 (4) | 0.0219 (2) | |
| H17 | 0.38266 (12) | 0.52785 (17) | 0.35539 (4) | 0.0262 (3)* | |
| O18 | 0.60006 (10) | −0.02676 (13) | 0.27362 (3) | 0.0286 (2) | |
| C19 | 0.70127 (16) | −0.1679 (2) | 0.29353 (4) | 0.0363 (3) | |
| H19a | 0.7335 (9) | −0.2609 (10) | 0.27322 (6) | 0.0545 (5)* | |
| H19b | 0.7878 (6) | −0.0977 (3) | 0.3070 (3) | 0.0545 (5)* | |
| H19c | 0.6519 (4) | −0.2418 (11) | 0.3140 (2) | 0.0545 (5)* | |
| O20 | 0.41893 (9) | 0.22626 (12) | 0.23544 (2) | 0.0241 (2) | |
| C21 | 0.32565 (14) | 0.37026 (19) | 0.21353 (4) | 0.0291 (3) | |
| H21a | 0.3150 (9) | 0.3372 (8) | 0.18431 (5) | 0.0436 (4)* | |
| H21b | 0.2278 (4) | 0.3696 (10) | 0.2238 (2) | 0.0436 (4)* | |
| H21c | 0.3702 (6) | 0.5028 (3) | 0.2175 (2) | 0.0436 (4)* | |
| O22 | 1.05851 (9) | 0.53419 (14) | 0.35250 (3) | 0.0282 (2) | |
| C23 | 1.00672 (13) | 0.37103 (19) | 0.32693 (4) | 0.0289 (3) | |
| H23a | 1.0801 (5) | 0.3403 (9) | 0.3079 (2) | 0.0433 (4)* | |
| H23b | 0.9924 (10) | 0.2541 (4) | 0.34395 (4) | 0.0433 (4)* | |
| H23c | 0.9122 (5) | 0.4069 (5) | 0.3114 (2) | 0.0433 (4)* | |
| O24 | 1.14269 (9) | 0.85526 (14) | 0.39014 (3) | 0.0324 (2) | |
| C25 | 1.20004 (16) | 1.0296 (2) | 0.41170 (4) | 0.0373 (3) | |
| H25a | 1.2970 (6) | 1.0637 (10) | 0.4028 (3) | 0.0559 (5)* | |
| H25b | 1.1312 (6) | 1.1410 (5) | 0.4058 (3) | 0.0559 (5)* | |
| H25c | 1.2110 (12) | 1.0031 (6) | 0.44120 (5) | 0.0559 (5)* | |
| C30 | 0.18953 (12) | 0.55150 (17) | 0.48065 (3) | 0.0206 (2) | |
| O31 | 0.26149 (9) | 0.45088 (13) | 0.45496 (3) | 0.0256 (2) | |
| H31 | 0.3443 (7) | 0.5047 (14) | 0.4531 (4) | 0.0384 (3)* | |
| O32 | 0.23373 (9) | 0.70574 (13) | 0.49758 (3) | 0.0272 (2) | |
| C33 | 0.04511 (12) | 0.45913 (17) | 0.48787 (3) | 0.0213 (2) | |
| H33 | 0.01702 (12) | 0.33727 (17) | 0.47454 (3) | 0.0256 (3)* |
1-(3,4-Dimethoxybenzyl)-6,7-dimethoxyisoquinoline–fumaric acid (2/1) (II). Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| N1 | 0.0193 (4) | 0.0212 (5) | 0.0225 (5) | −0.0011 (4) | 0.0023 (4) | −0.0005 (4) |
| C2 | 0.0174 (5) | 0.0194 (6) | 0.0189 (5) | −0.0011 (4) | −0.0002 (4) | 0.0019 (4) |
| C3 | 0.0178 (5) | 0.0175 (5) | 0.0188 (5) | −0.0022 (4) | −0.0011 (4) | 0.0029 (4) |
| C4 | 0.0191 (5) | 0.0184 (5) | 0.0213 (5) | −0.0041 (4) | 0.0002 (4) | 0.0003 (4) |
| C5 | 0.0196 (5) | 0.0252 (6) | 0.0207 (5) | −0.0036 (5) | 0.0024 (4) | 0.0016 (5) |
| C6 | 0.0226 (6) | 0.0275 (6) | 0.0209 (5) | −0.0114 (5) | 0.0002 (4) | 0.0043 (5) |
| C7 | 0.0271 (6) | 0.0196 (6) | 0.0209 (5) | −0.0084 (5) | −0.0022 (4) | 0.0012 (4) |
| C8 | 0.0227 (5) | 0.0175 (6) | 0.0189 (5) | −0.0024 (4) | −0.0025 (4) | 0.0035 (4) |
| C9 | 0.0280 (6) | 0.0178 (6) | 0.0214 (5) | −0.0016 (5) | −0.0012 (4) | −0.0014 (4) |
| C10 | 0.0251 (6) | 0.0211 (6) | 0.0236 (6) | 0.0010 (5) | 0.0024 (4) | −0.0019 (5) |
| C11 | 0.0183 (5) | 0.0190 (6) | 0.0250 (6) | −0.0048 (4) | 0.0041 (4) | 0.0004 (4) |
| C12 | 0.0144 (5) | 0.0185 (5) | 0.0251 (6) | −0.0061 (4) | 0.0034 (4) | −0.0014 (4) |
| C13 | 0.0163 (5) | 0.0178 (5) | 0.0272 (6) | −0.0009 (4) | 0.0014 (4) | 0.0009 (4) |
| C14 | 0.0180 (5) | 0.0177 (5) | 0.0271 (6) | 0.0001 (4) | 0.0045 (4) | −0.0026 (4) |
| C15 | 0.0172 (5) | 0.0194 (5) | 0.0234 (5) | −0.0031 (4) | 0.0022 (4) | −0.0007 (4) |
| C16 | 0.0180 (5) | 0.0195 (6) | 0.0290 (6) | 0.0011 (4) | 0.0006 (4) | 0.0001 (5) |
| C17 | 0.0185 (5) | 0.0192 (6) | 0.0282 (6) | −0.0003 (4) | 0.0040 (4) | −0.0046 (5) |
| O18 | 0.0330 (4) | 0.0255 (4) | 0.0267 (4) | 0.0120 (4) | 0.0012 (3) | −0.0044 (3) |
| C19 | 0.0401 (7) | 0.0313 (7) | 0.0368 (7) | 0.0189 (6) | 0.0001 (6) | −0.0051 (6) |
| O20 | 0.0265 (4) | 0.0225 (4) | 0.0228 (4) | 0.0041 (3) | 0.0006 (3) | −0.0012 (3) |
| C21 | 0.0370 (7) | 0.0237 (6) | 0.0259 (6) | 0.0057 (5) | 0.0000 (5) | 0.0030 (5) |
