Abstract
Background
Alzheimer’s disease neuropathologic change (ADNC) and Lewy pathology (LP) often coexist in cognitively impaired individuals. These pathologies’ relative distribution and severity may modify these individuals’ clinical presentation, cognitive profile, and prognosis. Therefore, we examined the contributions of LP and concomitant ADNC to disease survival and profiles of cognitive decline in preclinical and clinical stages in a large neuropathologically diagnosed group.
Methods
We evaluated 597 participants with LP and 491 participants with intermediate/high ADNC in the absence of LP from the National Alzheimer Coordinating Center (NACC) database. At baseline, 237 participants were cognitively normal (CN), 255 were diagnosed with mild cognitive impairment (MCI), and 596 with dementia. Cognition was assessed using three cognitive domain scores (i.e., Memory, Executive, and Language) from the NACC Uniform Dataset (UDS) neuropsychological test battery, MMSE, and Clinical Dementia Rating (CDR). Multivariate adaptive regression splines were used to evaluate associations between baseline cognitive scores and mean annual rate of change over two years. The likelihood of progression to MCI or dementia was assessed using Cox hazard models.
Results
Neocortical LP, independent of the clinical diagnosis, was associated with lower Executive and higher Language and Memory scores at baseline, whereas Braak V-VI neurofibrillary tangle pathology was associated with lower Memory and Language scores. Similarly, neocortical LP was associated with faster Executive decline, whereas Braak V-VI neurofibrillary tangle pathology was associated with faster Memory and Language decline. A clinical diagnosis of Lewy Body Dementia (i.e., a strong LP phenotype) was associated with the LP cognitive profile and shorter disease duration. Progression to incident MCI or dementia was primarily associated with the degree of tau pathology; neocortical LP or a diagnosis of Lewy Body Dementia only predicted progression when those with intermediate/high ADNC were excluded.
Conclusions
LP and ADNC differentially affected cross-sectional and longitudinal cognitive profiles in a large autopsy sample. Concomitant Braak V-VI neurofibrillary tangle pathology had a strong impact on clinical progression in those with LP, regardless of the initial stage. Thus, LB and ADNC co-pathology interact to affect cognitive domains that may be used to track Lewy Body disease longitudinally and as outcome measures in therapeutic trials.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13195-024-01628-z.
Keywords: Alzheimer disease, Tau, Lewy pathology, Lewy body disease, Dementia with Lewy bodies, α-synuclein, Cognition, Dementia
Background
With the recent development of in vivo biomarkers of α-synuclein associated with brain Lewy pathology (LP) [1], conducting clinical therapeutic trials that specifically target cognitive changes related to Lewy body disease (LBD) will soon be possible. These trials may span the spectrum of LBD-related cognitive decline from preclinical and prodromal stages (e.g., dementia with Lewy bodies-mild cognitive impairment (DLB-MCI) [2], Parkinson’s disease-mild cognitive impairment (PD-MCI) [3]) to frank dementia (e.g., dementia with Lewy bodies (DLB) [4], PD dementia (PDD) [5]). A factor complicating these trials is that LBD is frequently accompanied by Alzheimer’s disease (AD) neuropathological change (ADNC), especially if cognitive impairment is present [6–8]. At least 50% of individuals with DLB have AD co-pathology, whereas < 10% of PD without cognitive impairment present AD co-pathology. AD co-pathology leads to greater cognitive impairment, lower prevalence of DLB clinical features, faster cognitive decline, and shorter disease duration [6–10]. Conversely, AD dementia individuals frequently present Lewy pathology, including in autosomal dominant AD, leading to changes in cognitive profile [11]. Thus, LBD-targeting trials are likely to occur in the context of concomitant ADNC that could modify the clinical phenotype, cognitive deficit profile, and rate of decline. Understanding how LB and ADNC co-pathology interact is crucial to affect potential clinical and cognitive outcome measures used in LBD-targeting therapeutic trials.
Few autopsy-validated studies have examined the pattern and rate of LP-associated cognitive decline in the presence or absence of concomitant ADNC and those that have primarily focused on advanced stages of the disease [12, 13]. These few studies suggest that LBD is associated with a particularly rapid decline in attention, executive functions, and visuospatial abilities relative to declines in language and memory. This pattern is modified in those with concomitant ADNC who show additional rapid decline in memory comparable to those with AD alone. While these studies provide evidence that concomitant ADNC alters cognitive changes in those with LBD, they do not show if these alterations are expressed in early disease stages that are the focus of most clinical trials, nor do they provide estimates of annual decline in cognitive measures in individuals with LBD and varying degrees of concomitant ADNC. They also do not determine if the relationship between LP and the pattern and rate of decline in cognition depends upon the presence or absence of a strong LBD phenotype (i.e., a clinical diagnosis of Lewy Body Dementia).
In the present study, we compare baseline cognitive scores and cognitive decline over the assessments performed two years apart in individuals with or without LP and varying degrees of ADNC using data from autopsy-confirmed research participants in the Uniform Dataset (UDS) of the National Alzheimer’s Coordinating Center (NACC). The influence of the level of cognitive impairment (i.e., cognitively normal (CN), Mild Cognitive Impairment (MCI), or dementia) on these relationships is examined. We hypothesize that a specific profile of cognitive decline will be associated with LP (vs. no LP) and modified by concomitant ADNC, regardless of the stage of cognitive decline. In addition, we predict that a strong LBD phenotype (i.e., a diagnosis of Lewy Body Dementia) will be associated with the LB cognitive profile.
Methods
Participants
This study used cross-sectional and longitudinal data from the NACC database, which contains demographic, clinical, and neuropathological data from participants enrolled at 39 current and past NIA-funded Alzheimer’s Disease Research Centers (ADRCs). Participants were enrolled with any level of cognition, ranging from cognitively normal to dementia, and were evaluated annually using a standardized UDS protocol [14]. Brain autopsy was obtained for a subset of participants.
Each center conducted neuropathologic assessments following consensus guidelines and uploaded the data to the NACC UDS [15]. NIA-AA consensus guidelines for the neuropathologic evaluation of ADNC were applied [16]. Consensus guidelines for LBD were used to determine LP presence and assign a brainstem, limbic/transitional, or cerebral cortical stage [17]. Amygdala-restricted LP participants were excluded because studies have failed to show that this limited pathology impacts clinical features or cognitive trajectories.
