Abstract
The indicators for rapid assessment of the severity of acute cholangitis remain highly debated. Therefore, this study aimed to evaluate the efficacy of various inflammatory and immune-nutritional markers in predicting the severity of acute cholangitis. The prognostic roles of the following markers were investigated: Systemic Immune-Inflammatory Index (SII), Neutrophil-to-Lymphocyte Ratio (NLR), Platelet-to-Lymphocyte Ratio (PLR), Albumin (Alb), and Prognostic Nutritional Index (PNI). A total of 139 patients with acute cholangitis were included in the study. The inflammatory and immune-nutritional markers with better predictive efficacy were selected to construct a combined predictive score. According to the survival ROC curve analysis, the combined NLR and PNI score, termed PNS, demonstrated the best prognostic performance with an AUC of 0.853. Multivariable survival analysis identified the following independent prognostic factors: PNS (p = 0.010) and Prothrombin Time (PT) (p = 0.003). The results indicate that PNS = 2 is associated with a higher incidence of severe cholangitis.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12876-024-03560-w.
Keywords: Acute cholangitis, Neutrophil-to-Lymphocyte Ratio, Prognostic Nutritional Index
Introduction
Acute cholangitis, a severe inflammatory condition of the bile ducts, arises from biliary obstruction, leading to bile stasis and subsequent infection [1]. This obstruction elevates intraductal pressure, facilitating the translocation of bacteria or endotoxins from the bile into the systemic circulation [2]. The clinical presentation of acute cholangitis spans a broad spectrum, from mild cases amenable to conservative management to severe instances culminating in septic shock [3].Clinicians generally recognize the favorable prognosis associated with mild acute cholangitis. However, the stakes are high with severe forms of the disease; delayed recognition and differentiation can escalate mortality rates and precipitate serious complications. This underscores the necessity for prompt and accurate diagnosis, followed by appropriate therapeutic interventions.While emergency intervention is a cornerstone in the management of acute cholangitis, it is neither universally necessary nor always practical [4]. A robust predictive scoring system becomes indispensable, empowering clinicians to stratify patients based on prognosis and ensuring vigilant monitoring for those at high risk in an intensive care setting. The stratification also addresses the variability in medical center capabilities. Not all facilities have the technical prowess or resources to perform emergency Endoscopic Retrograde Cholangiopancreatography (ERCP) [5]. Early identification of patients unlikely to benefit from pharmacological therapy allows for their expedited referral to tertiary centers equipped for advanced interventions. This proactive approach minimizes the risk of mortality and morbidity associated with clinical deterioration and transfer delays.
The evaluation of inflammatory disease severity holds pivotal importance in medicine. It informs treatment decisions, guides resource allocation, aids in prognosis evaluation, and is crucial for disease monitoring. Accurate assessment is the linchpin that ensures optimal patient care and management, reflecting a commitment to precision in the medical field.In the quest to refine patient care, major medical centers globally have pioneered a variety of disease severity scoring systems. These tools, designed to gauge the gravity of medical conditions, have become integral to clinical decision-making. Notably, systems like APACHE II and III, ARDS score, MODS score, GCS (Glasgow Coma Scale), and IP-10 have gained prominence [6–10]. Their implementation has been pivotal in curbing the morbidity and mortality rates tied to the diseases they monitor, marking a significant leap forward in medical practice.
In recent years, a multitude of inflammatory markers such as NLR (Neutrophil-to-Lymphocyte Ratio), PLR, SII, and IL-6 have emerged to predict the progression of inflammatory diseases and cancer. Among these, NLR has been extensively studied for its role in inflammation. Research suggests that NLR offers better sensitivity than total white blood cell count (WBC) for the diagnosis and stratification of systemic infections, sepsis, and bacteremia [11–14]. NLR also holds predictive value for the severity of acute pancreatitis and cholecystitis [14]. However, studies exploring the correlation between NLR and the severity of acute cholangitis are scarce. In this article, we will evaluate the utility of NLR in predicting the severity of acute cholangitis.
Inflammation, inherently catabolic, is intricately linked to the body’s nutritional status. Nutritional markers are pivotal across various domains, including health management, disease prevention, treatment, and clinical nutrition. Notably, indices like the Prognostic Nutritional Index (PNI), Nutritional Risk Index (NRI), NUTRIC score, CONUT score, and ALB score have emerged as vital tools for medical professionals to evaluate patients’ nutritional and immunological health. These markers consider serum albumin levels, lymphocyte counts, and weight fluctuations, aiding in crafting tailored nutritional interventions that enhance patient care and disease management.The PNI, in particular, stands out as a widely recognized and predictive measure of nutritional status, offering insights into a patient’s nourishment and immune capabilities. It is instrumental in forecasting postoperative outcomes and mortality risks. Calculated from serum albumin and peripheral blood lymphocyte count, the PNI serves as a barometer for the immune-nutritional health of individuals with gastrointestinal diseases. Retrospective studies endorse the PNI’s efficacy in gauging the severity of acute pancreatitis, with lower scores correlating with adverse prognoses and elevated mortality rates.Current research on the PNI’s role in cholangitis predominantly revolves around its therapeutic and management implications. Investigations into the effectiveness of Elafibranor in treating primary biliary cholangitis and discussions on the latest strides in managing primary sclerosing cholangitis are examples of such studies. Yet, the correlation between PNI fluctuations and the severity of acute cholangitis remains underreported, signaling a gap ripe for exploration.
In our retrospective clinical study, we have innovated a predictive scoring system that synergizes multiple inflammatory markers with nutritional indices. This system empowers clinicians to swiftly conduct personalized risk assessments for individuals presenting with acute cholangitis. By enhancing patient management and expediting clinical interventions, it serves as a critical tool for the immediate identification of severe cholangitis cases, ensuring prompt triage and medical treatment. The deployment of this scoring system is pivotal in halting disease progression and elevating patient outcomes.
Clinical data and methods
Study population and data collection
Study site and setting
This study was conducted at Shanxi Bethune Hospital, a tertiary grade-A hospital located in Taiyuan City, Shanxi Province. The hospital serves as a major referral center, with patients accessing treatment through two primary routes: local residents seeking care via outpatient services or hospitalization, and out-of-town patients typically referred by other hospitals for specialized care. Acute cholangitis patients are first assessed at the emergency department, where they are screened and admitted if they meet diagnostic criteria. Those requiring specialized care are referred to the Hepatobiliary and Pancreatic Surgery Department, where the treatment of cholangitis is primarily managed. This department has extensive experience in diagnosing and treating acute cholangitis, providing a comprehensive range of treatment options.