| O22 | 0.0229 (4) | 0.0333 (5) | 0.0300 (4) | −0.0099 (4) | 0.0098 (3) | −0.0059 (4) |
| C23 | 0.0282 (6) | 0.0286 (7) | 0.0314 (6) | −0.0063 (5) | 0.0111 (5) | −0.0056 (5) |
| O24 | 0.0281 (4) | 0.0398 (5) | 0.0303 (4) | −0.0218 (4) | 0.0071 (3) | −0.0050 (4) |
| C25 | 0.0372 (7) | 0.0431 (8) | 0.0313 (6) | −0.0279 (6) | 0.0021 (5) | −0.0028 (6) |
| C30 | 0.0217 (5) | 0.0200 (6) | 0.0200 (5) | −0.0001 (4) | 0.0019 (4) | 0.0010 (4) |
| O31 | 0.0217 (4) | 0.0257 (4) | 0.0307 (4) | −0.0044 (3) | 0.0092 (3) | −0.0064 (3) |
| O32 | 0.0252 (4) | 0.0246 (5) | 0.0326 (4) | −0.0063 (3) | 0.0071 (3) | −0.0078 (4) |
| C33 | 0.0223 (5) | 0.0197 (6) | 0.0218 (5) | −0.0019 (4) | 0.0014 (4) | −0.0011 (4) |
1-(3,4-Dimethoxybenzyl)-6,7-dimethoxyisoquinoline–fumaric acid (2/1) (II). Geometric parameters (Å, º)
| N1—C2 | 1.3245 (14) | C15—C16 | 1.3817 (16) |
| N1—C10 | 1.3652 (15) | C15—O20 | 1.3688 (13) |
| C2—C3 | 1.4195 (15) | C16—H16 | 0.9500 |
| C2—C11 | 1.5053 (15) | C16—C17 | 1.3984 (16) |
| C3—C4 | 1.4187 (16) | C17—H17 | 0.9500 |
| C3—C8 | 1.4172 (16) | O18—C19 | 1.4288 (15) |
| C4—H4 | 0.9500 | C19—H19a | 0.9800 |
| C4—C5 | 1.3670 (15) | C19—H19b | 0.9800 |
| C5—C6 | 1.4386 (17) | C19—H19c | 0.9800 |
| C5—O22 | 1.3561 (14) | O20—C21 | 1.4285 (14) |
| C6—C7 | 1.3670 (17) | C21—H21a | 0.9800 |
| C6—O24 | 1.3533 (14) | C21—H21b | 0.9800 |
| C7—H7 | 0.9500 | C21—H21c | 0.9800 |
| C7—C8 | 1.4211 (16) | O22—C23 | 1.4296 (15) |
| C8—C9 | 1.4145 (16) | C23—H23a | 0.9800 |
| C9—H9 | 0.9500 | C23—H23b | 0.9800 |
| C9—C10 | 1.3602 (17) | C23—H23c | 0.9800 |
| C10—H10 | 0.9500 | O24—C25 | 1.4372 (15) |
| C11—H11a | 0.9900 | C25—H25a | 0.9800 |
| C11—H11b | 0.9900 | C25—H25b | 0.9800 |
| C11—C12 | 1.5183 (15) | C25—H25c | 0.9800 |
| C12—C13 | 1.4011 (16) | C30—O31 | 1.3064 (14) |
| C12—C17 | 1.3819 (16) | C30—O32 | 1.2230 (14) |
| C13—H13 | 0.9500 | C30—C33 | 1.4884 (16) |
| C13—C14 | 1.3880 (16) | O31—H31 | 0.8400 |
| C14—C15 | 1.4120 (16) | C33—C33i | 1.319 (2) |
| C14—O18 | 1.3684 (14) | C33—H33 | 0.9500 |
| C10—N1—C2 | 119.83 (10) | C16—C15—C14 | 119.43 (10) |
| C3—C2—N1 | 121.62 (10) | O20—C15—C14 | 115.67 (10) |
| C11—C2—N1 | 117.02 (9) | O20—C15—C16 | 124.90 (10) |
| C11—C2—C3 | 121.27 (10) | H16—C16—C15 | 119.68 (7) |
| C4—C3—C2 | 122.15 (10) | C17—C16—C15 | 120.64 (10) |
| C8—C3—C2 | 118.28 (10) | C17—C16—H16 | 119.68 (7) |
| C8—C3—C4 | 119.55 (10) | C16—C17—C12 | 120.38 (10) |
| H4—C4—C3 | 119.75 (6) | H17—C17—C12 | 119.81 (7) |
| C5—C4—C3 | 120.49 (10) | H17—C17—C16 | 119.81 (7) |
| C5—C4—H4 | 119.75 (7) | C19—O18—C14 | 117.12 (9) |
| C6—C5—C4 | 120.02 (10) | H19a—C19—O18 | 109.5 |
| O22—C5—C4 | 125.12 (11) | H19b—C19—O18 | 109.5 |
| O22—C5—C6 | 114.86 (10) | H19b—C19—H19a | 109.5 |
| C7—C6—C5 | 120.18 (10) | H19c—C19—O18 | 109.5 |
| O24—C6—C5 | 114.08 (10) | H19c—C19—H19a | 109.5 |
| O24—C6—C7 | 125.74 (11) | H19c—C19—H19b | 109.5 |
| H7—C7—C6 | 119.75 (7) | C21—O20—C15 | 116.43 (9) |
| C8—C7—C6 | 120.51 (10) | H21a—C21—O20 | 109.5 |
| C8—C7—H7 | 119.75 (7) | H21b—C21—O20 | 109.5 |
| C7—C8—C3 | 119.20 (10) | H21b—C21—H21a | 109.5 |
| C9—C8—C3 | 118.26 (10) | H21c—C21—O20 | 109.5 |
| C9—C8—C7 | 122.54 (10) | H21c—C21—H21a | 109.5 |
| H9—C9—C8 | 120.43 (6) | H21c—C21—H21b | 109.5 |
| C10—C9—C8 | 119.14 (10) | C23—O22—C5 | 116.42 (9) |
| C10—C9—H9 | 120.43 (7) | H23a—C23—O22 | 109.5 |
| C9—C10—N1 | 122.73 (11) | H23b—C23—O22 | 109.5 |
| H10—C10—N1 | 118.63 (6) | H23b—C23—H23a | 109.5 |
| H10—C10—C9 | 118.63 (7) | H23c—C23—O22 | 109.5 |
| H11a—C11—C2 | 108.51 (6) | H23c—C23—H23a | 109.5 |
| H11b—C11—C2 | 108.51 (6) | H23c—C23—H23b | 109.5 |
| H11b—C11—H11a | 107.5 | C25—O24—C6 | 117.14 (10) |
| C12—C11—C2 | 115.05 (9) | H25a—C25—O24 | 109.5 |
| C12—C11—H11a | 108.51 (5) | H25b—C25—O24 | 109.5 |