Participants were selected for our analyses if neuropathologic data were available and they had completed at least two annual clinical evaluations that included a Clinical Dementia Rating (CDR), Mini-Mental State Exam (MMSE), or UDS Neuropsychological Test Battery [18] within a two-year period. All participants with limbic or neocortical LP who met these criteria formed one group (n = 597). A comparison group of participants without LP (n = 491) and the required clinical and neuropathological data was selected, stratifying by clinical diagnosis (CN, MCI, and dementia) and matching demographic characteristics. Because we were evaluating the effect of AD, MCI, and dementia, participants in the second group had to have Braak stage ≥ III. We performed repeated random sampling, stratified by diagnosis, and selected the sample with the closest age, education, and sex distribution to the participants with LP. TDP-43 pathology was not included in the analyses due to the low number of participants that had this data (60.4%). The demographic, clinical, and neuropathologic characteristics of study participants for each group, stratified by clinical diagnosis, are shown in Table 1. Study approval was obtained from the Institutional Review Board (IRB) for each participating NACC site. The NACC database has a waiver of HIPAA Authorization. The Houston Methodist Neurological Institute IRB confirmed that the current analysis met exempt criteria.
Table 1.
Demographic, clinical, and neuropathologic characteristics of study participants stratified by clinical diagnosis and presence or absence of Lewy pathology
| Lewy Pathology (n = 597) |
No Lewy Pathology (n = 491) |
p-value | ||
|---|---|---|---|---|
| Sex (Female %) | 38.7% | 41.8% | 0.34 | |
| Age (Years) | 75 [68–80] | 74 [67–78] | 0.19 | |
| Education (Years) | 16 [14–18] | 16 [13–18] | 0.013 | |
| APOE ε4 (%) | 52% | 52.5% | 0.91 | |
| Clinical Diagnosis (%) | CN | 21.8% | 21.8% | 0.054 |
| MCI | 26.1% | 20.2% | ||
| Dementia | 52.1% | 58% | ||
| Final diagnosis of LBD (%) | 22.8% | 3.9% | < 0.0001 | |
| Tau NFT (%) | Braak 0-II | 15.4% | 8.4% | 0.0006 |
| Braak III-IV | 24.8% | 19.1% | ||
| Braak V-VI | 59.8% | 72.5% | ||
| Lewy Pathology (%) | Limbic | 40.5% | 0% | NA |
| Neocortical | 59.5% | 0% | ||
CN: Cognitively Normal; LBD: Lewy Body Dementia; MCI: Mild Cognitive Impairment; NFT: Neurofibrillary Tangles. The median (25th and 75th percentiles) was provided for age and education
Procedure
At each annual UDS evaluation, participants completed the CDR, MMSE, and a UDS Neuropsychological Test Battery that included measures of memory (immediate and delayed Logical Memory Test), language (Boston Naming Test, category fluency test), executive functions (Trail-Making Test parts A and B, Digit Symbol Substitution Test), and attention (forward and backward Digit Span). The tests are described in detail elsewhere [18]. For our analyses, we used the Executive, Memory, and Language standardized domain scores developed as part of the ADSP Phenotype Harmonization Consortium [19]. Clinical diagnoses of normal cognition, MCI, or dementia were made at each center either by a single clinician or a consensus group of clinicians after a review of all information available from the clinical evaluation. Primary and contributing etiology was assigned, based on established clinical guidelines, for participants with MCI or dementia.
We estimated the yearly change in the cognitive domain scores by calculating the difference in scores obtained during visits within two years and dividing the difference by the time interval estimated from the visit dates. We compared differences in baseline profiles and profiles of decline in cognitive domain scores (Memory, Executive, Language) in those with or without LP [19] and varying degrees of ADNC. We selected Braak tau neurofibrillary pathology stages (versus amyloid β (Aβ) pathology) to stage ADNC since it correlates more strongly with cognitive outcomes than AD Aβ scores [20].
Statistical analysis
Demographic and neuropathological data were summarized using median and interquartile range (IQR) for continuous variables or proportions for categorical data. Linear regressions evaluated group differences (Limbic or Neocortical LP vs. No LP) in covariate-adjusted (age, sex, and education) models. Power transformations were applied as needed to normalize the distribution of the residuals.
A multivariate adaptive regression splines (MARS) model evaluated the association between baseline scores (predictor) and longitudinal changes (averaged over two visits performed two years apart) in the CDR-SB, MMSE, and cognitive domain scores (dependent variables). Additional predictors for these models were age, tau Braak stage, LP (limbic or neocortical), sex, and education. The MARS algorithm is a nonparametric statistical method that partitions training data into piecewise linear segments [21]. The model identifies points, called knots, which define segments via hinge functions. These hinge functions delimit the range in the x-axis with independent linear regression models for each segment. If multiple variables affect the studied variable, the model can select an interaction of ≥ 2 hinge functions. MARS models are calculated with forward and backward passes. MARS starts with the intercept term, representing the mean of all values. The forward pass sequentially evaluates pairs of basis functions applied to different hinge functions to minimize the residual error. As indicated, linear regressions are used to calculate the terms. Once the maximum number of terms or the change in residual error is too small to change, the modeling concludes. To avoid overfitting, a backward pass prunes the least effective term at each step using the generalized cross-validation criterion, which is an approximate leave-one-out cross-validation error metric. To identify the best model, we explored level 2 interactions for the evaluated predictors (age and Braak stage, for example) and allowed up to 11 terms to be included in the model. A 5-fold cross-validation with five repeats was used to select the best model based on the lowest root mean square error [22]. The results are summarized in Figs. 1 and 2; the “other” category includes the neuropathological groups that showed the same associations between the different cognitive domains (Fig. 1) or the rate of progression (Fig. 2). A Cox proportional hazard model evaluated clinical progression to MCI and/or dementia and survival; the model included age, tau Braak stage, LP (limbic or neocortical), and sex as predictors. Two sub-analyses were performed to determine (1) if the relationship between LP and cognitive profiles or progression is modified by the presence or absence of a high AD pathology burden (i.e., tau Braak stages lower vs. higher than V-VI) and (2) if a clinical diagnosis of Lewy body dementia is associated with a particular cognitive profile or progression in individuals with LP (i.e., is the Lewy body dementia phenotype associated with a distinct cognitive profile). Cox proportional hazard model p-values were considered statistically significant if the two-tailed distribution was < 0.05. Analyses were performed using R version 4.2.1.
