Abstract
Objectives
To examine the evidence for the efficacy and glucocorticoid sparing effect of oral anti-leukotrienes taken daily as add-on therapy to inhaled glucocorticoids in patients with asthma.
Design
Systematic review of randomised controlled trials of children and adults with asthma comparing the addition of anti-leukotrienes or placebo to inhaled glucocorticoids.
Main outcome measures
The rate of exacerbations of asthma requiring rescue systemic glucocorticoids when the intervention was compared to the same or double dose of inhaled glucocorticoids, and the glucocorticoid sparing effect when the intervention was aimed at tapering the glucocorticoid.
Results
Of 376 citations, 13 were included: 12 in adult patients and one in children. The addition of licensed doses of anti-leukotrienes to inhaled glucocorticoids resulted in a non-significant reduction in the risk of exacerbations requiring systemic steroids (two trials; relative risk 0.61, 95% confidence interval 0.36 to 1.05). No trials comparing the use of anti-leukotrienes with double the dose of inhaled glucocorticoids could be pooled. The use of anti-leukotrienes resulted in no overall group difference in the lowest achieved dose of inhaled glucocorticoids (three trials; weighted mean difference –44.43 μg/day, –147.87 to 59.02: random effect model) but was associated with a reduction in withdrawals owing to poor asthma control (four trials; relative risk 0.56, 0.35 to 0.89).
Conclusions
The addition of anti-leukotrienes to inhaled glucocorticoids may modestly improve asthma control compared with inhaled glucocorticoids alone but this strategy cannot be recommended as a substitute for increasing the dose of inhaled glucocorticoids. The addition of anti-leukotrienes is possibly associated with superior asthma control after tapering of glucocorticoids, but the glucocorticoids sparing effect cannot be quantified at present.
What is already known on this topic
Anti-leukotrienes are increasingly being used as add-on therapy to inhaled glucocorticoids
No systematic review of randomised controlled trials has examined the evidence to support this treatment strategy
What this study adds
There is a shortage of relevant trials testing anti-leukotrienes at licensed doses as add-on therapy to inhaled glucocorticoids in patients, particularly children
Introduction
Inhaled glucocorticoids are the cornerstone of asthma management because of their efficacy, tolerance, and relatively rapid onset of action compared with other anti-inflammatory drugs.1 When the control of asthma is poor, other drugs such as long acting ß2 agonists and anti-leukotrienes can be added to inhaled glucocorticoids.2–4 Anti-leukotrienes are a new class of anti-inflammatory drugs that interfere with the production of cysteinyl leukotrienes (5-lipoxygenase inhibitors) or the receptors (leukotriene receptors antagonists). Cysteinyl leukotrienes stimulate the production of airway secretions, cause microvascular leakage, and enhance eosinophilic migration in the airways.5 Thus the combination of anti-leukotrienes and inhaled glucocorticoids may enhance the control of asthma by reducing bronchoconstriction and inflammation of the airways.
The efficacy of anti-leukotrienes in the management of asthma has yet to be established. No firm recommendations about their use are made in current national guidelines for asthma.2–4 However, recent randomised controlled trials have provided evidence of the efficacy of anti-leukotrienes as add-on therapy to inhaled glucocorticoids in persistent asthma.6–9
We examined the safety and efficacy of oral anti-leukotrienes as add-on therapy to inhaled glucocorticoids in children and adults with asthma to quantify the improvement in asthma control achieved over inhaled steroids alone (at the same or double the dose) and the glucocorticoid sparing effect when inhaled steroids are tapered.
Methods
Identification of trials
We searched Medline, Embase, Cinahl, and Central (Cochrane controlled trials register) databases up to August 2001 using the following MeSH, full text, and keyword terms: [asthma, wheez*, or respiratory sounds] and [random*, trial, placebo*, comparative study, controlled study, double-blind, or single-blind] and [leukotriene*, anti-leukotriene*, or *lukast*] and [inhaled* and [steroid*, corticosteroid*, fluticasone*, triamcinolone*, dexa*, deca*, gluticasone, beclomethasone*, budesonide*, flunisolide, bronalide, flixotide, or triamcinolone]. We checked the references of all identified trials and review articles, and we searched the abstract books of the international meeting of the American Thoracic Society for 1998, 1999, and 2000. We contacted the international headquarters of pharmaceutical companies producing anti-leukotrienes to obtain or to identify unpublished trials.
Study selection
We included trials if they met the following criteria: they were randomised controlled trials, they pertained to children and adults with asthma who were taking inhaled glucocorticoids for maintenance, they compared the addition of anti-leukotrienes or placebo daily to inhaled glucocorticoids for a minimum of 28 days, and they documented measures of efficacy other than compliance. The primary outcome measures were the number of exacerbations of asthma requiring rescue systemic glucocorticoids when the intervention was compared with the same or an increased dose of inhaled glucocorticoids and the change from the baseline dose of inhaled glucocorticoids required to maintain control when the intervention was aimed to establish the steroid sparing effect. Secondary outcomes were changes in pulmonary function tests, symptoms, use of rescue ß2 agonists, quality of life, exacerbations requiring hospital admission, adverse effects, and withdrawals.
