Abstract
Background
Despite their ability to regenerate as well as autografts, the use of nerve allografts is limited by the need for immunosuppression and the risk of disease transmission. Further, decellularized allografts lacking Schwann cells limit axonal regeneration in long nerve defects. This study evaluated sciatic nerve regeneration in rats implanted with cold- or cryopreserved allografts, and examined the effects of FK506, an immunosuppressant that targets calcineurin function, on motor recovery.
Methods
Sixty-five male Lewis rats were divided into five groups of 13, each with a 10-mm sciatic nerve gap. Group I received an autograft, whereas Groups II and III received allografts pretreated with cryopreservation and cold preservation, respectively. Groups IV and V were also implanted with cryo- and cold-preserved allografts, but were treated with a low dose of FK506. Motor regeneration was assessed at 20 weeks by the measurement of ankle contracture, compound muscle action potential, maximal isometric tetanic force, wet muscle weight of the tibialis anterior, peroneal nerve histomorphometry, and immunohistochemistry of the reconstructed sciatic nerve.
Results
Similar motor recovery was observed between the autografts and both types of allografts. The groups treated with FK506 showed improved recovery, particularly in terms of ankle angle and tibialis anterior muscle weight. Histomorphometry revealed a superior myelinated fiber area and nerve ratio in the cold-preserved allograft group, while Group II displayed a less well-organized morphology.
Conclusion
This study demonstrates that cold- or cryopreserved nerve allografts represent effective alternatives to autografts for peripheral nerve reconstruction, with low-dose FK506 enhancing motor recovery without necessitating immunosuppression.
Level of Evidence I
Basic Science Level I.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13018-024-05343-1.
Keywords: Cryopreservation, Cold preservation, Allograft, Immunosuppression, Nerve reconstruction, FK506
Background
Nerve autografts facilitate excellent nerve regeneration, often outperforming artificial and biological substitutes [1–3]. However, their use is limited by donor site morbidity and the limited availability of autologous nerve tissue. Nerve allografts offer an alternative, serving as temporary scaffolds for host nerve regeneration with outcomes comparable to those of autografts [4–6]. Further, nerve allografts, which provide an unlimited source of graft material, allow the repair of significant long segmental nerve injuries [7–9]. Despite these advantages, nerve allografts have not gained widespread acceptance in the decades since their development, owing to the need for immunomodulatory therapy and the risk of disease transmission [7].
Recently, decellularization of allograft nerves has gained attention as a strategy to eliminate the need for immunosuppressive therapy by removing the cellular components that trigger the immune response. This process involves the removal of cellular debris, antigens, and other immunogenic material, while preserving the extracellular matrix, which serves as a scaffold for nerve regeneration [10]. Recent studies have further shown that decellularized nerve allografts can effectively support nerve repair in short gaps. However, their application to long-gap nerve injuries remains limited, particularly those exceeding 3 cm in size [7, 11, 12]. This is primarily due to the absence of live Schwann cells, which are critical for axonal regeneration and remyelination [13]. Current research is exploring methods to overcome these limitations, such as repopulating decellularized scaffolds with Schwann or stem cells, to enhance their regenerative potential, and expand their clinical applicability to longer nerve gaps.
A series of experimental studies have focused on pretreatment methods for peripheral nerve grafts, such as cold preservation [14, 15] and cryopreservation [16, 17], to decrease antigenicity or eliminate cellular components. Evans et al. [17] previously reported that controlled cryopreservation can lower the immune response and reduce graft rejection in patients undergoing procedures aimed at preserving Schwann cell viability [17]. Squintani et al. [16] further demonstrated that cryopreserved nerve allografts harvested from cadaveric donors can effectively restore nerve function, without the need for immunosuppressive treatments in patients who require extensive reconstruction of the brachial and lumbosacral plexuses.