| C12—C11—H11b | 108.51 (6) | H25b—C25—H25a | 109.5 |
| C13—C12—C11 | 119.63 (10) | H25c—C25—O24 | 109.5 |
| C17—C12—C11 | 121.16 (10) | H25c—C25—H25a | 109.5 |
| C17—C12—C13 | 119.20 (10) | H25c—C25—H25b | 109.5 |
| H13—C13—C12 | 119.55 (6) | O32—C30—O31 | 124.73 (10) |
| C14—C13—C12 | 120.90 (10) | C33—C30—O31 | 113.12 (10) |
| C14—C13—H13 | 119.55 (7) | C33—C30—O32 | 122.15 (10) |
| C15—C14—C13 | 119.43 (10) | H31—O31—C30 | 109.5 |
| O18—C14—C13 | 125.11 (10) | C33i—C33—C30 | 122.17 (13) |
| O18—C14—C15 | 115.46 (10) | H33—C33—C30 | 118.91 (6) |
| N1—C2—C3—C4 | −177.32 (10) | C5—C6—C7—C8 | 1.10 (13) |
| N1—C2—C3—C8 | 4.06 (12) | C5—C6—O24—C25 | −175.75 (11) |
| N1—C2—C11—C12 | 113.81 (10) | C6—C7—C8—C9 | 179.76 (11) |
| N1—C10—C9—C8 | 2.72 (13) | C7—C8—C9—C10 | 179.60 (11) |
| C2—C3—C4—C5 | −178.66 (11) | C11—C12—C13—C14 | −179.55 (10) |
| C2—C3—C8—C7 | 177.22 (10) | C11—C12—C17—C16 | 179.76 (10) |
| C2—C3—C8—C9 | −1.70 (12) | C12—C13—C14—C15 | −0.40 (12) |
| C2—C11—C12—C13 | 133.10 (9) | C12—C13—C14—O18 | 179.50 (10) |
| C2—C11—C12—C17 | −47.48 (11) | C12—C17—C16—C15 | 0.01 (13) |
| C3—C4—C5—C6 | 2.05 (12) | C13—C14—C15—C16 | −0.41 (12) |
| C3—C4—C5—O22 | −177.78 (10) | C13—C14—C15—O20 | −179.63 (10) |
| C3—C8—C7—C6 | 0.89 (12) | C13—C14—O18—C19 | −0.69 (14) |
| C3—C8—C9—C10 | −1.52 (12) | C14—C15—C16—C17 | 0.61 (12) |
| C4—C5—C6—C7 | −2.60 (13) | C14—C15—O20—C21 | −177.02 (10) |
| C4—C5—C6—O24 | 177.69 (11) | C30—C33—C33i—C30i | 180.00 (14) |
| C4—C5—O22—C23 | 9.21 (14) |
Symmetry code: (i) −x, −y+1, −z+1.
1-(3,4-Dimethoxybenzyl)-6,7-dimethoxyisoquinoline–fumaric acid (2/1) (II). Hydrogen-bond geometry (Å, º)
| D—H···A | D—H | H···A | D···A | D—H···A |
| O31—H31···N1 | 0.84 (1) | 1.73 (1) | 2.5687 (12) | 175 (1) |
| C9—H9···O32ii | 0.95 (1) | 2.27 (1) | 3.2191 (14) | 176 (1) |
Symmetry code: (ii) −x+1, −y+2, −z+1.
1-(3,4-Dimethoxybenzyl)-6,7-dimethoxyisoquinoline–fumaric acid (2/3) (I). Crystal data
| C20H22NO4·1.5C4H4O4 | Z = 2 |
| Mr = 513.48 | F(000) = 540 |
| Triclinic, P1 | Dx = 1.405 Mg m−3 |
| a = 9.5290 (2) Å | Cu Kα radiation, λ = 1.54184 Å |
| b = 10.5445 (3) Å | Cell parameters from 6061 reflections |
| c = 12.6509 (4) Å | θ = 3.6–68.1° |
| α = 91.606 (2)° | µ = 0.92 mm−1 |
| β = 104.980 (2)° | T = 100 K |
| γ = 97.823 (2)° | Block, clear light colourless |
| V = 1213.87 (6) Å3 | 0.17 × 0.08 × 0.03 mm |
1-(3,4-Dimethoxybenzyl)-6,7-dimethoxyisoquinoline–fumaric acid (2/3) (I). Data collection
| XtaLAB Synergy, Single source at home/near, HyPix3000 diffractometer | 4366 independent reflections |
| Radiation source: micro-focus sealed X-ray tube, PhotonJet (Cu) X-ray Source | 3457 reflections with I > 2σ(I) |
| Mirror monochromator | Rint = 0.026 |
| Detector resolution: 10.0000 pixels mm-1 | θmax = 68.0°, θmin = 3.6° |
| ω scans | h = −11→5 |
| Absorption correction: multi-scan (CrysAlisPro; Rigaku OD, 2022) | k = −12→12 |
| Tmin = 0.862, Tmax = 1.000 | l = −15→15 |
| 10089 measured reflections |
1-(3,4-Dimethoxybenzyl)-6,7-dimethoxyisoquinoline–fumaric acid (2/3) (I). Refinement
| Refinement on F2 | Hydrogen site location: mixed |
| Least-squares matrix: full | H atoms treated by a mixture of independent and constrained refinement |
| R[F2 > 2σ(F2)] = 0.047 | w = 1/[σ2(Fo2) + (0.0609P)2 + 0.410P] where P = (Fo2 + 2Fc2)/3 |
| wR(F2) = 0.133 | (Δ/σ)max < 0.001 |
| S = 1.06 | Δρmax = 0.38 e Å−3 |
| 4366 reflections | Δρmin = −0.39 e Å−3 |
| 382 parameters | Extinction correction: SHELXL2019/2 (Sheldrick, 2015b), Fc*=kFc[1+0.001xFc2λ3/sin(2θ)]-1/4 |
| 282 restraints | Extinction coefficient: 0.0009 (3) |
| Primary atom site location: structure-invariant direct methods |
1-(3,4-Dimethoxybenzyl)-6,7-dimethoxyisoquinoline–fumaric acid (2/3) (I). Special details