We reviewed each identified citation, and we obtained the full text of all definite or possible randomised controlled trials, irrespective of language. Trials were assessed independently by two reviewers (FMD and Giselle Hicks or Ritz Kakuma) and, if they met the inclusion criteria, the quality of the methods and data were extracted. The quality of the methods of each trial was assessed with the Jadad's instrument, which evaluates the reported quality of randomisation, blinding, and description of withdrawals and dropouts on a scale from 0 (worst) to 5 (best).10 The reviewers were blind to authors' names, affiliations, journals, date of publication, and sources of financial support. Disagreement was settled by consensus. Confirmation of the methods and data extraction of included trials was sought from the authors, the funding pharmaceutical companies, or both.
Statistical analyses
The trials were divided into three protocols, according to the relative dose of inhaled glucocorticoids used by the control group (same, double, tapered). The trials were further stratified by the anti-leukotriene used and its dosage. Treatment effects for dichotomous outcomes are reported as pooled relative risks with the fixed effect model or, in case of heterogeneity, the random effect model.11,12 We assumed equivalence if the relative risk estimate and its confidence interval were between 0.9 and 1.1. The weighted mean difference is reported for continuous outcomes with the same unit of measure; when an outcome was reported in different units we used the standardised mean difference, reported as standard deviations. We tested the homogeneity of effect sizes with the Dersimonian and Laird method, with P=0.10 as the cut-off point for significance; heterogeneity is reported when identified.12 Owing to low power for detecting heterogeneity with few trials, we reported any difference in the conclusion related to the choice of the fixed or random models, when observed. To detect possible biases, the funnel plot symmetry was examined for trials contributing data to the main outcomes.13 The mean daily dose of inhaled corticosteroids was converted in “μg of beclomethasone equivalent”, when 1 μg of beclomethasone dipropionate is equivalent to 1 μg of budesonide or 0.5 μg of fluticasone dipropionate or 2 μg of triamcinolone acetonide or 2 μg of flunisolide.3 All estimates are reported with 95% confidence intervals.
In addition to the dose and anti-leukotrienes used, five factors were a priori believed to potentially influence the magnitude or direction of treatment response: baseline dose of inhaled glucocorticoids, age, severity of the asthma, duration of anti-leukotriene use, and asthma triggers. Whenever possible, these factors were examined as possible sources of heterogeneity. For the primary outcome, sensitivity analyses were performed to determine the effect of publication status and quality of the methods on results. The meta-analysis was performed with MetaView, version 4.1 (Cochrane Review Manager, Cochrane Collaboration, Oxford).
Results
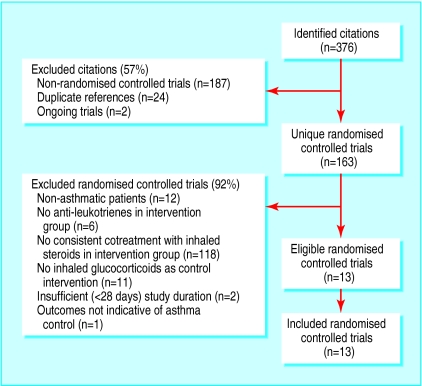
Of the 376 identified citations, 363 publications did not meet the inclusion criteria (fig 1). All available data from the remaining 13 trials (one study in children and 12 in adults; six unpublished as of August 2001) were included in the review (table 1). Within each protocol patients were relatively comparable across trials for age, sex, and severity of asthma at baseline, with the following exceptions. The protocol comparing the addition of anti-leukotriene to the same dose of inhaled glucocorticoids included the only identified trial in children.14 In this protocol, two trials abruptly reduced the dose of inhaled glucocorticoids at the time of randomisation to elicit poor control; although they reported normal spirometric values at baseline, the severity of the patients' asthma after randomisation was probably comparable to that of the other trials.7,15 In the second protocol, comparing anti-leukotriene use to a double dose of inhaled glucocorticoids, patients enrolled in one study had higher spirometric values than those enrolled in the other16,17; in both trials, patients were considered symptomatic on the basis of a high daytime symptom score and the use of rescue β2 agonists. The quality of methods of 10 trials was rated high (⩾4) and was confirmed by authors in all cases; in the remaining three trials allocation was not concealed (table 2).
Figure 1.
Flow diagram of trials selected for inclusion in review. Cochrane Library has list of excluded randomised controlled trials, with reason for ineligibility
Table 1.