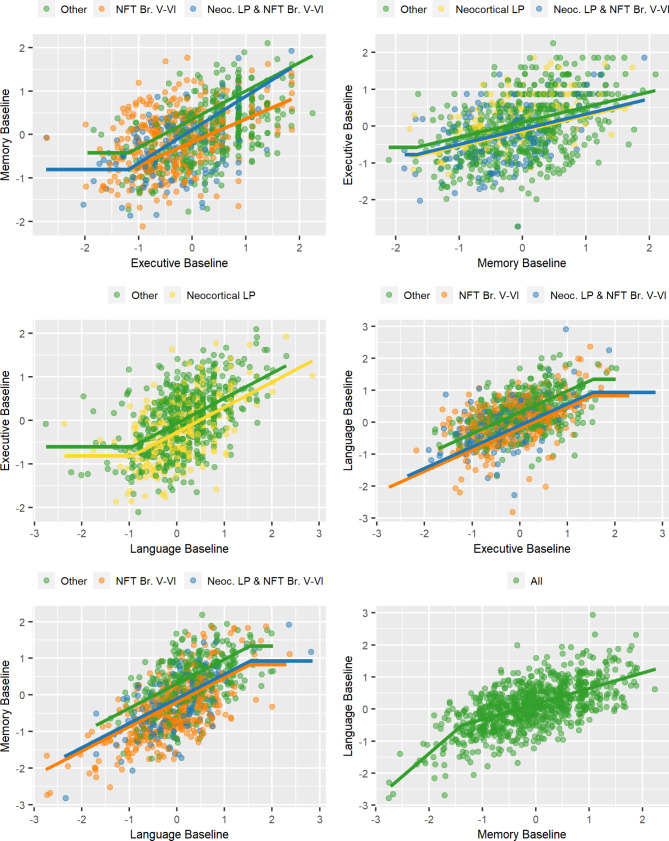
Fig. 1.
Cross-sectional neuropsychological associations. Associations between cognitive domain scores as a function of pathology (neurofibrillary tangle -NFT- Braak and Lewy pathology stage-LP-) using multivariate adaptive regression splines (MARS). The “Other” category includes neuropathologically defined groups that showed no differences among each other and were not selected as an independent predictor in the evaluated model
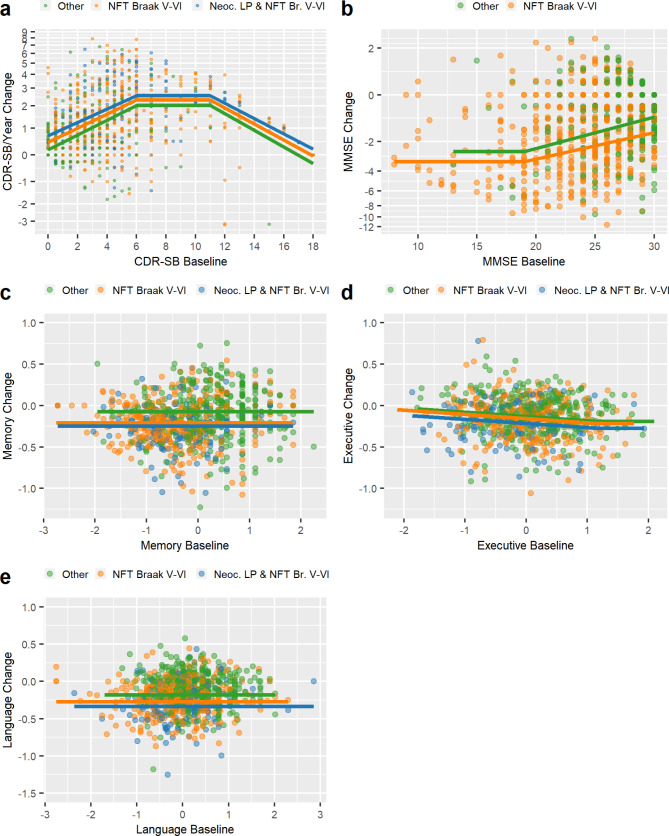
Fig. 2.
Longitudinal cognitive change. Yearly change (averaged over two years) in Clinical Dementia Rating-Sum of Boxes (CDR-SB) scores (a), Mini-Mental State Exam (MMSE) scores (b), and Memory (c), Executive (d), and Language (e) Domain scores (y-axis) versus their respective baseline score (x-axis) as a function of pathology status. The “Other” category includes neuropathologically defined groups that showed no differences among each other and were not selected as an independent predictor in the evaluated model
Informed consent
Written informed consent was obtained from all participants and their study co-participants at each ADRC. Institutional review board approval was obtained at each ADRC to allow de-identified clinical and neuropathological data to be entered into the NACC UDS database and made available for secondary analyses.
Data availability
Anonymized data can be requested from the NACC (https://naccdata.org/requesting-data/data-request-process).
Results
Clinical and pathological characteristics
Table 1 shows baseline demographic, clinical, and neuropathologic data for participants with and without LP. As expected from our matching procedure, the groups did not differ in average age or education, and there were similar proportions of females and those with at least one APOE ε4 allele in each group. There were also similar distributions of individuals who were CN or diagnosed with MCI or dementia at baseline in the two groups. At the last clinical visit before the autopsy, a clinical diagnosis of Lewy body dementia was more likely in the LP (27.0%) than in the No LP group (4.8%)(p < 0.0001). The group without Lewy pathology had higher Braak V-VI tau neurofibrillary tangle scores.
Influence of Lewy Pathology on Baseline Cognitive Scores by Diagnostic Group.
Limbic or neocortical LP did not affect baseline CDR-SB scores (i.e., add to effects of Braak tau neurofibrillary tangle stages) in analyses stratified by clinical diagnosis (p > 0.24, Supplemental Fig. 1A). Similar results were obtained for MMSE, although neocortical LP was associated with higher MMSE scores in the CN group (p < 0.001, Supplemental Fig. 1B) but not in the MCI or dementia groups. Neocortical LP was associated with higher Memory domain scores (p = 0.0089) and lower Executive function domain scores (p = 0.0094) in the dementia group but not in the CN or MCI groups (Supplemental Fig. 2) in analyses adjusted for sex, age, education, and Braak neurofibrillary tangle stage. A clinical diagnosis of Lewy body dementia in the participants with dementia was not associated with memory or executive domain scores (p˃0.05).