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
1-(3,4-Dimethoxybenzyl)-6,7-dimethoxyisoquinoline–fumaric acid (2/3) (I). Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | Occ. (<1) | |
| N1 | 0.46197 (17) | 0.81758 (17) | 0.51609 (18) | 0.0444 (5) | |
| H1 | 0.543685 | 0.850236 | 0.500466 | 0.053* | |
| C2 | 0.3393 (2) | 0.79572 (18) | 0.43509 (19) | 0.0359 (5) | |
| C3 | 0.20717 (19) | 0.74256 (17) | 0.45885 (17) | 0.0303 (4) | |
| C4 | 0.07358 (18) | 0.71542 (17) | 0.37492 (16) | 0.0286 (4) | |
| H4 | 0.073022 | 0.730035 | 0.301067 | 0.034* | |
| C5 | −0.05396 (18) | 0.66853 (17) | 0.39956 (16) | 0.0289 (4) | |
| C6 | −0.05444 (19) | 0.64893 (17) | 0.51115 (17) | 0.0301 (4) | |
| C7 | 0.0738 (2) | 0.67300 (17) | 0.59334 (17) | 0.0326 (4) | |
| H7 | 0.072747 | 0.659166 | 0.667034 | 0.039* | |
| C8 | 0.2077 (2) | 0.71842 (17) | 0.56852 (17) | 0.0320 (4) | |
| C9 | 0.3442 (2) | 0.7438 (2) | 0.6496 (2) | 0.0430 (5) | |
| H9 | 0.349081 | 0.726778 | 0.723680 | 0.052* | |
| C10 | 0.4681 (2) | 0.7924 (2) | 0.6211 (2) | 0.0492 (6) | |
| H10 | 0.559426 | 0.808756 | 0.675412 | 0.059* | |
| C11 | 0.3489 (2) | 0.83103 (18) | 0.3232 (2) | 0.0404 (5) | |
| H11A | 0.440287 | 0.891577 | 0.330194 | 0.049* | |
| H11B | 0.264971 | 0.875706 | 0.289528 | 0.049* | |
| C12 | 0.3477 (2) | 0.71580 (18) | 0.24786 (19) | 0.0356 (5) | |
| C13 | 0.2463 (2) | 0.69676 (18) | 0.14486 (19) | 0.0359 (5) | |
| H13 | 0.176960 | 0.754304 | 0.123955 | 0.043* | |
| C14 | 0.2456 (2) | 0.59499 (19) | 0.07283 (18) | 0.0352 (4) | |
| C15 | 0.3481 (2) | 0.50903 (18) | 0.10481 (17) | 0.0338 (4) | |
| C16 | 0.4475 (2) | 0.52812 (18) | 0.20657 (17) | 0.0342 (4) | |
| H16 | 0.515980 | 0.470031 | 0.228377 | 0.041* | |
| C17 | 0.4487 (2) | 0.63203 (18) | 0.27815 (18) | 0.0343 (4) | |
| H17 | 0.518893 | 0.645061 | 0.347593 | 0.041* | |
| O18 | 0.15271 (16) | 0.56999 (14) | −0.02976 (13) | 0.0416 (4) | |
| C19 | 0.0454 (2) | 0.6537 (2) | −0.0642 (2) | 0.0451 (5) | |
| H19A | −0.018009 | 0.622919 | −0.137013 | 0.068* | |
| H19B | 0.095157 | 0.740628 | −0.067495 | 0.068* | |
| H19C | −0.014298 | 0.654835 | −0.011816 | 0.068* | |
| O20 | 0.33975 (16) | 0.41301 (14) | 0.02746 (12) | 0.0415 (4) | |
| C21 | 0.4403 (3) | 0.3231 (2) | 0.05620 (19) | 0.0458 (5) | |
| H21A | 0.429616 | 0.263692 | −0.007301 | 0.069* | |
| H21B | 0.419471 | 0.274827 | 0.116846 | 0.069* | |
| H21C | 0.540978 | 0.369063 | 0.078816 | 0.069* | |
| O22 | −0.18676 (13) | 0.63998 (14) | 0.32630 (12) | 0.0370 (3) | |
| C23 | −0.1929 (2) | 0.6669 (3) | 0.21502 (19) | 0.0514 (6) | |
| H23A | −0.295060 | 0.649292 | 0.170510 | 0.077* | |
| H23B | −0.133712 | 0.612563 | 0.186378 | 0.077* | |
| H23C | −0.154234 | 0.757365 | 0.212015 | 0.077* | |
| O24 | −0.18929 (14) | 0.60799 (13) | 0.52392 (12) | 0.0375 (3) | |
| C25 | −0.2027 (3) | 0.6004 (2) | 0.63391 (19) | 0.0451 (5) | |
| H25A | −0.305854 | 0.573898 | 0.632334 | 0.068* | |
| H25B | −0.167756 | 0.684678 | 0.673616 | 0.068* | |
| H25C | −0.143486 | 0.537523 | 0.670996 | 0.068* | |
| C34 | 0.6558 (2) | 1.03159 (19) | 0.13490 (18) | 0.0369 (5) | |
| O35 | 0.60430 (18) | 0.93880 (15) | 0.18930 (13) | 0.0484 (4) | |
| H35 | 0.654420 | 0.946279 | 0.254974 | 0.073* | |
| O36 | 0.76487 (17) | 1.10891 (15) | 0.17335 (14) | 0.0477 (4) | |
| C37 | 0.5660 (2) | 1.03429 (18) | 0.02071 (18) | 0.0368 (5) | |
| H37 | 0.605453 | 1.088822 | −0.026367 | 0.044* | |
| C38 | 0.9160 (2) | 0.9597 (2) | 0.8450 (2) | 0.0504 (6) | |
| O39A | 0.9425 (5) | 1.0085 (3) | 0.7594 (3) | 0.0410 (9) | 0.598 (9) |
| H39A | 0.883696 | 0.969476 | 0.703076 | 0.062* | 0.598 (9) |
| O39B | 0.9637 (9) | 1.0502 (9) | 0.7980 (9) | 0.091 (3) | 0.402 (9) |
| O40A | 0.8223 (6) | 0.8669 (5) | 0.8449 (3) | 0.0554 (11) | 0.598 (9) |