Characteristics of included trials
| Trials | No of patients | Publication status | Industry sponsored trials | Mean age (years) | % male | Baseline FEV1 (mean % predicted) | Atopy | Dose optimisation before randomisation | Drug (dose) of anti- leukotrienes‡ | Inhaled glucocorticoids
|
Duration of treatment (weeks) | Intention to treat analyses | Reported outcomes
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Drug | Intervention group | Control group | Exacerbations requiring systemic steroids | Steroid dose reduction | |||||||||||||||
| Anti-leukotrienes versus placebo as add-on therapy to inhaled glucocorticoids | |||||||||||||||||||
| Laviolette et al9 | 393 | Yes | Merck | 40 | 54 | 72 | 75 | Montelukast (10 mg once daily) | Beclomethasone diproprionate | 400 μg | 400 μg | 16 | Yes | No | |||||
| Simons et al14† |
279 | Yes | Merck | 10 | 67 | 78 | 72 | Montelukast (5 mg once daily) | Budesonide | 400 μg | 400 μg | 4 | Yes | Yes | |||||
| Tamaoki et al7 | 79 | Yes | No | 48 | 43 | 80* | Not reported | Pranlukast (450 mg twice daily) | Beclomethasone diproprionate | 750 μg | 750 μg | 6 | Not reported | Yes | |||||
| Tomita et al18 | 41 | Yes | Not reported | 50 | 60 | 88* | 66 | Pranlukast (450 mg once daily) | Beclomethasone diproprionate | 400 μg | 400 μg | 8 | Not reported | No | |||||
| Virchow et al8 | 368 | Yes | Astra-Zeneca | 48 | 51 | 64 | 46 | Zafirlukast (80 mg twice daily) | Beclomethasone diproprionate | 1598 ± 381 μg | 1650 ± 456 μg | 6 | Yes | Yes | |||||
| Wada et al15 | 80 | Yes | No | 50 | 50 | 74 | 53 | Pranlukast (225 mg twice daily) | Beclomethasone diproprionate | 1048 ± 237 μg | 1127 ± 307 μg | 4 | Not reported | No | |||||
| Anti-leukotrienes as add-on therapy to inhaled glucocorticoids versus double dose of inhaled glucocorticoids | |||||||||||||||||||
| Nayak et al17 | 394 | No | Astra-Zeneca | 39 | 38 | 67 | 94 | Zafirlukast (40 mg or 80 mg twice daily) | Beclomethasone diproprionate | 400 μg | 800 μg | 13 | No | Yes | |||||
| Ringdal et al16 | 440 | No | Astra-Zeneca | 41 | 49 | 85 | Not reported | Zafirlukast (20 mg or 80 mg twice daily) | Beclomethasone diproprionate | 400-500 μg | 800-1000 μg | 12 | No | Yes | |||||
| Anti-leukotrienes versus placebo as add-on therapy to tapering doses of inhaled glucocorticoids | |||||||||||||||||||
| Baba et al21 | 24 | No | Not reported | Not reported | Not reported | Not reported |
Not reported | Not reported | Pranlukast (not reported) | Beclomethasone diproprionate | Not reported | Not reported | Not reported | Not reported | No | No | |||
| Bateman et al19 | 359 | No | Astra Zeneca | 42 | 45 | 2.6L | 48 | 0 | Zafirlukast (20 mg twice daily) | Beclomethasone diproprionate or Budesonide | 400-750 μg | 400-750 μg | 20 | Yes | No | Yes | |||
| Laitinen et al20 | 262 | No | Astra Zeneca | 44 | 42 | 2.5L | 43 | 2 weeks to 3 months | Zafirlukast (20 mg twice daily) | Beclomethasone diproprionate or Budesonide | 800-2000 μg | 800-2000 μg | 12 | No | No | Yes | |||
| Lofdahl et al6 | 226 | Yes | Merck | 40 | 43 | 83 | Not reported | ⩽7 weeks | Montelukast (10 mg once daily) | Various‡ | 300-3000 μg | 300-3000 μg | 12 | Yes | No | Yes | |||
| Shingo et al22 | 22 | No | Merck | 39 | 41 | 84 | Not reported | 0 | Montelukast (10 mg once daily) | Various§ | 1600 μg | 1350 μg | 8 | Yes | Yes | No | |||
FEV1=forced expiratory volume in one second. Anti-leukotriene licensed doses for adults: Montelukast: 10 mg once daily (5 mg for children aged 5 to 14 years), Pranlukast 225 mg twice daily, Zafirlukast 20 mg twice daily. No trial reported use of inhaled glucocorticoids propelled by hydrofluorocarbons. *Reported spirometry before abrupt reduction by half of maintenance dose of inhaled glucocorticoids.† Cross over study. ‡Reported use of beclomethasone dipropionate (16%), budesonide (22%), flunisolide(15%), fluticasone propionate (7%), and triamcinolone acetonide (40%). Corticosteroids dose reduction not been provided in μg of “chlorofluorocarbon propelled beclomethasone diproprionate equivalent” (T R Reiss, personal communication, 2000). §Reported use of triamcinolone acetonide (72%), flunisolide (18%), and beclomethasone dipropionate (9%).
Table 2.