Influence of Lewy pathology on baseline cognitive domain profiles
In AD and LBD, there is a progressive cognitive decline that might start in a single domain but then progressively affect additional domains. To explore if there are different degrees of impairment in the cognitive domains, we evaluated the impact of LP on cognitive domain profiles at baseline by comparing each possible cognitive domain pair across the three cognitive domains (Memory vs. Executive vs. Language). By comparing the impairment in each cognitive domain, we tested whether a cognitive domain has a relatively greater impairment than another. Figure 1 shows the relationships between various pairs of cognitive domain scores as a function of the presence or absence of neocortical LP in individuals with Braak tau stage V-VI AD pathology. Neocortical LP was associated with a profile of lower Executive domain scores than Memory (-0.24) or Language (-0.19) domain scores, indicating that individuals with neocortical Lewy pathology present a more prominent dysexecutive profile compared to the relative impairment in the Memory and Language domains. In contrast, Braak V-VI neurofibrillary tangle stages were associated with lower Memory (-0.24) and Language (-0.19) domain scores than Executive domain scores. Neocortical LP did not modify this relationship between Executive scores and Memory or Language scores. Participants with neocortical LP and Braak V-VI pathology had Memory scores similar to those of the low pathology group (i.e., no LP and Braak stage ≤ IV) when overall cognition was only mildly affected; however, they showed greater memory impairment relative to impairment in language or executive functions than did the low pathology group in those with more advanced cognitive impairment.
When participants with LP were excluded from the analyses, the model selected the same neuropathological variables as above as predictors of the relationship between Executive scores and Memory or Language scores. That is, Braak V-VI neurofibrillary tangle score was associated with a profile of lower Memory and Language domain scores than Executive domain scores. When the analyses only included participants with LP, a Lewy body dementia diagnosis was associated with a profile of lower Executive (-0.27) and Language (-0.17) scores compared to Memory scores, and neocortical LP was associated with a profile of lower Executive function scores than Memory (-0.20) or Language (-0.12) scores. However, only a Lewy body dementia diagnosis predicted relatively preserved Memory versus Executive scores (0.22). Intermediate levels of Lewy (i.e., limbic only) or AD (i.e., neurofibrillary tangle Braak III-IV only) pathologies alone were not associated with differences in the relationship between the cognitive domains. Age was selected as a covariate in several of the models, but none of the models selected sex.
Influence of Lewy pathology on longitudinal cognitive change
The annual rate of increase (worsening) in CDR-SB scores differed as a function of the baseline score, with a peak rate of about 2.0 points/year when the baseline CDR-SB score was between 6 and 11 points (Fig. 2). In this peak range, the rate of change was faster in those with neurofibrillary tangle Braak V-VI (2.3 points/year) or Braak V-VI and neocortical LP (2.5 points/year) than in those with low pathology (Braak < V and no LP). The model did not select age, sex, gender, or education. There was no difference in the rate of worsening on the CDR-SB as a function of a final clinical diagnosis of Lewy body dementia in those with Braak V-VI tau and neocortical LP (data not shown). The annual rate of decline on the MMSE also differed as a function of baseline score, with a faster decline in those with lower baseline scores, asymptoting at about − 2.58 points/year at a baseline MMSE of 19 or less. The annual rate of decline on the MMSE was greater in those with Braak V-VI tau (with or without LP) than in those with low pathology (Braak < V and no LP), regardless of the baseline starting point. There was no difference in the rate of MMSE decline as a function of a final diagnosis of Lewy body dementia in those with Braak V-VI tau and neocortical LP (data not shown).
The annual rate of decline in the Memory and Language domain scores did not vary as a function of the baseline score (Fig. 2). Memory and Language scores declined faster in those with Braak V-VI tau pathology (-0.21 s.d./year and − 0.27 s.d./year, respectively) or Braak V-VI tau and neocortical LP (-0.25 s.d./year and − 0.34 s.d./year, respectively) than in those with low pathology (Braak < V and no LP). The annual rate of decline on the Executive domain score was faster in those with higher baseline scores beginning at a baseline score of 1.2 s.d. and was faster in those with Braak V-VI tau pathology (-0.22 s.d./year) or Braak V-VI tau and neocortical LP (-0.28 s.d./year) than in those with low pathology (Braak < V and no LP) (-0.19 s.d./year). LP did not predict longitudinal change in Memory or Language domain scores (i.e., was not selected by the MARS model) when analyses were limited to participants who were tau Braak < V with or without LP (data not shown).
Influence of Lewy pathology on progression to MCI or dementia
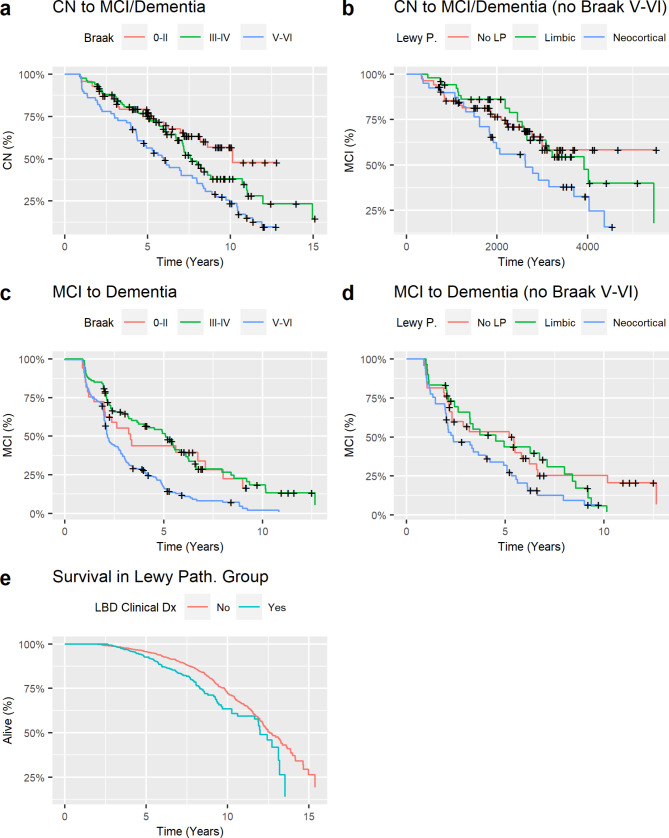
During a median follow-up of 7.7 years (IQR: 5.6–9.9 years), 131 of the CN participants (57.7%) progressed to MCI or dementia. Progression from CN to MCI or dementia was faster in individuals with Braak V-VI tau pathology than in those with Braak < V tau pathology, regardless of LP status (Table 2; Fig. 3). Neocortical LP significantly predicted clinical progression when analyses were restricted to those with Braak < V tau pathology. During a median follow-up of 6.2 years (IQR: 4.2–8.4 years), 262 of the MCI participants (87.3%) progressed to dementia. The same associations were observed in the progression from MCI to dementia. A clinical diagnosis of Lewy body dementia was associated with shorter survival to death in those with LP (H.R.= 1.53, 95% C.I.=1.18–1.99, p-value = 0.0014).