| O40B | 0.8172 (9) | 0.8612 (7) | 0.8033 (9) | 0.095 (3) | 0.402 (9) |
| H40B | 0.804 (10) | 0.868 (7) | 0.7354 (12) | 0.142* | 0.402 (9) |
| C41A | 1.0105 (5) | 1.0188 (5) | 0.9512 (4) | 0.0314 (11) | 0.598 (9) |
| H41A | 1.088580 | 1.085283 | 0.951450 | 0.038* | 0.598 (9) |
| C41B | 0.9522 (9) | 0.9608 (9) | 0.9704 (8) | 0.047 (2) | 0.402 (9) |
| H41B | 0.898144 | 0.898230 | 1.002913 | 0.057* | 0.402 (9) |
| C30 | 0.7944 (2) | 0.94153 (17) | 0.48749 (18) | 0.0334 (4) | |
| O31 | 0.69127 (18) | 0.92626 (15) | 0.40213 (14) | 0.0491 (4) | |
| O32 | 0.77542 (17) | 0.91662 (14) | 0.57896 (13) | 0.0448 (4) | |
| C33A | 0.9361 (4) | 0.9954 (2) | 0.4628 (4) | 0.0278 (11) | 0.742 (11) |
| H33A | 0.933667 | 1.023721 | 0.391822 | 0.033* | 0.742 (11) |
| C33B | 0.9632 (11) | 0.9786 (7) | 0.5352 (10) | 0.030 (3) | 0.258 (11) |
| H33B | 1.010435 | 0.971108 | 0.610235 | 0.036* | 0.258 (11) |
1-(3,4-Dimethoxybenzyl)-6,7-dimethoxyisoquinoline–fumaric acid (2/3) (I). Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| N1 | 0.0222 (8) | 0.0367 (9) | 0.0710 (14) | 0.0039 (6) | 0.0085 (8) | −0.0155 (9) |
| C2 | 0.0237 (9) | 0.0261 (9) | 0.0583 (13) | 0.0035 (7) | 0.0131 (9) | −0.0100 (8) |
| C3 | 0.0228 (8) | 0.0239 (8) | 0.0446 (12) | 0.0056 (6) | 0.0094 (8) | −0.0053 (8) |
| C4 | 0.0246 (8) | 0.0280 (9) | 0.0347 (10) | 0.0044 (7) | 0.0105 (8) | −0.0023 (7) |
| C5 | 0.0223 (8) | 0.0281 (9) | 0.0354 (10) | 0.0033 (7) | 0.0072 (7) | −0.0049 (7) |
| C6 | 0.0272 (9) | 0.0252 (9) | 0.0406 (11) | 0.0032 (7) | 0.0145 (8) | −0.0021 (8) |
| C7 | 0.0372 (10) | 0.0276 (9) | 0.0349 (11) | 0.0093 (7) | 0.0107 (8) | 0.0002 (8) |
| C8 | 0.0286 (9) | 0.0263 (9) | 0.0402 (11) | 0.0097 (7) | 0.0052 (8) | −0.0047 (8) |
| C9 | 0.0397 (11) | 0.0355 (11) | 0.0476 (13) | 0.0164 (8) | −0.0039 (9) | −0.0101 (9) |
| C10 | 0.0257 (10) | 0.0407 (12) | 0.0709 (17) | 0.0117 (8) | −0.0066 (10) | −0.0190 (11) |
| C11 | 0.0317 (10) | 0.0281 (9) | 0.0681 (15) | −0.0007 (7) | 0.0282 (10) | −0.0027 (9) |
| C12 | 0.0284 (9) | 0.0271 (9) | 0.0590 (14) | 0.0022 (7) | 0.0260 (9) | 0.0019 (9) |
| C13 | 0.0296 (9) | 0.0301 (9) | 0.0567 (13) | 0.0094 (7) | 0.0236 (9) | 0.0100 (9) |
| C14 | 0.0332 (10) | 0.0347 (10) | 0.0450 (12) | 0.0082 (8) | 0.0211 (9) | 0.0106 (8) |
| C15 | 0.0362 (10) | 0.0319 (9) | 0.0418 (12) | 0.0106 (8) | 0.0218 (9) | 0.0071 (8) |
| C16 | 0.0334 (9) | 0.0323 (10) | 0.0445 (12) | 0.0113 (7) | 0.0199 (9) | 0.0060 (8) |
| C17 | 0.0270 (9) | 0.0332 (10) | 0.0475 (12) | 0.0029 (7) | 0.0197 (8) | 0.0002 (8) |
| O18 | 0.0433 (8) | 0.0432 (8) | 0.0438 (9) | 0.0172 (6) | 0.0150 (7) | 0.0102 (7) |
| C19 | 0.0401 (11) | 0.0467 (12) | 0.0550 (14) | 0.0166 (9) | 0.0175 (10) | 0.0168 (11) |
| O20 | 0.0536 (8) | 0.0386 (8) | 0.0389 (8) | 0.0213 (7) | 0.0164 (7) | 0.0036 (6) |
| C21 | 0.0624 (14) | 0.0410 (12) | 0.0420 (13) | 0.0261 (10) | 0.0182 (11) | 0.0036 (10) |
| O22 | 0.0212 (6) | 0.0521 (8) | 0.0353 (8) | 0.0007 (5) | 0.0062 (5) | −0.0040 (6) |
| C23 | 0.0283 (10) | 0.0872 (18) | 0.0347 (12) | 0.0023 (10) | 0.0051 (9) | 0.0011 (12) |
| O24 | 0.0320 (7) | 0.0389 (7) | 0.0457 (9) | −0.0017 (5) | 0.0213 (6) | −0.0004 (6) |
| C25 | 0.0530 (13) | 0.0430 (12) | 0.0507 (14) | 0.0094 (10) | 0.0322 (11) | 0.0067 (10) |
| C34 | 0.0422 (11) | 0.0301 (10) | 0.0452 (12) | 0.0068 (8) | 0.0227 (9) | 0.0024 (8) |
| O35 | 0.0550 (9) | 0.0476 (9) | 0.0401 (9) | −0.0056 (7) | 0.0147 (7) | 0.0075 (7) |
| O36 | 0.0445 (8) | 0.0464 (9) | 0.0528 (10) | −0.0001 (7) | 0.0169 (7) | 0.0068 (7) |
| C37 | 0.0462 (11) | 0.0294 (9) | 0.0420 (12) | 0.0073 (8) | 0.0237 (9) | 0.0034 (8) |
| C38 | 0.0472 (13) | 0.0539 (14) | 0.0506 (15) | 0.0289 (11) | 0.0036 (11) | −0.0042 (11) |
| O39A | 0.0466 (17) | 0.0416 (16) | 0.0310 (18) | 0.0077 (12) | 0.0045 (15) | −0.0136 (12) |