Methodological quality of included trials
| Trials
|
Randomisation*
|
Blinding†
|
Withdrawals or dropouts‡
|
Jadad's quality score10
|
Methods confirmed
|
Withdrawals (%)
|
|
|---|---|---|---|---|---|---|---|
| Intervention group | Control group | ||||||
| Anti-leukotrienes versus placebo as add-on therapy to inhaled glucocorticoids | |||||||
| Laviolette et al9 | 2 | 2 | 1 | 5 | Yes | 8 | 11 |
| Simons et al14 | 2 | 2 | 0 | 4 | Yes | 3 | 2 |
| Tamaoki et al7 | 1 | 2 | 1 | 4 | No | 2 | 8 |
| Tomita et al18 | 1 | 0 | 0 | 1 | No | Not reported | Not reported |
| Virchow et al8 | 2 | 2 | 1 | 5 | Yes | 18 | 18 |
| Wada et al15 | 1 | 0 | 1 | 2 | No | 8 | 18 |
| Anti-leukotrienes as add-on therapy to inhaled glucocorticoids versus double dose of inhaled glucocorticoids | |||||||
| Nayak et al17 | 2 | 2 | 1 | 5 | Yes | 14 | 16 |
| Ringdal et al16 | 2 | 2 | 1 | 5 | Yes | 11 | 6 |
| Anti-leukotrienes versus placebo as add-on therapy to tapering doses of inhaled glucocorticoids | |||||||
| Baba et al21 | 1 | 0 | 0 | 1 | No | Not reported | Not reported |
| Bateman et al19 | 2 | 2 | 1 | 5 | Yes | 20 | 22 |
| Laitinen et al20 | 2 | 2 | 1 | 5 | Yes | 15 | 14 |
| Lofdahl et al6 | 2 | 2 | 1 | 5 | Yes | 15 | 27 |
| Shingo et al22 | 2 | 2 | 1 | 5 | Yes | 10 | 8 |
2=randomised, appropriate method; 1=randomised, method not described; 0=not randomised.†2=double blind, blinding appropriate; 1=double blind, blinding not reported; 0=no blinding.‡1=described by group; 0=not described by group.
Anti-leukotrienes versus placebo as add-on therapy to inhaled glucocorticoids
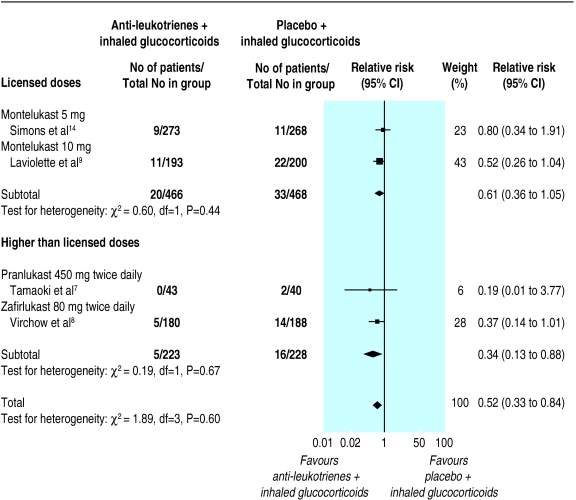
Although four7–9,14 of the six15,18 identified trials contributed data to the primary outcome, only two tested anti-leukotrienes (montelukast; Singulair, Merck Frosst) at licensed doses.9,14 With the addition of licensed doses of anti-leukotrienes to glucocorticoids, a non-significant reduction in the risk of exacerbations requiring systemic steroids was observed (relative risk 0.61, 95% confidence interval 0.36 to 1.05). The only paediatric trial did not show any significant group difference. When higher doses were examined, the addition of pranlukast (Ono, Japan), or zafirlukast (Accolate, Astra Zeneca) reduced the risk of exacerbations requiring systemic steroids by 66% (relative risk 0.34, 0.13 to 0.88) (fig 2). The number needed to treat was 20 (11 to 100). Within each stratum the results were homogeneous despite the different doses and anti-leukotrienes tested, age, baseline dose of inhaled glucocorticoids, and duration of anti-leukotriene use. No evidence was found of systematic bias identified by the measure of funnel plot asymmetry (intercept 0.17, –3.22 to 3.55).
Figure 2.
Anti-leukotrienes versus placebo as add-on therapy to inhaled glucocorticoids
Pooling of the two trials testing the use of licensed doses of montelukast for four or 16 weeks showed significant but modest group differences in favour of anti-leukotrienes in the change from baseline in morning peak expiratory flow rate (weighted mean difference 7.71 l/min, 2.98 to 12.44), use of ß2 agonists (–0.32 puffs/day, –0.56 to –0.08), and eosinophil counts (–0.07 × 109/l, –0.14 to 0.00; random effect model).9,14 No significant group difference was observed in the change in forced expiratory volume in one second (0.07 litres, –0.01 to 0.16) or in the risk of overall withdrawals (relative risk 0.91, 0.54 to 1.53), withdrawals owing to adverse effects (0.65, 0.26 to 1.66), increased liver enzyme concentrations (1.02, 0.36 to 2.88), headache (1.16, 0.86 to 1.57), and nausea (0.45, 0.19 to 1.07). No death was reported.
Pooling of the two trials of higher than licensed doses of pranlukast or zafirlukast for six weeks showed a significant group difference favouring the addition of anti-leukotrienes to inhaled corticosteroids. This was shown in the magnitude of improvement from baseline in forced expiratory volume in one second (weighted mean difference 0.10 litres; 0.01 to 0.20), peak expiratory flow (27.2 l/min, 18.6 to 35.8), use of rescue β2 agonists (–0.43, –0.22 to –0.63), and asthma symptoms (standardised mean difference –0.46, –0.25 to –0.66).7,8 No group difference in overall adverse events or nausea was observed; insufficient number of trials prevented pooling of data for other adverse effects.