Table 2.
Survival models. Prediction of clinical progression in Cox hazards models
| With APOE | CN to MCI/Dementia | |||
|---|---|---|---|---|
| All | Exclude Braak V-VI (n = 147) | |||
| Variable | HR (95% CI) | Pr(>|z|) | HR (95% CI) | Pr(>|z|) |
| Sex (Male) | 1.0 [0.70–1.42] | 0.99 | 1.05 [0.65–1.70] | 0.84 |
| Age (years) | 0.99 [0.96–1.02] | 0.57 | 1 [0.97–1.05] | 0.78 |
| Education (yrs) | 0.99 [0.93–1.06] | 0.80 | 0.98 [0.9–1.07] | 0.62 |
| NFT Braak III-IV | 1.30 [0.77–2.20] | 0.33 | 1.18 [0.69–2.04] | 0.55 |
| NFT Braak V-VI | 1.98 [1.19–3.29] | 0.009 | - | - |
| Limbic LP | 0.74 [0.47–1.16] | 0.19 | 1.04 [0.57–1.9] | 0.91 |
| Neocortical LP | 1.34 [0.88–2.05] | 0.17 | 1.74 [0.96–3.1] | 0.068 |
| APOE ε4 | 1.77 [1.20–2.60] | 0.004 | 1.60 (0.93–2.75) | 0.090 |
| MCI to Dementia | ||||
| All | Exclude Braak V-VI ( n = 113) | |||
| Variable | HR (95% CI) | Pr(>|z|) | HR (95% CI) | Pr(>|z|) |
| Sex (Male) | 1.04 [0.78–1.38] | 0.80 | 1.12 [0.69–1.83] | 0.67 |
| Age (years) | 0.99 [0.98–1.01] | 0.49 | 0.99 [0.96–1.02] | 0.53 |
| Education (yrs) | 1.03 [0.99–1.09] | 0.12 | 1.03 [0.96–1.1] | 0.39 |
| NFT Braak III-IV | 0.84 [0.50–1.41] | 0.51 | 0.97 [0.56–1.67] | 0.91 |
| NFT Braak V-VI | 1.78 [1.12–2.83] | 0.015 | - | - |
| Limbic LP | 1.02 [0.74–1.42] | 0.82 | 1.30 [0.69–2.46] | 0.41 |
| Neocortical LP | 1.23 [0.92–1.65] | 0.21 | 2.05 [1.15–3.66] | 0.015 |
| APOE ε4 | 1.14 [0.87–1.51] | 0.35 | 1.04 (0.64–1.69) | 0.88 |
HR: Hazard ratio; LP: Lewy pathology; NFT: Neurofibrillary tangles
Fig. 3.
Disease progression. Progression from cognitively normal (CN) to mild cognitive impairment (MCI) or dementia in the overall group (a) and after excluding participants with Braak stage V-VI neurofibrillary tangle pathology (b); and progression from MCI to dementia in the overall group (c) and after excluding participants with Braak stage V-VI neurofibrillary tangle pathology (d). Survival in individuals with a clinical diagnosis of Lewy body dementia (LBD, blue) or other dementia (red) (e)
Discussion
Our results indicate that LP and ADNC, represented by Braak V-VI tau neurofibrillary tangle stages, were differentially associated with cognitive impairment and decline in individuals with LBD, independent of the level of cognitive impairment (e.g., preclinical/prodromal or frank dementia) or clinical diagnosis (i.e., syndromic presentation). Across the disease severity spectrum, neocortical LP was associated with a profile of greater deficits in executive functions than in memory or language abilities. In contrast, Braak V-VI tau neurofibrillary tangle stages were associated with a profile of greater deficits in memory and language abilities than in executive functions. Similarly, longitudinal analyses showed that a faster decline in executive functions was primarily associated with neocortical LP, while a faster decline in memory and language abilities was associated with higher levels of tau pathology (i.e., higher Braak stage). These results are similar to those from neuropathological studies that have compared more advanced stages of LBD and AD [12, 13] and extended them to early stages of the disease, including preclinical, prodromal, and mild dementia states. Results are also consistent with previous findings showing that individuals with a clinical diagnosis of DLB and positive AD biomarkers perform worse on cognitive testing [6, 23] and decline faster on cognitive tests [7, 8] than those with negative AD biomarkers. These cognitive changes may be independent of core features of DLB since some previous studies of individuals with dementia and LP [7, 10, 24] found no decrease in DLB core features in the presence of ADNC co-pathology [25, 26]. In addition, our results suggest that the effects of LP on cognition may be independent of the clinical phenotype, as the impact of LP was still present once participants with Lewy body dementia were excluded.
Various types of co-pathology are usual in cognitively impaired older individuals [27, 28], and each pathology may contribute to cognitive decline, lower the individual threshold for pathology to become symptomatic [27], and modify the clinical profile [29]. Differences in the type and frequency of co-pathologies could explain discrepancies between studies that have examined cognitive profiles and decline in individuals with LP. A recent study, for example, showed that the presence of LP and vascular co-pathologies led to greater executive-memory impairment in individuals with dementia and ADNC compared to those without co-pathology [7]. Vascular pathology is only marginally addressed in the UDS neuropathology database, but there was no relationship between the presence of atherosclerosis and Braak stage in individuals with LBD in additional analyses (data not shown).