| O39B | 0.069 (4) | 0.150 (6) | 0.068 (6) | 0.054 (5) | 0.020 (4) | 0.054 (5) |
| O40A | 0.079 (3) | 0.075 (3) | 0.0154 (18) | 0.0210 (18) | 0.0145 (19) | 0.0043 (17) |
| O40B | 0.086 (4) | 0.082 (4) | 0.082 (6) | 0.042 (3) | −0.048 (4) | −0.058 (4) |
| C41A | 0.034 (2) | 0.035 (2) | 0.031 (3) | 0.0118 (16) | 0.0154 (18) | 0.0083 (17) |
| C41B | 0.043 (4) | 0.050 (4) | 0.048 (5) | 0.021 (3) | 0.002 (3) | 0.005 (3) |
| C30 | 0.0262 (9) | 0.0246 (9) | 0.0509 (13) | 0.0034 (7) | 0.0140 (9) | −0.0030 (8) |
| O31 | 0.0561 (9) | 0.0458 (9) | 0.0434 (9) | 0.0058 (7) | 0.0104 (8) | 0.0035 (7) |
| O32 | 0.0540 (9) | 0.0415 (8) | 0.0426 (9) | 0.0160 (7) | 0.0147 (7) | 0.0036 (7) |
| C33A | 0.0264 (19) | 0.0261 (13) | 0.032 (2) | 0.0037 (10) | 0.0103 (15) | 0.0011 (11) |
| C33B | 0.033 (5) | 0.026 (4) | 0.030 (7) | 0.006 (3) | 0.003 (3) | 0.001 (3) |
1-(3,4-Dimethoxybenzyl)-6,7-dimethoxyisoquinoline–fumaric acid (2/3) (I). Geometric parameters (Å, º)
| N1—H1 | 0.8800 | C21—H21A | 0.9800 |
| N1—C2 | 1.328 (3) | C21—H21B | 0.9800 |
| N1—C10 | 1.350 (3) | C21—H21C | 0.9800 |
| C2—C3 | 1.415 (3) | O22—C23 | 1.432 (3) |
| C2—C11 | 1.496 (3) | C23—H23A | 0.9800 |
| C3—C4 | 1.419 (3) | C23—H23B | 0.9800 |
| C3—C8 | 1.416 (3) | C23—H23C | 0.9800 |
| C4—H4 | 0.9500 | O24—C25 | 1.433 (3) |
| C4—C5 | 1.364 (3) | C25—H25A | 0.9800 |
| C5—C6 | 1.434 (3) | C25—H25B | 0.9800 |
| C5—O22 | 1.351 (2) | C25—H25C | 0.9800 |
| C6—C7 | 1.372 (3) | C34—O35 | 1.324 (2) |
| C6—O24 | 1.351 (2) | C34—O36 | 1.211 (3) |
| C7—H7 | 0.9500 | C34—C37 | 1.480 (3) |
| C7—C8 | 1.416 (3) | O35—H35 | 0.8400 |
| C8—C9 | 1.420 (3) | C37—C37i | 1.331 (4) |
| C9—H9 | 0.9500 | C37—H37 | 0.9500 |
| C9—C10 | 1.361 (4) | C38—O39A | 1.280 (4) |
| C10—H10 | 0.9500 | C38—O39B | 1.235 (5) |
| C11—H11A | 0.9900 | C38—O40A | 1.231 (4) |
| C11—H11B | 0.9900 | C38—O40B | 1.300 (5) |
| C11—C12 | 1.520 (3) | C38—C41A | 1.476 (6) |
| C12—C13 | 1.398 (3) | C38—C41B | 1.533 (10) |
| C12—C17 | 1.383 (3) | O39A—H39A | 0.8400 |
| C13—H13 | 0.9500 | O40B—H40B | 0.843 (5) |
| C13—C14 | 1.386 (3) | C41A—C41Aii | 1.362 (9) |
| C14—C15 | 1.413 (3) | C41A—H41A | 0.9500 |
| C14—O18 | 1.364 (3) | C41B—C41Bii | 1.218 (17) |
| C15—C16 | 1.378 (3) | C41B—H41B | 0.9500 |
| C15—O20 | 1.368 (2) | C30—O31 | 1.248 (3) |
| C16—H16 | 0.9500 | C30—O32 | 1.246 (3) |
| C16—C17 | 1.399 (3) | C30—C33A | 1.504 (3) |
| C17—H17 | 0.9500 | C30—C33B | 1.555 (10) |
| O18—C19 | 1.432 (2) | C33A—C33Aiii | 1.322 (8) |
| C19—H19A | 0.9800 | C33A—H33A | 0.9500 |
| C19—H19B | 0.9800 | C33B—C33Biii | 1.32 (2) |
| C19—H19C | 0.9800 | C33B—H33B | 0.9500 |
| O20—C21 | 1.427 (2) | ||
| C2—N1—H1 | 118.2 | O18—C19—H19C | 109.5 |
| C2—N1—C10 | 123.56 (18) | H19A—C19—H19B | 109.5 |
| C10—N1—H1 | 118.2 | H19A—C19—H19C | 109.5 |
| N1—C2—C3 | 118.9 (2) | H19B—C19—H19C | 109.5 |
| N1—C2—C11 | 117.53 (17) | C15—O20—C21 | 116.92 (17) |
| C3—C2—C11 | 123.60 (18) | O20—C21—H21A | 109.5 |
| C2—C3—C4 | 121.08 (19) | O20—C21—H21B | 109.5 |
| C2—C3—C8 | 119.45 (18) | O20—C21—H21C | 109.5 |
| C8—C3—C4 | 119.47 (16) | H21A—C21—H21B | 109.5 |
| C3—C4—H4 | 119.8 | H21A—C21—H21C | 109.5 |
| C5—C4—C3 | 120.47 (18) | H21B—C21—H21C | 109.5 |
| C5—C4—H4 | 119.8 | C5—O22—C23 | 116.43 (14) |
| C4—C5—C6 | 120.03 (17) | O22—C23—H23A | 109.5 |
| O22—C5—C4 | 125.26 (18) | O22—C23—H23B | 109.5 |
| O22—C5—C6 | 114.68 (15) | O22—C23—H23C | 109.5 |
| C7—C6—C5 | 120.41 (16) | H23A—C23—H23B | 109.5 |
| O24—C6—C5 | 113.56 (16) | H23A—C23—H23C | 109.5 |
| O24—C6—C7 | 126.02 (18) | H23B—C23—H23C | 109.5 |
| C6—C7—H7 | 119.9 | C6—O24—C25 | 117.25 (16) |
| C6—C7—C8 | 120.11 (19) | O24—C25—H25A | 109.5 |
| C8—C7—H7 | 119.9 | O24—C25—H25B | 109.5 |
| C3—C8—C9 | 117.70 (18) | O24—C25—H25C | 109.5 |
| C7—C8—C3 | 119.43 (17) | H25A—C25—H25B | 109.5 |