Anti-leukotrienes as add-on therapy to inhaled glucocorticoids versus double dose inhaled glucocorticoids
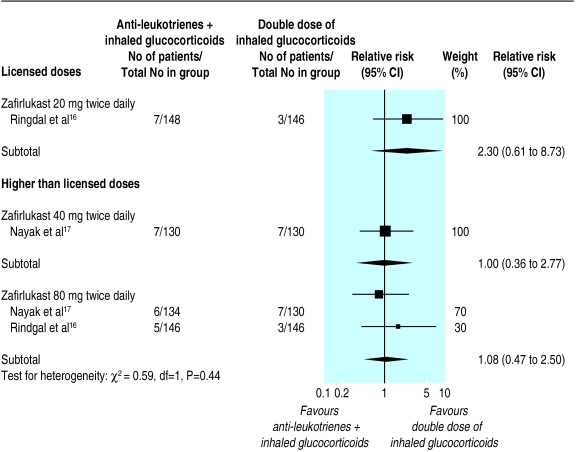
The data from two unpublished trials, each testing two different doses of zafirlukast, were analysed.16,17 Pooling of data was only possible for zafirlukast at four times the licensed dose. No apparent group difference was found in the risk of an exacerbation requiring systemic steroids after 12 weeks of treatment with zafirlukast 80 mg twice daily (relative risk 1.08, 0.47 to 2.50); thewidth of this confidence interval exceeded our definition of equivalence (fig 3). The small number of trials prevented subgroup and sensitivity analyses.
Figure 3.
Anti-leukotrienes as add-on therapy to inhaled glucocorticoids versus double dose of inhaled glucocorticoids. There was no aggregation of data across different doses of zafirlukast
No group difference was found in secondary outcomes, including change from baseline forced expiratory volume in one second, peak expiratory flow, symptom score, use of short acting β2 agonists, withdrawal due to poor asthma control, or hospital admission. Zafirlukast (80 mg twice daily) was associated with an increased risk of increased liver enzyme concentrations (5.36, 1.40 to 20.44) and of withdrawal due to adverse events (2.77, 1.02 to 7.58)—that is, 1 in every 25 (95% confidence interval 14 to 100) patients and 1 in every 33 (16 to ∞) patients treated with high dose zafirlukast would have an increase in liver enzyme concentrations and withdrawals due to adverse events, respectively. In contrast, a double dose of beclomethasone was associated with a higher risk of oral moniliasis compared with anti-leukotrienes (7.1, 1.3 to 33; number needed to harm 33, 17 to 100).
Anti-leukotrienes versus placebo as add-on therapy to tapered doses of inhaled glucocorticoids
The data from four of the five identified trials testing licensed doses of anti-leukotrienes were provided in sufficient detail to be analysed.6,19–21 The assessment of the glucocorticoid sparing effect of anti-leukotrienes depends on showing adequate and comparable control of asthma between the intervention and control groups after tapering. After 12 weeks of treatment, two trials of zafirlukast reported no significant group difference in final mean symptom scores and use of ß2 agonists.19,20 Trends approaching significance favouring the intervention were observed in the final forced expiratory volume in one second (weighted mean difference 0.12 litres,–0.02 to 0.27) and final peak expiratory flow (14.47 l/min, –4.54 to 33.48). Two trials testing montelukast failed to report sufficient data to confirm comparable asthma control after steroid tapering.6,22 Pooling of the four trials showed a noticeable reduction (relative risk 0.56, 0.35 to 0.89) in the rate of withdrawal owing to poor asthma control in the group treated with anti-leukotrienes, suggesting better asthma control with the combination therapy.
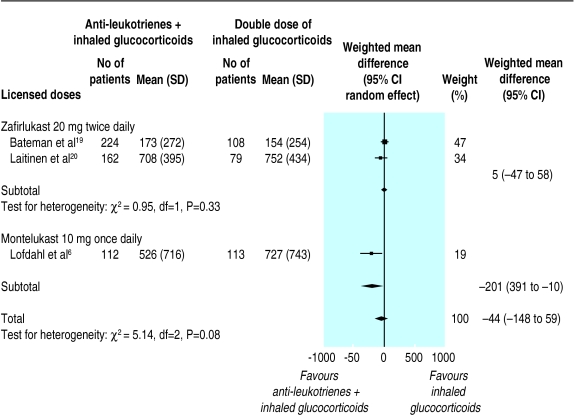
After 12 to 20 weeks of treatment, no overall group difference was observed in change from the baseline dose of inhaled glucocorticoid required to maintain asthma control (three trials; weighted mean difference 1.87%, –3.52 to 7.27). When the lowest tolerated dose of inhaled glucocorticoids was considered, no meaningful group difference was observed either (–44.43 μg/day, –147.87 to 59.02; random effect model) (fig 4). Heterogeneity was apparent among trials: the two trials of zafirlukast reported no group difference whereas the trial of montelukast reported a significant reduction of 200 μg/day in favour of anti-leukotrienes. Based on the relative potency and distribution of inhaled steroids used in the montelukast trial (table 1), the 200 μg/day would translate to an approximate corticosteroid sparing effect of 160 μg/day of beclomethasone equivalent.3 The small number of trials and their design prevented the clear attribution of the source of heterogeneity; trials differed not only in the dose and anti-leukotriene used but also in the baseline dose and inhaled glucocorticoid used, dose optimisation period, weaning protocol, and intention to treat analysis. The rate of complete glucocorticoid weaning was similar between groups (three trials, relative risk 1.18, 0.95 to 1.47).
Figure 4.