Study population
This study retrospectively analyzed the clinical data of acute cholangitis patients who visited Shanxi Bethune Hospital and met the TG18 diagnostic criteria between January 2014 and January 2024.The inclusion criteria were: 1) Adults aged ≥ 18 years, admitted within 72 h of symptom onset; 2) Diagnosis of acute cholangitis confirmed according to TG18 criteria; 3) Availability of complete clinical data for analysis (including medical history, laboratory results, etc.). Upon admission, all patients underwent a comprehensive clinical evaluation to confirm the diagnosis and ensure the consistency of the collected data. Exclusion criteria included: 1) Patients with concurrent acute inflammatory diseases (such as liver abscess, pneumonia, etc.) or malignant tumors, as these conditions could confound the inflammatory markers; 2) Patients who had received antibiotic treatment prior to hospitalization, as such treatment could affect inflammatory markers (e.g., NLR, SII), potentially influencing the accuracy of the study’s results; 3) Patients with missing clinical data, who were excluded to maintain the integrity and reliability of the data. All included patients were treated by a specialized team from the Hepatobiliary and Pancreatic Surgery Department and were not referred to other hospitals during the study period, ensuring the homogeneity of the study population.
Management of cholangitis by surgeons and specialists
In this study, all patients with acute cholangitis were managed by a specialized team in the Hepatobiliary and Pancreatic Surgery Department. The surgeons involved in this study are highly experienced, with many years of clinical practice specifically in managing cholangitis and other biliary diseases. They hold advanced qualifications, including board certifications in Hepatobiliary and Pancreatic Surgery. The team’s experience spans across diagnosis, treatment planning, surgical intervention, and postoperative management. This specialized expertise ensures that all patients receive consistent, high-quality care in line with current best practices for managing acute cholangitis.
This study received approval from the hospital institutional review board, and informed consent was waived.
Statistical methods
This study performed statistical analyses using R 4.3.3 software and Microsoft Excel 2019. Descriptive statistics for continuous variables are expressed as means, and categorical variables as percentages (%). Chi-square and Fisher’s exact tests were used to analyze differences between categorical variables, while that follow a normal distribution.. The normality of quantitative data distributions was assessed using the Kolmogorov–Smirnov test. For variables with skewed distributions, the median with interquartile range (IQR) was used to describe the data, and the Mann–Whitney U test was employed for comparisons. Continuous variables included NLR, PLR, SII, Alb, PNI, BMI, PLT, WBC, ALT, AST, TBIL, DBIL, Hb, Scr, PT, height, weight, and age. Gender, DM, smoking, and TG18 (severity of cholangitis) were considered categorical variables.
To estimate the required sample size, we used a sample size calculation method based on an expected area under the curve (AUC) of 0.75. According to the sample size formula recommended in the literature, and considering 80% statistical power and a significance level of 0.05, we estimated that at least 100–150 patients were necessary to achieve reliable conclusions. Receiver operating characteristic (ROC) curves were plotted, and the area under the curve (AUC) was calculated to determine the optimal cutoff values. Additionally, 95% confidence intervals (CIs) were calculated.
Results
Patient characteristics
From the initial pool of 165 patients screened for acute cholangitis, exclusions were made for incomplete clinical data (10 patients), concurrent inflammatory diseases (6 patients), and prior antibiotic treatment (10 patients), which could skew inflammatory biomarker accuracy. This resulted in a study cohort of 139 patients (Fig. 1). As depicted in Fig. 1, Table 1 delineates the baseline demographics and cholangitis severity across the study population. The cohort was balanced in gender, with 63 males and 76 females, and a median age of 64 years, spanning from 18 to 93 years. Notably, a history of smoking was more prevalent among the patients, suggesting a potential risk factor. The cases of acute cholangitis were predominantly mild/moderate (87 cases, 62.5%) as opposed to severe (52 cases, 37.5%). The univariate regression analysis showed that males have a higher risk of severe acute cholangitis, but this difference did not reach statistical significance. Importantly, the analysis revealed no significant disparities in baseline characteristics when stratified by disease severity(Table 1a).
Fig. 1.
Flow diagram of cases enrolled in this study
Table 1.
Correlation and clinicopathological characteristics
| a | ||||
| mild/moderate | severe | p.overall | ||
| N = 87 | N = 52 | |||
| Age(years) | 64.3 (14.1) | 62.9 (17.2) | 0.615 | |
| gender: | 0.743 | |||
| Female | 49 (56.3%) | 27 (51.9%) | ||
| Male | 38 (43.7%) | 25 (48.1%) | ||
| DM: | 0.879 | |||
| YES | 36 (41.4%) | 23 (44.2%) | ||
| NO | 51 (58.6%) | 29 (55.8%) | ||
| smoking: | 0.569 | |||
| YES | 59 (67.8%) | 32 (61.5%) | ||
| NO | 28 (32.2%) | 20 (38.5%) | ||
| Height(cm) | 1.68 (0.05) | 1.69 (0.05) | 0.426 | |
| Weight(kg) | 74.4 (7.62) | 75.1 (8.39) | 0.641 | |
| BMI(kg/m2) | 26.3 (1.28) | 26.3 (1.51) | 0.991 | |
| PLT(× 10^9/L) | 208 (60.6) | 176 (90.6) | 0.023 | |
| WBC(× 10^9/L) | 6.18 (2.36) | 9.33 (5.18) | < 0.001 | |
| ALT(IU/L) | 153 (246) | 141 (181) | 0.756 | |
| AST(IU/L) | 132 (273) | 119 (150) | 0.703 | |
| TBIL(μmol/L) | 0.65 (1.07) | 1.02 (0.80) | 0.022 | |