FK506, also known as tacrolimus, is widely recognized for its potent immunosuppressive effects driven by T-cell inhibition; however, recent studies have highlighted its significant neuroregenerative properties. These neuroregenerative effects are mediated by various pathways and molecules, including FK binding protein 52 (FKBP 52), growth-associated protein 43 (GAP 43), and heat shock protein 56 (HSP 56), all of which play critical roles in nerve growth and repair [18–21]. Recent animal studies have further shown that continuous low-dose administration of FK506 following sciatic nerve allografting can result in a functional recovery comparable to that of autografts [22, 23]. These findings underscore the therapeutic potential of FK506 not only as an immunosuppressant, but also as a potent neuroregenerative agent, which could play a pivotal role in improving outcomes in peripheral nerve reconstruction.
In the present study, we compared the motor recovery following sciatic nerve reconstruction in model rats separated into three different groups: autograft, cold, and cryopreserved nerve allografts. Additionally, we investigated the effects of administering a continuous low dose of FK506 following the grafting procedure on nerve allografts pre-treated with either cold or cryopreservation. Ultimately, our goal was to establish an effective nerve graft substitute, through a comprehensive assessment of motor recovery, to facilitate a better understanding of the associated recovery mechanisms.
Methods
Experimental design
This study was approved by the Institutional Animal Care and Use Committee (IACUC) of Dankook University (DKU-16-039). A total of sixty-five male Lewis (LEW) rats and twenty-six male) rats weighing 300–400 g (10 weeks old) were used as models. Animal strains were chosen based on strong major histocompatibility complex mismatches. The LEW rats were randomized into five groups, as follows:
Group I (n = 13): Unilateral 10-mm sciatic nerve gap, repaired with an ipsilateral autologous graft, serving as the control group.
Group II (n = 13): The nerve gap was reconstructed with a 10-mm allograft pretreated with cryopreservation in RPMI 1640 solution containing cryoprotectant for 12 weeks.
Group III (n = 13): Nerve gap reconstructed with a 10-mm allograft preserved in cold University of Wisconsin (UW) solution (SPS-1®: Orange Recovery Systems, Inc., Chicago, IL) for 7 weeks.
Group IV (n = 13): Nerve gap reconstructed with a cryopreserved allograft (12 weeks) and treated with low-dose FK506 (0.1 mg/kg) until sacrifice.
Group V (n = 13): Nerve gap reconstructed with a UW-preserved allograft (7 weeks) and treated with low-dose FK506 (0.1 mg/kg) until sacrifice.
The sides of the surgical procedures were randomized.
Twenty weeks after surgery, motor nerve regeneration was evaluated by measuring the rate of ankle contracture, compound muscle action potential (CMAP), maximal isometric tetanic force (MITF), wet muscle weight of the tibialis anterior (TA), and through histomorphometric and immunohistochemical analyses of the peroneal nerve.
Surgical procedure
The rats were anesthetized with isoflurane (Forane; Choongwae Pharma, Seoul, Korea). The sciatic nerve on the experimental side of each rat was exposed through a lateral skin incision. Bilateral sciatic donor nerves were harvested for pretreatment with either cold or cryopreservation as inlay grafts, and donor SD rats were killed using an overdose of pentobarbital. The recipient sciatic nerve was fully exposed from the inferior margin of the piriformis muscle to a point 5-mm distal to the bifurcation of the peroneal and distal tibial branches. Subsequently, a 10-mm segment of the nerve was excised and repaired by interposing a 10-mm nerve segment obtained from the ipsilateral side for Group I, or pretreated allografts from SD donor rats for the other groups, using 10–0 nylon epineural sutures under an operating microscope. All rats had a sciatic nerve gap bridged by reversing the excised nerve segment (Fig. 1).
Fig. 1.