Anti-leukotrienes versus placebo with tapering doses of inhaled glucocorticoids
No group difference was found in the number of overall withdrawals, withdrawals owing to adverse effects (relative risk 1.07, 0.57 to 2.03), increased liver enzyme concentrations (2.13, 0.80 to 5.68), headache (0.90, 0.64 to 1.26), or nausea (1.14, 0.49 to 2.67). The similarity between groups in the number of overall adverse effects met the review's definition of equivalence (four trials; 0.98, 0.91 to 1.05). A significantly increased risk of serious adverse events as defined by the criteria of the Federal Drug Administration was associated with zafirlukast at licensed doses (2.47, 1.53 to 3.97).19,20,23 No death was reported.
Discussion
Asthma control
The addition of licensed doses of anti-leukotrienes to inhaled glucocorticoids results in a non-significant reduction in the risk of exacerbations of asthma requiring systemic glucocorticoids, with modest group differences in peak expiratory flow, use of ß2 agonists, and eosinophil counts in favour of anti-leukotrienes. A beneficial effect of anti-leukotrienes was more apparent with higher doses than with licensed doses of pranlukast or zafirlukast, when a 66% reduction in exacerbations requiring rescue glucocorticoids was documented. No statistical heterogeneity was observed despite variation in the dose and anti-leukotrienes used, dose of beclomethasone (750-2000 μg/day), age, duration of treatment, and intention to treat analysis. Evidence thus suggests a modest effect of licensed doses of montelukast as add-on therapy to inhaled glucocorticoids in children and adults with asthma.
In symptomatic patients, however, most doctors would consider an alternative to the current treatment; many would increase the dose of inhaled glucocorticoids or consider additional therapy. Surprisingly, only two 12 week trials, both unpublished, compared the combination of zafirlukast (20, 40, or 80 mg twice daily) and low doses of beclomethasone (400-500 μg/day) with a double dose of inhaled glucocorticoids.16,17 With only one trial considering zafirlukast at the licensed dose, no pooling of data was possible. The effects of adding zafirlukast at four times the licensed dose were similar (although they did not meet the review's equivalence criteria) to increasing the dose of beclomethasone by 400-500 μg/day with respect to risk of exacerbations requiring systemic steroids, lung function, symptoms, and night waking. With no identified trials testing anti-leukotrienes at the licensed dose, the efficacy of adding these drugs to current therapy compared with modestly increasing the dose of inhaled glucocorticoids has yet to be determined.
Glucocorticoid sparing effect
Also of interest is whether the addition of anti-leukotrienes allows a meaningful reduction in the dose of inhaled glucocorticoids required to maintain control. Data from four trials of high quality methods, all using licensed doses of anti-leukotrienes, provided some answers.6,19,20,22 In adults well controlled on various doses (300-3000 μg/day) of inhaled glucocorticoids, treatment for 12 weeks combining oral anti-leukotrienes with inhaled steroids daily did not reduce the dose of inhaled steroids any more than did placebo.6,19,20 No significant group differences were observed in the lowest tolerated dose of inhaled steroids or the proportion of patients with complete steroid withdrawal. Pooling of trials resulted in statistical heterogeneity only with the lowest tolerated dose of inhaled glucocorticoids: the trial testing montelukast was associated with a significant additional reduction of 200 μg/day of inhaled glucocorticoids (or 160 μg/day of chlorofluorocarbon propelled beclomethasone equivalent), whereas the two trials testing zafirlukast showed no group difference.
To establish the overall or relative efficacy of anti-leukotrienes, the level of asthma control achieved after glucocorticoid tapering must be similar among groups. In fact, patients treated with anti-leukotrienes seemed to have better control than those given placebo, with a significant 44% reduction in withdrawal owing to poor asthma control and trends towards a modestly higher forced expiratory volume in one second and peak expiratory flow after tapering. Possible explanations for the heterogeneity include not only the anti-leukotriene used but also trial specific designs likely to influence the level of asthma control after tapering such as dose optimisation period before randomisation, tapering protocols, baseline dose of inhaled glucocorticoids, and intention to treat analysis; the small number of trials could not permit the precise identification of the source of heterogeneity. A longer tapering period, for example, might have permitted greater reduction in the dose of inhaled steroids or shown better asthma control in favour of anti-leukotrienes. Although there are insufficient data to make a firm conclusion, based on the upper confidence limit for either anti-leukotriene used (fig 4) the maximal glucocorticoid sparing effect of anti-leukotrienes would probably be less than 400 μg/day. This agrees with a previous systematic review showing that the use of anti-leukotrienes as a single agent is less effective than using 400 μg/day of beclomethasone.24 In one trial inhaled steroids were tapered by 500-600 μg/day in both the intervention and control groups before randomisation, and the placebo group achieved a similar reduction after randomisation.6 Clearly, the magnitude of reduction of glucocorticoid dose achievable without any other treatment outweighs that shown to date with anti-leukotrienes.