| DBIL(μmol/L) | 0.30 (0.62) | 0.56 (0.53) | 0.012 | |
| Hb(g/L) | 131 (16.2) | 128 (23.8) | 0.384 | |
| Scr(μmol/L) | 0.81 (0.17) | 1.21 (1.10) | 0.014 | |
| PT(s) | 11.2 (0.60) | 13.1 (2.04) | < 0.001 | |
| Alb(g/L) | 38.3 (6.90) | 32.4 (8.25) | < 0.001 | |
| NLR | 4.82 (7.93) | 12.8 (13.7) | < 0.001 | |
| PLR | 181 (122) | 229 (137) | 0.042 | |
| SII | 912 (1254) | 2013 (2088) | 0.001 | |
| PNI | 45.4 (7.40) | 37.1 (8.45) | < 0.001 | |
| b | ||||
| [ALL] | mild/moderate | severe | p.overall | |
| N = 139 | N = 87 | N = 52 | ||
| SII | 1324 (1696) | 912 (1254) | 2013 (2088) | 0.001 |
| PNI | 42.3 (8.77) | 45.4 (7.40) | 37.1 (8.45) | < 0.001 |
| NLR | 7.82 (11.1) | 4.82 (7.93) | 12.8 (13.7) | < 0.001 |
| Alb | 36.1 (7.93) | 38.3 (6.90) | 32.4 (8.25) | < 0.001 |
| PLR | 199 (129) | 181 (122) | 229 (137) | 0.042 |
Laboratory indicators and their association with the severity of acute cholangitis
Our study reinforces the growing body of evidence that biomarkers such as NLR (Neutrophil-to-Lymphocyte Ratio), PLR (Platelet-to-Lymphocyte Ratio), and SII (Systemic Immune-Inflammation Index) possess significant predictive value for the severity of acute cholangitis. The baseline data analysis revealed significant differences in the levels of five inflammatory biomarkers among patients with varying severities of acute cholangitis. These biomarkers include the Systemic Immune-Inflammation Index (SII) (p < 0.001), Prognostic Nutritional Index (PNI) (p < 0.001), Neutrophil-to-Lymphocyte Ratio (NLR) (p < 0.001), Albumin (Alb) (p < 0.001), and Platelet-to-Lymphocyte Ratio (PLR) (p = 0.042). Notably, the values of NLR and SII in the severe group were significantly higher than those in the mild group, more than twice as high, indicating that the systemic inflammatory response and immune dysregulation in severe patients are more pronounced. This may signal the severity of the disease and poor clinical prognosis. The data variability among patients with severe acute cholangitis was considerable, suggesting significant inter-individual differences in immune responses, nutritional status, and inflammatory control, which may reflect the instability of the body under severe infection conditions. Table 1b Univariate regression analysis corroborated these findings, underscoring the close association between these biomarkers and the severity of acute cholangitis, with all five biomarkers demonstrating a significant correlation (p < 0.001).(Table 2)Notably, all inflammatory biomarkers (SII, NLR, PLR) exhibited an upward trend correlating with the increasing severity of acute cholangitis. Conversely, nutritional indicators (PNI, Alb) trended downward as the severity escalated.
Table 2.
Risk Factor Analysis (Cox Regression) for Predicting Severe Cholangitis
| Variates | HR (风险比) | 95% CI | p-Value |
|---|---|---|---|
| Univariate | |||
| Age(years) | 1.002 | 0.99–1.01 | 0.706 |
| Height(cm) | 0.355 | 0.05–2.43 | 0.544 |
| Weight(kg) | 0.996 | 0.97–1.01 | 0.72 |
| PLT(× 10^9/L) | 1.002 | 0.99–1.00 | 0.093 |
| WBC(× 10^9/L) | 0.942 | 0.90–0.98 | < 0.001 |
| ALT(IU/L) | 1.000 | 0.99–1.00 | 0.806 |
| AST(IU/L) | 1.000 | 0.99–1.00 | 0.754 |
| TBIL(μmol/L) | 0.810 | 0.65–0.99 | 0.067 |
| DBIL(μmol/L) | 1.419 | 0.51–3.89 | 0.999 |
| Hb(g/L) | 1.002 | 0.99–1.01 | 0.521 |
| Scr(μmol/L) | 0.777 | 0.56–1.06 | 0.085 |
| PT(s) | 0.739 | 0.62–0.87 | < 0.001 |
| Alb(g/L) | 1.033 | 1.01–1.05 | < 0.001 |
| gender | 0.937 | 0.64–1.35 | 0.704 |
| DM | 1.043 | 0.74–1.46 | 0.804 |
| smoking | 0.905 | 0.62–1.30 | 0.575 |
| PNI | 1.040 | 1.02–1.05 | < 0.001 |
| NLR | 0.975 | 0.95–0.99 | < 0.001 |
| SII | 0.999 | 0.99–1.00 | < 0.001 |
| PLR | 0.999 | 0.99–1.00 | < 0.001 |
| PNS | 0.514 | 0.39–0.67 | 0.124 |
| Multivariate | |||
| WBC(× 10^9/L) | 0.968 | 0.90–1.05 | 0.409 |
| PNS | 0.604 | 0.41–0.89 | 0.010 |
| PNI | 1.002 | 0.92–1.10 | 0.967 |
| NLR | 1.007 | 0.98–1.03 | 0.621 |
| SII | 1.000 | 1.00–1.00 | 0.749 |
| PT(s) | 0.826 | 0.73–0.94 | 0.003 |
| Alb(g/L) | 1.008 | 0.93–1.09 | 0.843 |
Construction of a simplified predictive scoring system
Addressing the challenges of using Alb, PNI, NLR, SII, and PLR to accurately classify acute cholangitis severity, and the lack of standardized laboratory references, our study embraced ROC analysis for both inflammatory and nutritional markers. The ROC curves, illustrated in Figs. 2, reveal that the AUCs for NLR, PLR, and SII are 0.759, 0.620, and 0.680 respectively. Figures 2 and 3 presents AUCs for PNI and Alb at 0.798 and 0.750 respectively. These factors, despite their varied accuracy, aid in identifying severe acute cholangitis at specific cutoffs (Fig. 3). Notably, NLR emerged as the superior inflammatory marker with a cutoff of 5.595, aligning with other research and affirming the validity of our optimal threshold [15–17]. For nutritional assessment, PNI stood out with a cutoff of 41.175.Building on these insights, we devised a straightforward scoring system, termed PNS.The scoring criteria for PNI and NLR are as follows: PNI > 41.175 is assigned 0 points, and PNI < 41.175 is assigned 1 point; NLR > 5.595 is assigned 1 point, and NLR < 5.595 is assigned 0 points. Based on these criteria, all patients were classified into three groups based on their total score: 0 points (n = 69) representing mild cholangitis, 1 point (n = 34) representing moderate cholangitis, and 2 points (n = 36) representing severe cholangitis. A comprehensive ROC analysis juxtaposing PNS, NLR, and PNI—as depicted in Fig. 4—yielded AUCs of 0.853, 0.759, and 0.798 respectively. (Fig. 4)The PNS score, when applied as a predictive measure for severe cholangitis, achieved a specificity of 72.4% and a sensitivity of 88.5%. Multivariate regression analysis underscored its statistical significance with a p-value of 0.010 (Table 2).
Fig. 2.