Regarding global measures of cognition and function, a higher Braak stage (i.e., greater ADNC) was associated with faster annual decline on both the MMSE and CDR-SB, regardless of the level of baseline cognitive impairment. In contrast, LP had little additional impact beyond that of ADNC; that is, the rate of decline on the CDR-SB or MMSE was similar in those with ADNC with or without LP. These results indicate the primacy of high ADNC in driving the global cognitive decline in LBD when it co-occurs with LP. Neocortical LP was, however, associated with greater decline (i.e., a faster increase) in CDR-SB, but not MMSE, when the level of concomitant ADNC was low. The additional impairment observed in CDR-SB but not in MMSE could be related to functional impairment captured by the CDR-SB but not by cognitive scales. Furthermore, the MMSE may be relatively insensitive to LP since it only marginally assesses executive function and visuospatial ability.
As with cognitive and functional test scores, the rate of progression in clinical diagnostic status from normal cognition to MCI to dementia was primarily driven by high ADNC, although LP was significantly associated with clinical progression when participants with the highest Braak stages (i.e., Braak V-VI) were excluded from the analysis. An association between LP and cognitive decline is consistent with two recent studies that showed baseline CSF α-synuclein positivity determined by seeding amplification assays [30, 31] was an independent predictor of cognitive decline in early stages of AD or LBD. A clinical diagnosis of Lewy body dementia at baseline or follow-up was associated with shorter survival in individuals with LP, independently of the degree of ADNC. This could be related to motor dysfunction and dysautonomia often associated with the LBD phenotype, which could affect overall progression and risk of death [32, 33]. We did not observe a significant association between a clinical diagnosis of Lewy body dementia and cognitive decline profiles when analyses were limited to individuals with LP, perhaps due to insufficient power or imprecise clinical diagnosis since criteria for Lewy body dementia have relatively low sensitivity [34].
Our analyses showed that Memory, Language, and Executive cognitive domain scores declined over two years at a relatively uniform rate across levels of severity of baseline cognitive impairment. The rate of decline on the more global MMSE, in contrast, showed an increasing rate of decline (from about 2 points/year to 3 points/year) as the baseline severity of cognitive impairment increased until a baseline score of 19, where the annual rate of decline plateaued at about 3 points/year. A similar pattern was observed for the CDR-SB, where the annual rate of decline increased (from about 0.5 points/year to about 2 points/year) as baseline severity increased up to a baseline CDR-SB of about 6. Rates of decline then plateaued at about 2 points/year with increasing severity between a baseline CDR-SB of 6 to 12 points (and declined towards the ceiling thereafter). This suggests that these cognitive screening and monitoring tools commonly used in clinical practice and clinical trials may be less sensitive to change in early than later stages of disease, and this could lead to a greater relative response to treatment in more advanced stages for a similar absolute benefit [35]. This could also explain conflicting results regarding differences in the rate of progression during MCI stages of DLB versus AD [36–39].
Recent clinical trials have shown the benefit of monoclonal antibody treatments targeting Aβ in patients with MCI or early dementia who have a positive AD biomarker and an amnestic clinical profile [35, 40]. Further studies are needed to determine how these treatments might be influenced by LP co-pathology and how treating ADNC in patients with prodromal DLB might affect cognitive decline. Our results provide reference cognitive decline rates with standard deviations that could inform power calculations for clinical trials to address these questions. A subanalysis of the recent AscenD-LB phase 2a clinical trial suggested that AD co-pathology, defined based on plasma p-tau 181, led to decreased response to the treatment, underscoring the importance of co-pathologies in clinical trials [41].
While a strength of the present study is a long-term clinical and cognitive follow-up of a large autopsy series, several limitations should be considered. First, there may be variability in the application of diagnostic criteria across the different ADRCs, particularly in diagnosing Lewy body dementia. This could explain the low frequency of an LBD diagnosis, as previously reported [34], in addition to AD co-pathology modifying the clinical presentation [7, 24]. Second, autopsy rates vary across the ADRCs, and autopsy procedures may vary. Third, the NACC UDS neuropsychological test battery available for the current analyses (UDS 1.0 and 2.0) is limited and includes no visuospatial cognitive assessment. Fourth, the exclusion of amygdala-predominant cases could lead to some bias in our case selection. Amygdala-predominant Lewy pathology has been considered an isolated α-synucleinopathy and is not considered to lead to DLB or PD dementia [42, 43]; however, studies have shown that amygdala-predominant Lewy pathology is associated with decreased CA1 neuronal density and more severe TDP-43 pathology [44]. Finally, there is a lack of diversity among participants followed at the ADRCs, including among autopsied individuals, so further studies will be required to confirm whether these findings generalize.
Conclusions
LP and ADNC are highly prevalent co-pathologies that can modify clinical and cognitive deficit profiles and rates of progression. This may be especially relevant for clinical trials that may include biomarkers for the targeted pathology but not for coincident pathologies not targeted by the studied treatment. Our findings that concomitant high ADNC strongly impacts cognitive performance in individuals with LP and Lewy body dementia suggests that assessment of specific cognitive domains over 12 months could be helpful to track the differential impact of therapeutic trials that target AD or LB pathologies as well as combination therapies in the future [7].
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
J.B.T. is the Ann and Billy Harrison Centennial Chair in Alzheimer’s Research and is supported by the endowment. This research was supported by NIA/NIH grant P30 AG062429. This study used publicly available de-identified data; ethics declaration: not applicable. Dr. Armstrong receives research support from the NIH (R01AG068128, P30AG066506, R01NS121099, R44AG062072), the Florida Department of Health (grants 20A08, 24A14, 24A15), and as the local PI of a Lewy Body Dementia Association Research Center of Excellence. She serves on the DSMBs for the Alzheimer’s Therapeutic Research Institute/Alzheimer’s Clinical Trial Consortium and the Alzheimer’s Disease Cooperative Study. She has provided educational content for Medscape, Vindico CME, and Prime Inc.
Abbreviations
- Aβ
Amyloid β
- AD
Alzheimer’s disease
- ADNC
Alzheimer’s disease neuropathological change
- CDR
Clinical Dementia Rating
- CN
Cognitively normal
- DLB
Dementia with Lewy bodies
- H.R.
Hazard ratio
- IQR
Interquartile range
- LBD
Lewy body disease
- LP
Lewy pathology
- MARS
Multivariate adaptive regression splines
- MCI
Mild cognitive impairment
- MMSE
Mini-Mental State Exam
- PD
Parkinson’s disease
- PDD
Parkinson’s disease dementia
- UDS
Uniform dataset
Author contributions
J.B.T., D.G., D.V.S. wrote the manuscript. J.B.T. performed the analyses and prepared figures and tables. J.B.T., D.G., D.V.S, and M.J.A. participated in the conceptualization of the study, and edited and reviewed the manuscript.