Adverse effects
Montelukast at licensed doses was not associated with increased adverse effects. The 2.5-fold increased risk of serious adverse events, noted only in the tapering protocol in association with licensed doses of zafirlukast, raises concerns. Although the definition of serious events included those resulting in major disability, admission to hospital or prolongation of hospital stay, life threatening reaction, or death, the observed events were often linked with increased concentrations of liver enzymes prompting withdrawals (C Miller, personal communication, 2000). The fivefold increased risk of liver enzyme concentrations being increased and threefold increased risk of withdrawals owing to adverse events noted with higher than licensed doses of zafirlukast plead against using these drugs beyond the recommended doses. Other than the expected increased risk of oral moniliasis with double doses of inhaled steroids, no trials have examined important adverse effects associated with the prolonged use of inhaled glucocorticoids, such as osteopenia, adrenal suppression, and growth suppression in children; such documentation would have permitted a fairer comparison between the safety profile of the two treatments.
Strengths and limitations of the review
As with all systematic reviews, this meta-analysis is limited by the quantity and quality of existing data. Despite the abundance of literature on anti-leukotrienes, only 8% of randomised controlled trials were designed to assess the role of anti-leukotrienes as add-on therapy to inhaled glucocorticoids; most of the excluded trials compared anti-leukotrienes with placebo in groups of patients comprised of, or including, those naïve to steroids. Fortunately most (10 of 13) relevant trials were of high quality. Trials with low quality methods did not contribute to the review owing to insufficient reporting and thus could not bias the conclusions.15,18,22 Publication bias was evident; of the five trials involved in the glucocorticoid tapering protocol, only the one in favour of anti-leukotrienes is published6; exclusion of unpublished trials would have possibly led to an overestimation of the true glucocorticoid sparing effect of anti-leukotrienes. A thorough systematic search resulted in the identification of unpublished trials of high quality methods, increasing the power and scope of the review.25 The value of this review is strengthened by the direct confirmation of methods and extracted data from the authors or sponsors of nine of the 13 trials and the voluntary disclosure of data for five unpublished trials.16,17,19,20,22 Because the number and size of studies pooled under each protocol were small, the robustness of analyses of different inhaled glucocorticoids and anti-leukotrienes, doses, age, duration of intervention, and lung function could not be assessed. Clearly, these preliminary conclusions may be modified with accumulating data from future well designed, parallel group, long term (>20 weeks) randomised controlled trials; a prolonged (>16 weeks) dose optimisation period before randomisation and intention to treat analysis are key design features to clarify the glucocorticoid sparing effect of anti-leukotrienes. Updates of this review will be available in the Cochrane Library.
Conclusions
This systematic review summarises the best evidence available until August 2001 and emphasises the shortage of relevant trials testing the role of licensed doses of anti-leukotrienes as add-on therapy to inhaled glucocorticoids. Although no firm conclusion can be made, the addition of licensed doses of anti-leukotrienes to inhaled glucocorticoids may modestly improve the control of asthma, but there is little evidence to consider their use as a substitute to increasing the dose of inhaled glucocorticoids. In well controlled patients, the addition of anti-leukotrienes is possibly associated with superior asthma control after glucocorticoid tapering, but there is insufficient evidence to quantify the corticosteroid sparing effect. With only one paediatric trial showing little benefit, extrapolation of data to children remains speculative. Until further evidence is available, the gold standard of asthma treatment should remain the use of inhaled glucocorticoids at the lowest effective dose.
Acknowledgments
We thank Giselle Hicks and Ritz Kakuma for helping in the identification of eligible trials, assessment of methods and data extraction, and data entry, Christopher Miller and Susan Shaffer (Astra-Zeneca, USA) and Theodore F Reiss and G P Noonan (Merck Frosst, USA) for confirmation of methods and data extraction and providing data whenever possible, Toby Lasserson and Karen Blackhall (Cochrane Airways Review Group) for the literature search and ongoing support, Christopher Cates and Paul Jones for their constructive comments, and Keiji Hayashi for translating the Japanese articles.
Footnotes
Funding: FMD was supported by a salary award of the Fonds de la Recherche en Santé du Québec. Ritz Kakuma was supported by the Canadian Cochrane Network.
Competing interests: FMD has received travel support, research funds, and fees for speaking from both Zeneca Pharma, producer of zafirlukast, and from Merck Frosst, producer of montelukast. She has received some travel support for attending meetings, a research grant, and consulting fee from Glaxo Wellcome, producer of some inhaled corticosteroids preparations to which anti-leukotriene agents have been compared.