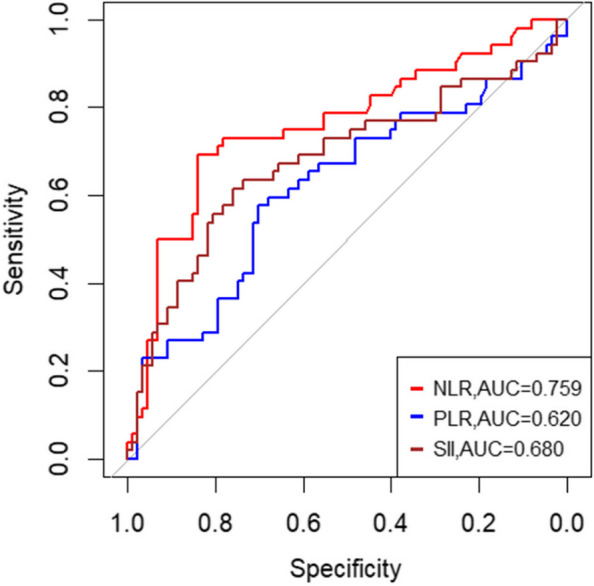
Comparison of the areas under the ROC curve for predicting severe acute cholangitis using different inflammatory markers
Fig. 3.
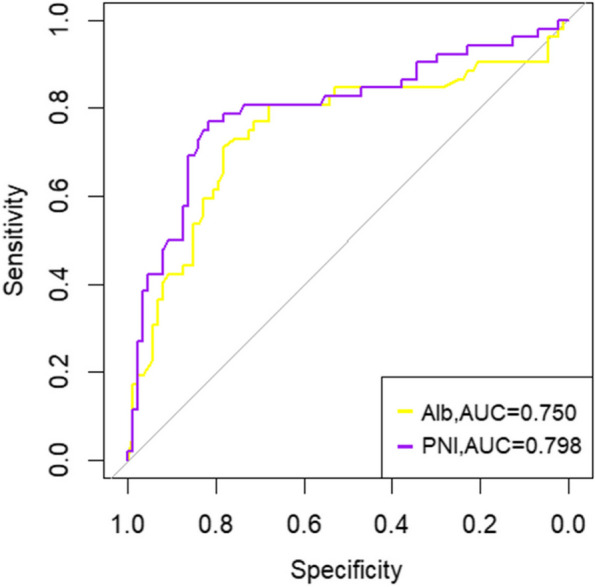
Comparison of the areas under the ROC curve for predicting severe acute cholangitis using different nutritional indicators
Fig. 4.
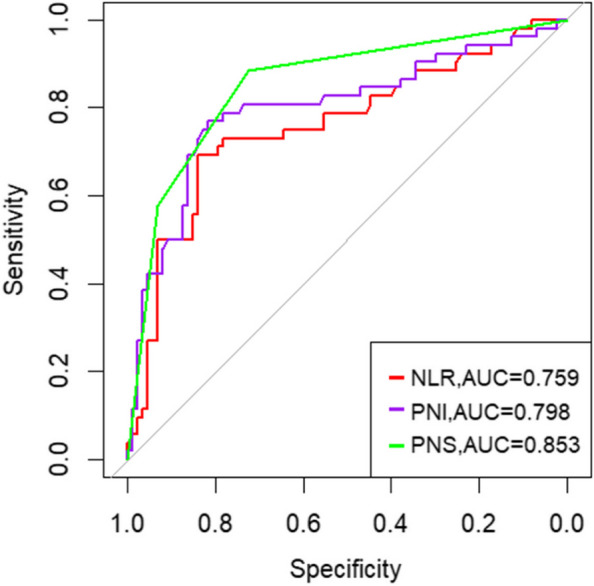
Comparison of the areas under the ROC curve for predicting severe acute cholangitis using individual indicators and pns combined score
Discussion
Charcot’s triad, comprising fever, biliary pain, and jaundice, has long been upheld as a pivotal diagnostic tool for acute cholangitis. However, the sensitivity of these criteria is notably low, limiting their utility in predicting disease severity. The traditional TG18 diagnostic criteria, encompassing a series of complex standards and indicators, necessitate a comprehensive evaluation of the patient’s clinical presentation, laboratory findings, and radiological results [18]. While TG18 is esteemed as a benchmark for assessing acute cholangitis severity, it mandates an assessment across six organ systems and incorporates intricate items such as vital signs and laboratory data. Consequently, these guidelines may not be expedient for rapid diagnosis in emergent clinical scenarios.
A plethora of studies have introduced diverse predictive models for severe acute cholangitis, incorporating risk factors such as non-alcoholic fatty liver disease (NAFLD), procalcitonin, adiponectin-2, IL-7, and NLR (Neutrophil-to-Lymphocyte Ratio). Mahmud et al. undertook an extensive analysis of 298 cases, highlighting the paramount risk posed by NAFLD in severe acute cholangitis [19]. In another study, Umefune et al. examined data from 213 patients, 29 classified as severe, and deduced that procalcitonin was notably effective in predicting severe acute cholangitis, evidenced by an impressive AUC of 0.9 and an optimal cutoff of 2.2 ng/mL(sensitivity of 0.97 and specificity of 0.73). Additionally, retrospective studies have underscored serum adiponectin-2’s superior performance as a biomarker over traditional inflammatory indices for risk stratification in acute cholangitis patients. Suwa et al.’s research also pointed to a significant association between diminished IL-7 levels and the gravity of acute cholangitis.However, the diagnostic process for NAFLD and the quantification of procalcitonin and adiponectin-2 are frequently hampered by the limitations of hospital equipment, barring many healthcare facilities from conducting such evaluations. This limitation underscores the pressing need for an economically feasible and universally applicable standard, seamlessly integrated into routine clinical practice, to precisely gauge the severity of acute cholangitis. Scarlat et al. utilized easily accessible non-invasive biomarkers and scoring systems to predict liver cirrhosis complications in a simple and accurate manner [20], thereby improving the accuracy and efficiency of clinical decision-making in complex cases and high-risk patients.
Confronted with the aforementioned diagnostic challenges, our team has formulated the PNS predictive scoring system. This novel approach was evaluated through a retrospective analysis of 139 hospitalized cases of acute cholangitis. We found that the PNS score, derived from the integration of NLR (Neutrophil-to-Lymphocyte Ratio) and PNI (Prognostic Nutritional Index), exhibits a robust predictive capacity for severe acute cholangitis. As the disease severity escalates, inflammatory biomarkers such as NLR, SII (Systemic Immune-Inflammation Index), and PLR (Platelet-to-Lymphocyte Ratio) significantly rise, whereas nutritional indicators like PNI and Alb (Albumin) markedly decline.