Funding
J.B.T. is the Ann and Billy Harrison Centennial Chair in Alzheimer’s Research and is supported by the endowment. This research was supported by NIA/NIH grant P30 AG062429. This study used publicly available de-identified data; ethics declaration: not applicable. Dr. Armstrong receives research support from the NIH (R01AG068128, P30AG066506, R01NS121099, R44AG062072), the Florida Department of Health (grants 20A08, 24A14, 24A15), and as the local PI of a Lewy Body Dementia Association Research Center of Excellence. She serves on the DSMBs for the Alzheimer’s Therapeutic Research Institute/Alzheimer’s Clinical Trial Consortium and the Alzheimer’s Disease Cooperative Study. She has provided educational content for Medscape, Vindico CME, and Prime Inc.
Data availability
Data used in this manuscript was obtained from the NACC database: https://naccdata.org/.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Groveman BR, Orru CD, Hughson AG, Raymond LD, Zanusso G, Ghetti B, et al. Rapid and ultra-sensitive quantitation of disease-associated alpha-synuclein seeds in brain and cerebrospinal fluid by alphaSyn RT-QuIC. Acta Neuropathol Commun. 2018;6(1):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McKeith IG, Ferman TJ, Thomas AJ, Blanc F, Boeve BF, Fujishiro H, et al. Research criteria for the diagnosis of prodromal dementia with Lewy bodies. Neurology. 2020;94(17):743–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Litvan I, Goldman JG, Troster AI, Schmand BA, Weintraub D, Petersen RC, et al. Diagnostic criteria for mild cognitive impairment in Parkinson’s disease: Movement Disorder Society Task Force guidelines. Mov Disorders: Official J Mov Disorder Soc. 2012;27(3):349–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McKeith IG, Boeve BF, Dickson DW, Halliday G, Taylor JP, Weintraub D, et al. Diagnosis and management of dementia with Lewy bodies: fourth consensus report of the DLB Consortium. Neurology. 2017;89(1):88–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Emre M, Aarsland D, Brown R, Burn DJ, Duyckaerts C, Mizuno Y, et al. Clinical diagnostic criteria for dementia associated with Parkinson’s disease. Mov Disorders: Official J Mov Disorder Soc. 2007;22(12):1689–707. quiz 837. [DOI] [PubMed] [Google Scholar]
- 6.Ferreira D, Przybelski SA, Lesnick TG, Lemstra AW, Londos E, Blanc F, et al. beta-amyloid and tau biomarkers and clinical phenotype in dementia with Lewy bodies. Neurology. 2020;95(24):e3257–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Toledo JB, Abdelnour C, Weil RS, Ferreira D, Rodriguez-Porcel F, Pilotto A, et al. Dementia with Lewy bodies: impact of co-pathologies and implications for clinical trial design. Alzheimer’s Dement J Alzheimer’s Assoc. 2023;19(1):318–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abdelnour C, van Steenoven I, Londos E, Blanc F, Auestad B, Kramberger MG, et al. Alzheimer’s disease cerebrospinal fluid biomarkers predict cognitive decline in lewy body dementia. Mov Disorders: Official J Mov Disorder Soc. 2016;31(8):1203–8. [DOI] [PubMed] [Google Scholar]
- 9.Toledo JB, Gopal P, Raible K, Irwin DJ, Brettschneider J, Sedor S, et al. Pathological alpha-synuclein distribution in subjects with coincident Alzheimer’s and Lewy body pathology. Acta Neuropathol. 2016;131(3):393–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferman TJ, Aoki N, Crook JE, Murray ME, Graff-Radford NR, van Gerpen JA, et al. The limbic and neocortical contribution of alpha-synuclein, tau, and amyloid beta to disease duration in dementia with Lewy bodies. Alzheimer’s Dement J Alzheimer’s Assoc. 2018;14(3):330–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Toledo JB, Cairns NJ, Da X, Chen K, Carter D, Fleisher A, et al. Clinical and multimodal biomarker correlates of ADNI neuropathological findings. Acta Neuropathol Commun. 2013;1(1):65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smirnov DS, Galasko D, Edland SD, Filoteo JV, Hansen LA, Salmon DP. Cognitive decline profiles differ in Parkinson disease dementia and dementia with Lewy bodies. Neurology. 2020;94(20):e2076–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ryman SG, Yutsis M, Tian L, Henderson VW, Montine TJ, Salmon DP, et al. Cognition at each stage of Lewy body disease with co-occurring Alzheimer’s disease pathology. J Alzheimer’s Disease: JAD. 2021;80(3):1243–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weintraub S, Salmon D, Mercaldo N, Ferris S, Graff-Radford NR, Chui H, et al. The Alzheimer’s disease centers’ Uniform Data Set (UDS): the neuropsychologic test battery. Alzheimer Dis Assoc Disord. 2009;23(2):91–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Besser LM, Kukull WA, Teylan MA, Bigio EH, Cairns NJ, Kofler JK, et al. The revised National Alzheimer’s Coordinating Center’s neuropathology form-available data and new analyses. J Neuropathol Exp Neurol. 2018;77(8):717–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Montine TJ, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Dickson DW, et al. National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease: a practical approach. Acta Neuropathol. 2012;123(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McKeith IG, Dickson DW, Lowe J, Emre M, O’Brien JT, Feldman H, et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology. 2005;65(12):1863–72. [DOI] [PubMed] [Google Scholar]
- 18.Weintraub S, Besser L, Dodge HH, Teylan M, Ferris S, Goldstein FC, et al. Version 3 of the Alzheimer disease centers’ neuropsychological test battery in the Uniform Data Set (UDS). Alzheimer Dis Assoc Disord. 2018;32(1):10–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mukherjee S, Choi SE, Lee ML, Scollard P, Trittschuh EH, Mez J, et al. Cognitive domain harmonization and cocalibration in studies of older adults. Neuropsychology. 2023;37(4):409–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boyle PA, Wilson RS, Yu L, Barr AM, Honer WG, Schneider JA, et al. Much of late life cognitive decline is not due to common neurodegenerative pathologies. Ann Neurol. 2013;74(3):478–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Friedman HF. Multivariate adaptive regression splines. Annals Stat. 1991;19(1):1–67. [Google Scholar]
- 22.Kuhn M. Building predictive models in R using the caret. J Stat Softw. 2008;28(5).