| C7—C8—C9 | 122.9 (2) | H25A—C25—H25C | 109.5 |
| C8—C9—H9 | 120.0 | H25B—C25—H25C | 109.5 |
| C10—C9—C8 | 120.0 (2) | O35—C34—C37 | 113.63 (18) |
| C10—C9—H9 | 120.0 | O36—C34—O35 | 124.4 (2) |
| N1—C10—C9 | 120.4 (2) | O36—C34—C37 | 121.98 (19) |
| N1—C10—H10 | 119.8 | C34—O35—H35 | 109.5 |
| C9—C10—H10 | 119.8 | C34—C37—H37 | 117.7 |
| C2—C11—H11A | 109.0 | C37i—C37—C34 | 124.6 (2) |
| C2—C11—H11B | 109.0 | C37i—C37—H37 | 117.7 |
| C2—C11—C12 | 113.08 (16) | O39A—C38—C41A | 116.0 (3) |
| H11A—C11—H11B | 107.8 | O39B—C38—O40B | 128.8 (6) |
| C12—C11—H11A | 109.0 | O39B—C38—C41B | 121.7 (6) |
| C12—C11—H11B | 109.0 | O40A—C38—O39A | 125.4 (3) |
| C13—C12—C11 | 119.44 (18) | O40A—C38—C41A | 118.6 (3) |
| C17—C12—C11 | 121.0 (2) | O40B—C38—C41B | 108.9 (6) |
| C17—C12—C13 | 119.51 (19) | C38—O39A—H39A | 109.5 |
| C12—C13—H13 | 119.6 | C38—O40B—H40B | 102 (4) |
| C14—C13—C12 | 120.86 (18) | C38—C41A—H41A | 118.9 |
| C14—C13—H13 | 119.6 | C41Aii—C41A—C38 | 122.3 (7) |
| C13—C14—C15 | 119.3 (2) | C41Aii—C41A—H41A | 118.9 |
| O18—C14—C13 | 125.05 (18) | C38—C41B—H41B | 118.9 |
| O18—C14—C15 | 115.65 (18) | C41Bii—C41B—C38 | 122.2 (15) |
| C16—C15—C14 | 119.54 (19) | C41Bii—C41B—H41B | 118.9 |
| O20—C15—C14 | 114.80 (19) | O31—C30—C33A | 110.7 (2) |
| O20—C15—C16 | 125.64 (17) | O31—C30—C33B | 145.1 (5) |
| C15—C16—H16 | 119.6 | O32—C30—O31 | 122.17 (17) |
| C15—C16—C17 | 120.76 (18) | O32—C30—C33A | 127.1 (2) |
| C17—C16—H16 | 119.6 | O32—C30—C33B | 92.6 (5) |
| C12—C17—C16 | 120.0 (2) | C30—C33A—H33A | 119.1 |
| C12—C17—H17 | 120.0 | C33Aiii—C33A—C30 | 121.9 (4) |
| C16—C17—H17 | 120.0 | C33Aiii—C33A—H33A | 119.1 |
| C14—O18—C19 | 117.23 (17) | C30—C33B—H33B | 122.1 |
| O18—C19—H19A | 109.5 | C33Biii—C33B—C30 | 115.7 (13) |
| O18—C19—H19B | 109.5 | C33Biii—C33B—H33B | 122.1 |
| N1—C2—C3—C4 | −178.96 (16) | C11—C12—C13—C14 | 177.90 (16) |
| N1—C2—C3—C8 | 2.3 (3) | C11—C12—C17—C16 | −178.71 (16) |
| N1—C2—C11—C12 | 103.3 (2) | C12—C13—C14—C15 | 0.6 (3) |
| C2—N1—C10—C9 | −1.0 (3) | C12—C13—C14—O18 | −178.84 (17) |
| C2—C3—C4—C5 | −177.63 (17) | C13—C12—C17—C16 | −0.9 (3) |
| C2—C3—C8—C7 | 175.96 (16) | C13—C14—C15—C16 | −0.4 (3) |
| C2—C3—C8—C9 | −2.8 (3) | C13—C14—C15—O20 | −179.14 (16) |
| C2—C11—C12—C13 | 127.20 (19) | C13—C14—O18—C19 | −1.7 (3) |
| C2—C11—C12—C17 | −55.0 (2) | C14—C15—C16—C17 | −0.5 (3) |
| C3—C2—C11—C12 | −77.7 (2) | C14—C15—O20—C21 | −179.53 (17) |
| C3—C4—C5—C6 | 1.4 (3) | C15—C14—O18—C19 | 178.86 (16) |
| C3—C4—C5—O22 | 179.76 (16) | C15—C16—C17—C12 | 1.1 (3) |
| C3—C8—C9—C10 | 1.5 (3) | C16—C15—O20—C21 | 1.8 (3) |
| C4—C3—C8—C7 | −2.8 (3) | C17—C12—C13—C14 | 0.1 (3) |
| C4—C3—C8—C9 | 178.42 (16) | O18—C14—C15—C16 | 179.10 (16) |
| C4—C5—C6—C7 | −2.2 (3) | O18—C14—C15—O20 | 0.3 (2) |
| C4—C5—C6—O24 | 177.01 (16) | O20—C15—C16—C17 | 178.15 (16) |
| C4—C5—O22—C23 | −2.8 (3) | O22—C5—C6—C7 | 179.25 (16) |
| C5—C6—C7—C8 | 0.5 (3) | O22—C5—C6—O24 | −1.5 (2) |
| C5—C6—O24—C25 | −172.83 (16) | O24—C6—C7—C8 | −178.63 (17) |
| C6—C5—O22—C23 | 175.66 (18) | O35—C34—C37—C37i | 10.4 (3) |
| C6—C7—C8—C3 | 2.0 (3) | O36—C34—C37—C37i | −168.9 (2) |
| C6—C7—C8—C9 | −179.28 (17) | O39A—C38—C41A—C41Aii | 176.4 (4) |
| C7—C6—O24—C25 | 6.3 (3) | O39B—C38—C41B—C41Bii | −12.6 (10) |
| C7—C8—C9—C10 | −177.25 (18) | O40A—C38—C41A—C41Aii | −5.6 (6) |
| C8—C3—C4—C5 | 1.1 (3) | O40B—C38—C41B—C41Bii | 175.7 (8) |
| C8—C9—C10—N1 | 0.4 (3) | O31—C30—C33A—C33Aiii | −171.1 (3) |
| C10—N1—C2—C3 | −0.3 (3) | O31—C30—C33B—C33Biii | 7.3 (12) |
| C10—N1—C2—C11 | 178.75 (18) | O32—C30—C33A—C33Aiii | 10.5 (4) |
| C11—C2—C3—C4 | 2.0 (3) | O32—C30—C33B—C33Biii | −176.6 (8) |
| C11—C2—C3—C8 | −176.73 (16) |
Symmetry codes: (i) −x+1, −y+2, −z; (ii) −x+2, −y+2, −z+2; (iii) −x+2, −y+2, −z+1.