Upon patient admission, a simple assessment of neutrophil count, lymphocyte count, and albumin levels, followed by the computation of NLR and PNI, culminates in the PNS score. This score serves as a prognostic tool to ascertain the severity of acute cholangitis. It enables clinicians to swiftly differentiate the severity levels of cholangitis using basic test data, thus facilitating the interpretation of risk assessments for both physicians and patients. This straightforward and expedient method enhances doctor-patient communication, ensures the prompt recognition of cholangitis progression, and informs tailored medical decisions for patients with diverse clinical presentations. Notably, for those with severe cholangitis, the expedited transfer to ICU wards has been instrumental in saving critical time, thereby diminishing the mortality rates associated with the condition.
In the realm of intensive care, procalcitonin (PCT) and C-reactive protein (CRP) have gained prominence as biomarkers. A Japanese study involving 213 patients showcased PCT’s superior predictive ability for severe acute cholangitis with an AUC of 0.9, outperforming both WBC (0.62) and CRP (0.7) [21]. Similarly, Lee et al.'s analysis of 204 acute cholangitis patients—26 with severe conditions—underscored procalcitonin’s significant predictive value for severe acute cholangitis, blood culture positivity, and clinical deterioration, with optimal cutoff values of 1.76, 0.68, and 3.77 respectively.Despite its predictive prowess, routine testing for serum procalcitonin is not commonplace across all medical institutions, primarily due to its high cost which limits its continuous use during hospitalization [22]. CRP, initially a tool for assessing disease severity and monitoring conditions like rheumatoid arthritis and secondary amyloidosis, has seen its role in clinical practice wane in favor of more cost-effective and easily applied markers such as CRP and ESR [23, 24].At Shanxi Bethune Hospital, CRP has not been established as the primary test for patients admitted with acute cholangitis, leaving its potential for predicting the severity of the condition an open question for future research.
The Neutrophil-to-Lymphocyte Ratio (NLR) serves as an indicator of the balance between neutrophils, markers of inflammation, and lymphocytes, key players in the immune response. An elevated NLR signifies a pronounced immune-inflammatory imbalance. Neutrophils, pivotal in orchestrating inflammatory responses, exacerbate systemic inflammation through the secretion of pro-inflammatory cytokines, potentially precipitating Systemic Inflammatory Response Syndrome (SIRS) and septic shock in severe acute cholangitis cases [25]. Concurrently, lymphocytes modulate this systemic inflammation, with persistent inflammatory states inducing lymphocyte redistribution and apoptosis, thereby diminishing lymphocyte counts [26]. Consequently, NLR mirrors the immune system’s dynamic shifts in acute cholangitis patients.Emerging research has linked a heightened NLR with adverse prognoses across various cancers [27]. A particular study underscored NLR’s association with the early detection of Severe Acute Pancreatitis (SAP). Within a cohort of 216 patients, the Area Under the Curve (AUC) for NLR and Platelet-to-Lymphocyte Ratio (PLR) stood at 0.894 and 0.728, respectively. An NLR threshold of 6.105 yielded a sensitivity of 92.9% and specificity of 76.1%, suggesting the combined NLR-PLR assessment’s potential in SAP’s early prediction.In a retrospective Korean study, Lee SH et al. evaluated 206 acute cholangitis patients, deducing NLR’s predictive capacity for severe illness, the necessity for vasopressors/inotropes in shock scenarios, and positive blood cultures. Continuous NLR monitoring facilitated temporal severity assessment, with an AUC of 0.81, surpassing both WBC and CRP. Presently [28], NLR is gaining traction in diverse research domains, particularly infectious diseases. In biliary infections, elevated NLR levels are indicative of severe over mild to moderate acute cholangitis [29]. Our findings corroborate this, with NLR predicting cholangitis severity exhibiting an AUC of 0.759, specificity of 83.9%, and sensitivity of 69.2%. This positions NLR as a viable, alternative laboratory marker for forecasting severe acute cholangitis.
The correlation between nutritional indicators and disease severity, as well as prognosis, is increasingly substantiated by recent studies. The Nutritional Risk Index (NRI), which amalgamates weight change and serum albumin levels, is lauded for its capacity to evaluate malnutrition risk across diverse patient demographics. Nonetheless, it may lack comprehensive applicability and sensitivity for certain patient subsets.The NUTRIC score, tailored for Intensive Care Unit (ICU) patients, features objective and readily accessible indicators [30]. However, its association with definitive nutritional status markers remains tenuous. The CONUT score (Control of Nutritional Status score), assessing nutritional status via serum albumin, cholesterol, and lymphocyte count, is instrumental in the prompt identification of malnutrition [31]. Yet, its broader application across various diseases is somewhat constrained.The ALB score (serum albumin score), a straightforward blood test, mirrors patients’ nutritional status and hepatic function. Its prognostic accuracy, however, is susceptible to confounding factors such as edema or hepatic pathology. Illustratively, one study examined the C-reactive protein to serum albumin ratio (CRP/ALB) [32], establishing its correlation with acute pancreatitis severity, organ failure, pancreatic necrosis, and mortality risk. ALB’s utility extends to other conditions; for instance, it was deemed a pivotal biomarker in a model predicting disease severity through serum biomarkers among patients with mild to moderate atopic dermatitis.
The Prognostic Nutritional Index (PNI), widely recognized for its predictive accuracy, reflects patients’ nutritional status and serves as a dual indicator of both nutrition and immune function. Commonly, PNI is employed to forecast postoperative complications and mortality, with lower scores correlating with adverse outcomes. Systemic inflammatory responses, characterized by increased vascular permeability, facilitate albumin translocation from the intravascular compartment. Inflammation suppresses hepatic albumin mRNA expression, curtailing albumin synthesis and accelerating its catabolism. Inflammatory cytokines, notably IL-6, inhibit albumin synthesis in hepatocytes while stimulating acute-phase proteins such as C-reactive protein production [33]. Lymphocyte dynamics are governed by inflammatory signaling pathways, with the NF-κB pathway being central to these processes. Activation by pathogens or tumor necrosis factors leads to the upregulation of pro-inflammatory cytokines like IL-1 and IL-6, which can induce lymphocytopenia [34]. In addition, the stress response induced by inflammation releases immunosuppressive substances such as corticosteroids, which inhibit the production of lymphocytes, further exacerbating the immunosuppressive state.The study by T. Kalaycı et al. further validated this point it out that PNI can serve as an important prognostic indicator for acute appendicitis in the super-elderly population [35].Empirical studies have demonstrated PNI’s and the Control of Nutritional Status (CONUT) score’s influence on the survival of multiple myeloma patients, with low PNI indicative of poor prognosis [36]. A multicenter retrospective study established a notable link between PNI and mortality risk in COVID-19 patients, pinpointing a PNI threshold of 33.405 below which the death risk escalates [37]. PNI has been validated as an independent predictor of all-cause and cardiovascular mortality in individuals with metabolic syndrome and heart failure.In the context of cholangitis, research has predominantly concentrated on therapeutic and management aspects. Investigations into Elafibranor’s efficacy in primary biliary cholangitis and advancements in primary sclerosing cholangitis management exemplify this focus [38]. However, the association between PNI alterations and acute cholangitis severity remains underexplored. Our study delves into this relationship, utilizing PNI as a prognostic tool for severe acute cholangitis. We ascertain that a diminished PNI is intricately linked with the manifestation of severe cholangitis.