References
- 1.Spahn JD, Leung DYM. The role of glucocorticoids in the management of asthma. Allergy Asthma Proc. 1996;17:341–350. doi: 10.2500/108854196778606365. [DOI] [PubMed] [Google Scholar]
- 2.The British guidelines on asthma management. Thorax. 1997;52:1–21S. [Google Scholar]
- 3.Murphy S, Sheffer AL, Pauwels R. National asthma education and prevention program. Expert panel report 2: guidelines for the diagnosis and management of asthma. National Institutes of Health publication 97-4051. Bethesda, MD: National Heart, Lung and Blood Institute; 1997. [Google Scholar]
- 4.Boulet LP, Becker A, Berube D, Beveridge R, Ernst P. Canadian asthma consensus report, 1999. Can Med Assoc J. 1999;161:1–72S. [PMC free article] [PubMed] [Google Scholar]
- 5.Piper PJ. Leukotrienes and the airways. Eur J Anaesthesiol. 1989;6:241–255. [PubMed] [Google Scholar]
- 6.Lofdahl CG, Reiss TF, Leff JA, Israel E, Noonan MJ, Finn AF, et al. Randomised, placebo controlled trial of effect of a leukotriene receptor antagonist, montelukast, on tapering inhaled corticosteroids in asthmatic patients. BMJ. 1999;319:87–90. doi: 10.1136/bmj.319.7202.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tamaoki J, Kondo M, Sakai N, Nakata J, Takemura H, Nagai A, et al. Leukotriene antagonist prevents exacerbation of asthma during reduction of high-dose inhaled corticosteroid. The Tokyo Joshi-Idai Asthma Research Group. Am J Respir Crit Care Med. 1997;155:1235–1240. doi: 10.1164/ajrccm.155.4.9105060. [DOI] [PubMed] [Google Scholar]
- 8.Virchow JC, Hassall SM, Summerton L, Harris A. Zafirlukast improves asthma control in patients receiving high-dose inhaled corticosteroids. Am J Respir Crit Care Med. 1997;156:578–585. doi: 10.1164/ajrccm.162.2.9905041. [DOI] [PubMed] [Google Scholar]
- 9.Laviolette M, Malmstrom K, Lu S, Chervinsky P, Pujet JC, Peszek I, et al. Montelukast added to inhaled beclomethasone in treatment of asthma. Am J Respir Crit Care Med. 1999;160:1862–1868. doi: 10.1164/ajrccm.160.6.9803042. [DOI] [PubMed] [Google Scholar]
- 10.Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized controlled trials: is blinding necessary? Control Clin Trials. 1995;134:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 11.Greenland S, Robins JM. Estimation of a common effect parameter from sparse follow-up data. Biometrics. 1985;41:55–68. [PubMed] [Google Scholar]
- 12.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 13.Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simons FER, Villa JR, Lee BW, Teper AM, Lyttle B, Aristizabal G, et al. Montelukast added to budesonide in children with persistent asthma: a randomised, double-blind, crossover study. J Pediatr. 2001;138:694–698. doi: 10.1067/mpd.2001.112899. [DOI] [PubMed] [Google Scholar]
- 15.Wada K, Minoguchi K, Kohno Y, Oda N, Matsuura T, Kawazu K, et al. Effect of a leukotriene receptor antagonist, pranlukast hydrate, on airway inflammation and airway hyperresponsiveness in patients with moderate to severe asthma. Allergol Int. 2000;49:63–68. [Google Scholar]
- 16.Ringdal N, White M, Harris A. Addition of zafirlukast (Accolate) compared with a double-dose of inhaled corticosteroids in patients with reversible airways obstruction symptomatic on inhaled corticosteroids. Am J Respir Crit Care Med. 2000;159(3 of part 2):639. . [Abstract.] [Google Scholar]
- 17.Nayak AS, Anderson P, Charous BL, Williams K, Simonson S. Equivalence of adding zafirlukast versus double-dose inhaled corticosteroids in asthmatic patients symptomatic on low-dose inhaled corticosteroids. J Allergy Clin Immunol. 1998;101(1 of part 2):S233. . [Abstract 965.] [Google Scholar]
- 18.Tomita T, Hashimoto K, Matsumoto S, Nakamoto T, Tokuyasu H, Yamasaki A, et al. Pranlukast allows reduction of inhaled steroid dose without deterioration in lung function in adult asthmatics. Arerugi. 1999;48:459–465. [PubMed] [Google Scholar]
- 19.Bateman ED, Holgate ST, Binks SM, Tarna IP. A multicentre study to assess the steroid-sparing potential of Accolate. Allergy. 1995;50(suppl 26):320. . [Abstract P-0709.] [Google Scholar]
- 20.Laitinen LA, Zetterstrom O, Holgate ST, Binks S, Whitney JG. Effects of Accolate in permitting reduced therapy with inhaled steroids: a multicenter trial in patients with doses of inhaled steroids optimised between 800 and 2000 mcg per day. Allergy. 1995;50(suppl 26):320. . [Abstract P-0710.] [Google Scholar]
- 21.Baba K, Hattori T, Sakakibara A, Kobayashi T, Takagi K. The usefulness of pranlukast or seratrodast for step-down of inhaled corticosteroid therapy in adult chronic asthma. Am Rev Resp Crit Care Med. 1999;159:A626. [Google Scholar]
- 22. Shingo S, Zhang J, Noonan N, Reiss TF, Leff JA. A standardized composite clinical score allows safe tapering of inhaled corticosteroids in an asthma clinical trial. [Theodore Reiss, personal communication, 2001.]
- 23. Anon. Code of Federal Regulations. US Government Printing Office via GPO Access. Title 21,volume 5; section 312.32 IND safety reports 2001, 69-71. www.fda.gov/cder/regulatory (accessed Aug 2001.)
- 24.Ducharme FM, Hicks G. Cochrane Library, Issue 2. Oxford: Update Software; 2001. Anti-leukotriene agents compared to inhaled corticosteroids in the management of recurrent and/or chronic asthma. [Google Scholar]
- 25.Thornton A, Lee P. Publication bias in meta-analysis: its causes and consequences. J Clin Epidemiol. 2000;53:207–216. doi: 10.1016/s0895-4356(99)00161-4. [DOI] [PubMed] [Google Scholar]