1-(3,4-Dimethoxybenzyl)-6,7-dimethoxyisoquinoline–fumaric acid (2/3) (I). Hydrogen-bond geometry (Å, º)
| D—H···A | D—H | H···A | D···A | D—H···A |
| N1—H1···O31 | 0.88 | 2.20 | 3.034 (3) | 159 |
| N1—H1···O32 | 0.88 | 2.18 | 2.919 (2) | 141 |
| O35—H35···O31 | 0.84 | 1.83 | 2.617 (2) | 156 |
| O39A—H39A···O32 | 0.84 | 1.67 | 2.502 (4) | 169 |
Selected geometric parameters (Å) for fumaric acid A, B, and C in compound (I)
| C30–O31 | 1.248 (2) |
| C30–O32 | 1.246 (3) |
| C34–O35 | 1.324 (3) |
| C34–O36 | 1.211 (2) |
| C38–O39Aa | 1.280 (5) |
| C38–O40Aa | 1.231 (6) |
References
- Baggio, R. F. & Baggio, S. (1973). Cryst. Struct. Commun.2, 251–253.
- Bharate, S. S. (2021). Pharm. Res.38, 1307–1326. [DOI] [PubMed]
- Bourhis, L. J., Dolomanov, O. V., Gildea, R. J., Howard, J. A. K. & Puschmann, H. (2015). Acta Cryst. A71, 59–75. [DOI] [PMC free article] [PubMed]
- Chen, J.-M., Wang, Z.-Z., Wu, C.-B., Li, S. & Lu, T.-B. (2012). CrystEngComm, 14, 6221–6229.
- Childs, S. L., Stahly, P. & Park, A. (2007). Mol. Pharm.4, 3, 323–338. [DOI] [PubMed]
- Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K. & Puschmann, H. (2009). J. Appl. Cryst.42, 339–341.
- Groom, C. R., Bruno, I. J., Lightfoot, M. P. & Ward, S. C. (2016). Acta Cryst. B72, 171–179. [DOI] [PMC free article] [PubMed]
- Kang, D., Qiang, G., Du, L. & Du, G. (2018). Papaverine. In Natural Small Molecule Drugs from Plants. Springer, Singapore.
- Kawajiri, T., Kijima, A., Iimuro, A., Ohashi, E., Yamakawa, K., Agura, K., Masuda, K., Kouki, K., Kasamatsu, K., Yanagisawa, S., Nakashima, S., Shibahara, S., Toyota, T., Higuchi, T., Suto, T., Oohara, T., Maki, T., Sahara, N., Fukui, N., Wakamori, H., Ikemoto, H., Murakami, H., Ando, H., Hosoya, M., Sato, M., Suzuki, Y., Nakagawa, Y., Unoh, Y., Hirano, Y., Nagasawa, Y., Goda, S., Ohara, T. & Tsuritani, T. (2023). ACS Cent. Sci. 9, 836–843 [DOI] [PMC free article] [PubMed]
- Kotte, L., Pendota, V., Sreedhar, B. & Nanubolu, J. B. (2023). CrystEngComm, 25, 2662–2678.
- Marek, J., Dostal, J. & Slavik, J. (1997). Z. Kristallogr. Cryst. Mater.211, 649–650.
- Reynolds, C. D., Palmer, R. A. & Gorinsky, B. (1974). J. Cryst. Mol. Struct.4, 213–225.
- Rigaku OD (2022). CrysAlis PRO. Rigaku Oxford Diffraction, Yarnton, England.
- Sheldrick, G. M. (2015a). Acta Cryst. A71, 3–8.
- Sheldrick, G. M. (2015b). Acta Cryst. C71, 3–8.
- Steiner, T. (1997). Chem. Commun. pp. 727–734.
- Stevens, J. S., Coultas, S., Jaye, C., Fischer, D. A. & Schroeder, S. L. M. (2020). Phys. Chem. Chem. Phys.22, 4916–4923. [DOI] [PubMed]
- Thipparaboina, R., Kumar, D., Mittapalli, S., Balasubramanian, S., Nangia, A. & Shastri, N. R. (2015). Cryst. Growth Des.15, 12, 5816–5826.
- Tothadi, S., Shaikh, T. R., Gupta, S., Dandela, R., Vinod, C. P. & Nangia, A. K. (2021). Cryst. Growth Des.21, 2, 735–747.
- Van Hulle, A., Amelinckx, S. & Dekeyser, W. (1953). Acta Cryst.6, 664–665.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) II, I. DOI: 10.1107/S2056989024009794/ox2007sup1.cif
Structure factors: contains datablock(s) II. DOI: 10.1107/S2056989024009794/ox2007IIsup3.hkl
Structure factors: contains datablock(s) I. DOI: 10.1107/S2056989024009794/ox2007Isup2.hkl
Supporting information file. DOI: 10.1107/S2056989024009794/ox2007Isup4.mol
Supporting information file. DOI: 10.1107/S2056989024009794/ox2007IIsup5.mol
Supporting information file. DOI: 10.1107/S2056989024009794/ox2007Isup6.cml
Supporting information file. DOI: 10.1107/S2056989024009794/ox2007IIsup7.cml
Additional supporting information: crystallographic information; 3D view; checkCIF report