The revelation that an elongated Prothrombin Time (PT) is positively associated with acute cholangitis severity is a compelling finding that resonates with prior research. This correlation may be attributed to several factors [39]:1.Inflammatory Response: An active inflammatory response can compromise liver functionality, disrupting the synthesis of essential clotting factors and consequently prolonging PT.2.Biliary Stasis: Cholangitis-induced biliary stasis can impede the intestinal absorption of vitamin K, a vital component for the production of clotting factors II, VII, IX, and X.3.Bacterial Infections: The presence of bacterial infections and their endotoxins can trigger the coagulation cascade, heightening the utilization of clotting factors and, in severe cases, precipitating Disseminated Intravascular Coagulation (DIC), further extending PT.These interconnected biological mechanisms collectively influence the coagulation profile of patients with acute cholangitis, serving as significant diagnostic and prognostic markers in clinical practice [40]. The insights gleaned from this study shed new light on the pathophysiological intricacies of acute cholangitis and hold the potential to refine patient management and therapeutic approaches.
This study represents the first exploration of the combined use of NLR (Neutrophil-to-Lymphocyte Ratio) and PLR (Platelet-to-Lymphocyte Ratio) as prognostic tools for acute cholangitis at the time of admission, which carries a degree of novelty. A notable strength of our methodology is the rigorous inclusion criteria, which excluded patients who had received antibiotic therapy prior to admission and those with concurrent acute inflammatory conditions. This approach minimized the potential interference of these factors on the inflammatory biomarkers, ensuring the reliability of the results. Furthermore, the strict control of data quality and the handling of missing data further enhanced the credibility of our findings.
However, this study has several limitations. First, differences in treatment protocols may lead to inconsistencies in patient outcomes, thereby affecting the generalizability of the results. The variation in treatment protocols, which depend on the clinical practices and experience of different centers, limits the external applicability of the findings. Second, the study covers a time span of approximately 10 years, during which clinical practices, diagnostic criteria, and treatment options may have changed, potentially influencing patient prognosis. Additionally, the retrospective design may introduce biases, such as data loss, temporal relationship bias, and inconsistencies in documentation, which could affect the accuracy of the data and the reliability of the analytical results. Furthermore, the sample size of this study is relatively small and conducted at a single institution, which may lead to selection bias. To improve the reliability and broader applicability of the findings, future studies should aim to expand the sample size and validate the results using multicenter datasets. Lastly, there is currently no consensus on the optimal cutoff values for NLR and PNI in acute cholangitis. Future research should focus on establishing specific thresholds for these biomarkers to enhance the accuracy of severity predictions.
Conclusion
Our investigation reveals that upon hospital admission, the Neutrophil-to-Lymphocyte Ratio (NLR) and Prognostic Nutritional Index (PNI) can be swiftly and cost-effectively determined. The synthesized PNS score, derived from NLR and PNI metrics, enhances the predictive accuracy for the severity of acute cholangitis. An elevated PNS score is indicative of an escalated risk for severe cholangitis, necessitating prompt and appropriate interventions, including optimal biliary drainage, surgical procedures, or expedited transfer to an Intensive Care Unit (ICU).
Looking ahead, the efficacy of the PNS as a prognostic indicator warrants further corroboration through prospective, multicenter studies with expansive patient cohorts. Such research endeavors will be instrumental in substantiating the PNS score’s role as a reliable marker for assessing the severity of acute cholangitis.
Supplementary Information
Acknowledgements
The authors would like to thank all the patients and investigators who participated in this study.
Disclosure
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
- NLR
Neutrophil-to-Lymphocyte Ratio
- PLR
Platelet-to-Lymphocyte Ratio
- SII
Systemic Immune-Inflammation Index
- PNI
Prognostic Nutritional Index
- WBC
White Blood Cell
- AST
Aspartate Aminotransferase
- ALT
Alanine Aminotransferase
- TBIL
Total Bilirubin
- DBIL
Direct Bilirubin
- PT
Prothrombin Time
- DM
Diabetes Mellitus
- PNS
Score Combined Prognostic Nutritional Index and Neutrophil-to-Lymphocyte Ratio
Authors’ contributions
The success of this study is attributed to the collective efforts of all authors. Dong Li and Jingchao Sun, as co-first authors, were responsible for the conceptual design, data acquisition, analysis, and interpretation of the research. Chao Qi was in charge of executing the study and drafting the manuscript. Xifeng Fu and Fei Gao, as corresponding authors, were responsible for reviewing and critically revising the manuscript. All authors participated in the final review of the article and approved the version to be published. Each author agrees to be accountable for all aspects of the work and has consented to submit to the chosen journal.
Funding
This study was funded by Shanxi Province “136 Revitalization Medical Project Construction Funds.And a scientific research project of Shanxi Provincial Health and Wellness Commission, the title of which is: Research on the molecular mechanism of YAP1 regulation of NGF to promote neuroinvasion in pancreatic cancer Standard No: 2023074. And the project: Exploratory studies on the expression of antitumor drug targets in liver, gallbladder and pancreatic tumors Item No:2021177.
Data availability
All data generated or analysed during this study are included in supplementary information files.
Declarations
Ethics approval and consent to participate
Informed consent was obtained from the patients and this study has been approved by the Ethics Committee of Shanxi Academy of Medical Sciences. The studies were conducted in accordance with the local legislation and institutional requirements. Ethics No.YXLL-2023–249. Our study complies with the Declaration of Helsinki.