- 23.Di Censo R, Abdelnour C, Blanc F, Bousiges O, Lemstra AW, van Steenoven I, et al. CSF tau proteins correlate with an atypical clinical presentation in dementia with Lewy bodies. J Neurol Neurosurg Psychiatry. 2020;91(1):109–10. [DOI] [PubMed] [Google Scholar]
- 24.Ferman TJ, Aoki N, Boeve BF, Aakre JA, Kantarci K, Graff-Radford J, et al. Subtypes of dementia with Lewy bodies are associated with alpha-synuclein and tau distribution. Neurology. 2020;95(2):e155–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gomperts SN, Locascio JJ, Marquie M, Santarlasci AL, Rentz DM, Maye J, et al. Brain amyloid and cognition in Lewy body diseases. Mov Disorders: Official J Mov Disorder Soc. 2012;27(8):965–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kantarci K, Lowe VJ, Boeve BF, Senjem ML, Tosakulwong N, Lesnick TG, et al. AV-1451 tau and beta-amyloid positron emission tomography imaging in dementia with Lewy bodies. Ann Neurol. 2017;81(1):58–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Toledo JB, Arnold SE, Raible K, Brettschneider J, Xie SX, Grossman M, et al. Contribution of cerebrovascular disease in autopsy confirmed neurodegenerative disease cases in the National Alzheimer’s coordinating centre. Brain. 2013;136(Pt 9):2697–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boyle PA, Yu L, Wilson RS, Leurgans SE, Schneider JA, Bennett DA. Person-specific contribution of neuropathologies to cognitive loss in old age. Ann Neurol. 2018;83(1):74–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boyle PA, Yu L, Leurgans SE, Wilson RS, Brookmeyer R, Schneider JA, et al. Attributable risk of Alzheimer’s dementia attributed to age-related neuropathologies. Ann Neurol. 2019;85(1):114–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Palmqvist S, Rossi M, Hall S, Quadalti C, Mattsson-Carlgren N, Dellavalle S et al. Cognitive effects of lewy body pathology in clinically unimpaired individuals. Nat Med. 2023;29(8):1971–8. [DOI] [PMC free article] [PubMed]
- 31.Quadalti C, Palmqvist S, Hall S, Rossi M, Mammana A, Janelidze S et al. Clinical effects of lewy body pathology in cognitively impaired individuals. Nat Med. 2023;29(8):1964–70. [DOI] [PMC free article] [PubMed]
- 32.Rodriguez-Porcel F, Wyman-Chick KA, Abdelnour Ruiz C, Toledo JB, Ferreira D, Urwyler P, et al. Clinical outcome measures in dementia with Lewy bodies trials: critique and recommendations. Transl Neurodegener. 2022;11(1):24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Taylor JP, McKeith IG, Burn DJ, Boeve BF, Weintraub D, Bamford C, et al. New evidence on the management of Lewy body dementia. Lancet Neurol. 2020;19(2):157–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nelson PT, Jicha GA, Kryscio RJ, Abner EL, Schmitt FA, Cooper G, et al. Low sensitivity in clinical diagnoses of dementia with Lewy bodies. J Neurol. 2010;257(3):359–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sims JR, Zimmer JA, Evans CD, Lu M, Ardayfio P, Sparks J et al. Donanemab in early symptomatic alzheimer disease: the TRAILBLAZER-ALZ 2 randomized clinical trial. JAMA: J Am Med Association. 2023;330(6):512–27. [DOI] [PMC free article] [PubMed]
- 36.van de Beek M, van Steenoven I, van der Zande JJ, Barkhof F, Teunissen CE, van der Flier WM, et al. Prodromal dementia with Lewy bodies: clinical characterization and predictors of progression. Mov Disorders: Official J Mov Disorder Soc. 2020;35(5):859–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hamilton CA, Matthews FE, Donaghy PC, Taylor JP, O’Brien JT, Barnett N, et al. Cognitive decline in mild cognitive impairment with Lewy bodies or Alzheimer disease: a prospective cohort study. Am J Geriatric Psychiatry: Official J Am Association Geriatric Psychiatry. 2021;29(3):272–84. [DOI] [PubMed] [Google Scholar]
- 38.Hamilton CA, Matthews FE, Donaghy PC, Taylor JP, O’Brien JT, Barnett N, et al. Prospective predictors of decline v. stability in mild cognitive impairment with Lewy bodies or Alzheimer’s disease. Psychol Med. 2021;51(15):2590–8. [DOI] [PubMed] [Google Scholar]
- 39.Hamilton CA, Matthews FE, Donaghy PC, Taylor JP, O’Brien JT, Barnett N, et al. Progression to dementia in mild cognitive impairment with Lewy bodies or Alzheimer disease. Neurology. 2021;96(22):e2685–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van Dyck CH, Swanson CJ, Aisen P, Bateman RJ, Chen C, Gee M, et al. Lecanemab in early Alzheimer’s disease. N Engl J Med. 2023;388(1):9–21. [DOI] [PubMed] [Google Scholar]
- 41.Alam JJ, Maruff P, Doctrow SR, Chu HM, Conway J, Gomperts SN, et al. Association of plasma phosphorylated tau with the response to neflamapimod treatment in patients with dementia with Lewy bodies. Neurology. 2023;101(17):e1708–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Uchikado H, Lin WL, DeLucia MW, Dickson DW. Alzheimer disease with amygdala Lewy bodies: a distinct form of alpha-synucleinopathy. J Neuropathol Exp Neurol. 2006;65(7):685–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sorrentino ZA, Goodwin MS, Riffe CJ, Dhillon JS, Xia Y, Gorion KM, et al. Unique alpha-synuclein pathology within the amygdala in Lewy body dementia: implications for disease initiation and progression. Acta Neuropathol Commun. 2019;7(1):142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gawor K, Tomé S, Vandenberghe R, Van Damme P, Vandenbulcke M, Otto M et al. Amygdala-predominant α-synuclein pathology exacerbates hippocampal neuron loss in Alzheimer’s disease. bioRxiv. 2024:2024.06.21.599515. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Anonymized data can be requested from the NACC (https://naccdata.org/requesting-data/data-request-process).
Data used in this manuscript was obtained from the NACC database: https://naccdata.org/.