Consent for publication
Not Applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Dong Li and Jingchao Sun contributed equally to this work.
Contributor Information
Xifeng Fu, Email: fxfyisheng@163.com.
Fei Gao, Email: gfgf2007@163.com.
References
- 1.Gardos G, Cole JO. Maintenance antipsychotic therapy: Is the cure worse than the disease? Am J Psychiatry. 1976;133(1):32–6. 10.1176/ajp.133.1.32. [DOI] [PubMed] [Google Scholar]
- 2.Miura F, Okamoto K, Takada T, Strasberg SM, Asbun HJ, Pitt HA, Gomi H, Solomkin JS, Schlossberg D, Han HS, Kim MH, Hwang TL, Chen MF, Huang WSW, Kiriyama S, Itoi T, Garden OJ, Liau KH, Horiguchi A, Yamamoto M. Tokyo Guidelines 2018: Initial management of acute biliary infection and flowchart for acute cholangitis. J Hepato Biliary Pancr Sci. 2018;25(1):31–40. 10.1002/jhbp.509 [DOI] [PubMed]
- 3.Lee YS, Cho KB, Park KS, Lee JY, Lee YJ. Procalcitonin as a decision-supporting marker of urgent biliary decompression in acute cholangitis. Dig Dis Sci. 2018;63(9):2474–9. 10.1007/s10620-018-4963-1. [DOI] [PubMed] [Google Scholar]
- 4.Csendes A, Diaz JC, Burdiles P, Maluenda F, Morales E. Risk factors and classification of acute suppurative cholangitis. Br J Surg. 2005;79(7):655–8. 10.1002/bjs.1800790720. [DOI] [PubMed] [Google Scholar]
- 5.An Z, Braseth AL, Sahar N. Acute cholangitis: causes, diagnosis, and management. Gastroenterol Clin North Am. 2021;50(2):403–14. 10.1016/j.gtc.2021.02.005. [DOI] [PubMed] [Google Scholar]
- 6.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: A severity of disease classification system. Crit Care Med. 1985;13(10):818–29. 10.1097/00003246-198510000-00009. [PubMed] [Google Scholar]
- 7.Knaus WA, Zimmerma JE, Wagner DP, Draper EA, Lawrence DE. APACHE—acute physiology and chronic health evaluation: A physiologically based classification system: Crit Care Med. 1981;9(8):591–597. 10.1097/00003246-198108000-00008 [DOI] [PubMed]
- 8.Villar J, Pérez-Méndez L, López J, Belda J, Blanco J, Saralegui I, Suárez-Sipmann F, López J, Lubillo S, Kacmarek RM, & on behalf of the HELP Network. An Early PEEP/F iO 2 Trial Identifies Different Degrees of Lung Injury in Patients with Acute Respiratory Distress Syndrome. Am J Respiratory Crit Care Med. 2007;176(8):795–804.10.1164/rccm.200610-1534OC [DOI] [PubMed]
- 9.Marshall JC, Cook DJ, Christou NV, Bernard GR, Sprung CL, Sibbald WJ. multiple organ dysfunction score: a reliable descriptor of a complex clinical outcome. Crit Care Med. 1995;23(10):1638–52. 10.1097/00003246-199510000-00007. [DOI] [PubMed] [Google Scholar]
- 10.Teasdale G, Jennett B. Assessment of coma and impaired consciousness. The Lancet. 1974;304(7872):81–4. 10.1016/S0140-6736(74)91639-0. [DOI] [PubMed] [Google Scholar]
- 11.Woo S, Lee W, Seol S, Kim D, Choi S. The accuracies of abdominal computed tomography and the neutrophil-to-lymphocyte ratio used to predict the development of clinically severe acute cholecystitis in elderly patients visiting an emergency department. Niger J Clin Pract. 2018;21(5):645. 10.4103/njcp.njcp_76_17. [DOI] [PubMed] [Google Scholar]
- 12.Jeon TJ, Park JY. Clinical significance of the neutrophil-lymphocyte ratio as an early predictive marker for adverse outcomes in patients with acute pancreatitis. World J Gastroenterol. 2017;23(21):3883. 10.3748/wjg.v23.i21.3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kong W, He Y, Bao H, Zhang W, Wang X. Diagnostic value of neutrophil-lymphocyte ratio for predicting the severity of acute pancreatitis: a meta-analysis. Dis Markers. 2020;2020:1–9. 10.1155/2020/9731854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kler A, Taib A, Hajibandeh S, Hajibandeh S, Asaad P. The predictive significance of neutrophil-to-lymphocyte ratio in cholecystitis: A systematic review and meta-analysis. Langenbeck’s Archives of Surgery. 2022;407(3):927–35. 10.1007/s00423-021-02350-2. [DOI] [PubMed] [Google Scholar]
- 15.Wang C, Yuan Y, Hunt RH. The association between helicobacter pylori infection and early gastric cancer: a meta-analysis. Am J Gastroenterol. 2007;102(8):1789–98. 10.1111/j.1572-0241.2007.01335.x. [DOI] [PubMed] [Google Scholar]
- 16.Thom C, Lewis N. Never say never: Identification of acute pulmonary embolism on non-contrast computed tomography imaging. Am J Emerg Med. 2017;35(10):1584.e1-1584.e3. 10.1016/j.ajem.2017.07.052. [DOI] [PubMed] [Google Scholar]
- 17.Xing J, Tian Y, Ji W, Wang X. Comprehensive evaluation of SPATS2 expression and its prognostic potential in liver cancer. Medicine. 2020;99(9):e19230. 10.1097/MD.0000000000019230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kiriyama S, Kozaka K, Takada T, Strasberg SM, Pitt HA, Gabata T, Hata J, Liau KH, Miura F, Horiguchi A, Liu KH, Su CH, Wada K, Jagannath P, Itoi T, Gouma DJ, Mori Y, Mukai S, Giménez ME, Yamamoto M. Tokyo Guidelines 2018: Diagnostic criteria and severity grading of acute cholangitis (with videos). J Hepato Biliary Pancreatic Sci. 2018;25(1):17–30. 10.1002/jhbp.512. [DOI] [PubMed] [Google Scholar]
- 19.Alwers E, Jia M, Kloor M, Bläker H, Brenner H, Hoffmeister M. Associations between molecular classifications of colorectal cancer and patient survival: a systematic review. Clin Gastroenterol Hepatol. 2019;17(3):402-410.e2. 10.1016/j.cgh.2017.12.038. [DOI] [PubMed] [Google Scholar]
- 20.Scarlata GGM, Ismaiel A, Gambardella ML, Leucuta DC, Luzza F, Dumitrascu DL, Abenavoli L. Use of non-invasive biomarkers and clinical scores to predict the complications of liver cirrhosis: a bicentric experience. Medicina. 2024;60(11):1854. 10.3390/medicina60111854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Umefune G, Kogure H, Hamada T, Isayama H, Ishigaki K, Takagi K, Akiyama D, Watanabe T, Takahara N, Mizuno S, Matsubara S, Yamamoto N, Nakai Y, Tada M, Koike K. Procalcitonin is a useful biomarker to predict severe acute cholangitis: A single-center prospective study. J Gastroenterol. 2017;52(6):734–45. 10.1007/s00535-016-1278-x. [DOI] [PubMed] [Google Scholar]
- 22.Kim K, Park Y, Hyun BG, Choi M, Park J. Recent Advances in Transparent Electronics with Stretchable Forms. Adv Mater. 2019;31(20):1804690. 10.1002/adma.201804690. [DOI] [PubMed] [Google Scholar]
- 23.Sonawane MD, Nimse SB. C-Reactive protein: A major inflammatory biomarker. Anal Methods. 2017;9(23):3400–13. 10.1039/C7AY00711F. [Google Scholar]
- 24.Mouliou DS. C-reactive protein: pathophysiology, diagnosis, false test results and a novel diagnostic algorithm for clinicians. Diseases. 2023;11(4):132. 10.3390/diseases11040132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tahtaci M, Koseoglu H, Alisik M, TayfurYurekli O, Tahtaci G, Erel O, Ersoy O. Association of low fecal elastase-1 and non-ulcer dyspepsia. J Clin Med. 2018;7(6):155. 10.3390/jcm7060155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hotchkiss RS, Nicholson DW. Apoptosis and caspases regulate death and inflammation in sepsis. Nat Rev Immunol. 2006;6(11):813–22. 10.1038/nri1943. [DOI] [PubMed] [Google Scholar]
- 27.Guthrie GJK, Charles KA, Roxburgh CSD, Horgan PG, McMillan DC, Clarke SJ. The systemic inflammation-based neutrophil–lymphocyte ratio: Experience in patients with cancer. Crit Rev Oncol Hematol. 2013;88(1):218–30. 10.1016/j.critrevonc.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 28.Zhou Z, Liu S, Zhang M, Zhou R, Liu J, Chang Y, Zhao Q. Overexpression of topoisomerase 2-alpha confers a poor prognosis in pancreatic adenocarcinoma identified by co-expression analysis. Dig Dis Sci. 2017;62(10):2790–800. 10.1007/s10620-017-4718-4. [DOI] [PubMed] [Google Scholar]
- 29.De Jager CPC, Wever PC, Gemen EFA, Kusters R, Van Gageldonk-Lafeber AB, Van Der Poll T, Laheij RJF. The Neutrophil-Lymphocyte Count Ratio in Patients with Community-Acquired Pneumonia. PLoS ONE. 2012;7(10):e46561. 10.1371/journal.pone.0046561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yoshihisa A, Kanno Y, Watanabe S, Yokokawa T, Abe S, Miyata M, Sato T, Suzuki S, Oikawa M, Kobayashi A, Yamaki T, Kunii H, Nakazato K, Suzuki H, Ishida T, Takeishi Y. Impact of nutritional indices on mortality in patients with heart failure. Open Heart. 2018;5(1):e000730. 10.1136/openhrt-2017-000730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matsukawa T, Suto K, Kanaya M, Izumiyama K, Minauchi K, Yoshida S, Oda H, Miyagishima T, Mori A, Ota S, Hashimoto D, Teshima T, & North Japan Hematology Study Group (NJHSG). Validation and comparison of prognostic values of GNRI, PNI, and CONUT in newly diagnosed diffuse large B cell lymphoma. Ann Hematol. 2020;99(12):2859–2868.10.1007/s00277-020-04262-5 [DOI] [PubMed]
- 32.Zhao Y, Xia W, Lu Y, Chen W, Zhao Y, Zhuang Y. Predictive value of the C-reactive protein/albumin ratio in severity and prognosis of acute pancreatitis. Front Surg. 2023;9:1026604. 10.3389/fsurg.2022.1026604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nicholson JP, Wolmarans MR, Park GR. The role of albumin in critical illness. Br J Anaesth. 2000;85(4):599–610. 10.1093/bja/85.4.599. [DOI] [PubMed] [Google Scholar]
- 34.Tak PP, Firestein GS. NF-κB: A key role in inflammatory diseases. J Clin Investig. 2001;107(1):7–11. 10.1172/JCI11830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kalayci T, Kartal, M. Significance of neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, serum albumin and prognostic nutritional index as predictors of morbidity in super-elderly patients operated on for acute appendicitis. Eur Rev Med Pharmacol Sci. 2022;26(3):820–827. 10.26355/eurrev_202202_27990 [DOI] [PubMed]
- 36.Ferraro MP, Gimeno-Vazquez E, Subirana I, Gómez M, Díaz J, Sánchez-González B, García-Pallarols F, Martínez L, Ble M, Molina L, Belarte LC, Abella E, Elosua R, Comín-Colet J, Salar A. Anthracycline-induced cardiotoxicity in diffuse large B-cell lymphoma: NT-proBNP and cardiovascular score for risk stratification. Eur J Haematol. 2019;102(6):509–15. 10.1111/ejh.13234. [DOI] [PubMed] [Google Scholar]
- 37.Nguyen LHT, Dang AK, Tran TV, Phan HT, Doan DAT, Nguyen LBT, Tran AM, Do TD, Nguyen TB, Nguyen TT, Nguyen BH, Le HT. The role of nutritional risk evaluation in predicting adverse outcomes among patients with severe COVID-19 in Vietnam. Front Nutr. 2023;10:1245816. 10.3389/fnut.2023.1245816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ray K. Resmetirom safe for nonalcoholic fatty liver disease. Nat Rev Gastroenterol Hepatol. 2024;21(1):2–2. 10.1038/s41575-023-00874-0. [DOI] [PubMed] [Google Scholar]
- 39.Du Z-Y. Middle segmental pancreatectomy: A safe and organ-preserving option for benign and low-grade malignant lesions. World J Gastroenterol. 2013;19(9):1458. 10.3748/wjg.v19.i9.1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Levi M, Van Der Poll T. Inflamm Coagulation. Crit Care Med. 2010;38:S26–34. 10.1097/CCM.0b013e3181c98d21. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analysed during this study are included in supplementary information files.