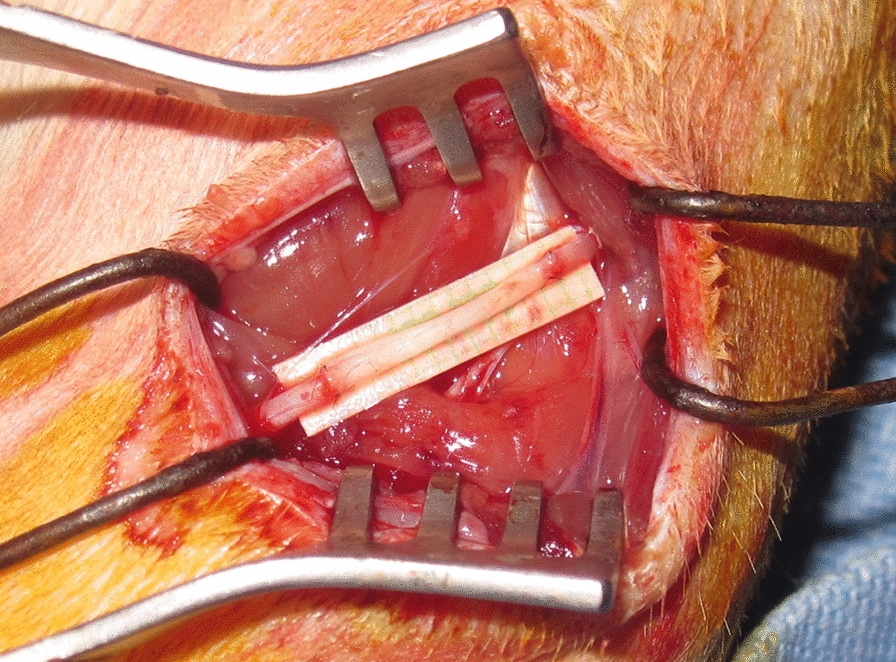
Reconstruction of a 10-mm sciatic nerve gap by reversing the excised or pretreated nerve segment
The muscle was approximated using 4–0 absorbable sutures, and the skin was closed using 4–0 nylon sutures. Postoperatively, the rats were kept warm with towels, and 300 mg/kg acetaminophen (equate™; Wal-Mart Stores, Inc., Bentonville, AR, USA) was added once to the water.
Processing of the cryo-preserved nerve allograft
Twenty-six segments of the 15-mm sciatic nerve obtained bilaterally from 26 SD rats were immersed in gentamycin solution for decontamination. The segments were then preserved in sterile polypropylene cryogenic vials, each containing a solution composed of RPMI1640 with L-glutamine, 10% dimethyl sulfoxide as cryoprotectant, and 2% human albumin. These were then placed in a liquid nitrogen computer-controlled freezer (ICECUBE 1860) that facilitates a controlled decrease in temperature (1 °C/min) to –140 °C. Dimethyl sulfoxide quickly penetrates the cells and binds to water molecules, forming a liquid phase that prevents the formation of ice crystals, which can cause mechanical stress on the cell walls and damage the cells. At very low temperatures, all biological processes and chemical reactions are suspended, while the cell architecture is preserved. After freezing, the tissues were stored at between –140 °C and –160 °C in liquid nitrogen vapor for at least 3 months. Before use, the nerves were thawed at 4 °C, and their structural integrity was confirmed.
Processing of cold-preservation
Twenty-six 15-mm sciatic nerve segments obtained bilaterally from donor SD rats were immersed in an antibiotic solution for decontamination. The nerves were subsequently transferred directly into sterile Petri dishes containing UW solution (SPS-1®: Orange Recovery Systems, Inc., Chicago, IL) with penicillin G (200,000 U/L), regular insulin (40 U/L), and dexamethasone (16 mg/L). The petri dishes with nerve segments were maintained at 4 °C until surgical implantation, or tissue fixation for 7 weeks. The solution was changed weekly for prolonged periods of cold preservation.
Preparation of FK506
FK506 (Prograf®, Astellas Pharma US, Inc., Deerfield, Ill.) was reconstituted with saline to a concentration of 0.1 mg/ml for subcutaneous injection. A dose of 0.1 mg/kg was administered daily to Groups IV and V, starting on the operative day, and continuing until sacrifice at 20 weeks. Doses were based on the animals’ body weights. The rats were carefully observed for dehydration and diarrhea, weighed weekly to ensure accurate dosing, and monitored for weight loss and morbidity.
Measurement and sacrifice procedure at twenty weeks
Ankle contracture
At 20 weeks, rats were re-anesthetized to ensure survival. The ankle contracture angle was determined on the experimental side by measuring the angle between the longitudinal axes of the tibia and foot with the ankle in maximal passive plantar flexion. This measurement was then compared with that of the contralateral normal side (Fig. 2).
Fig. 2.
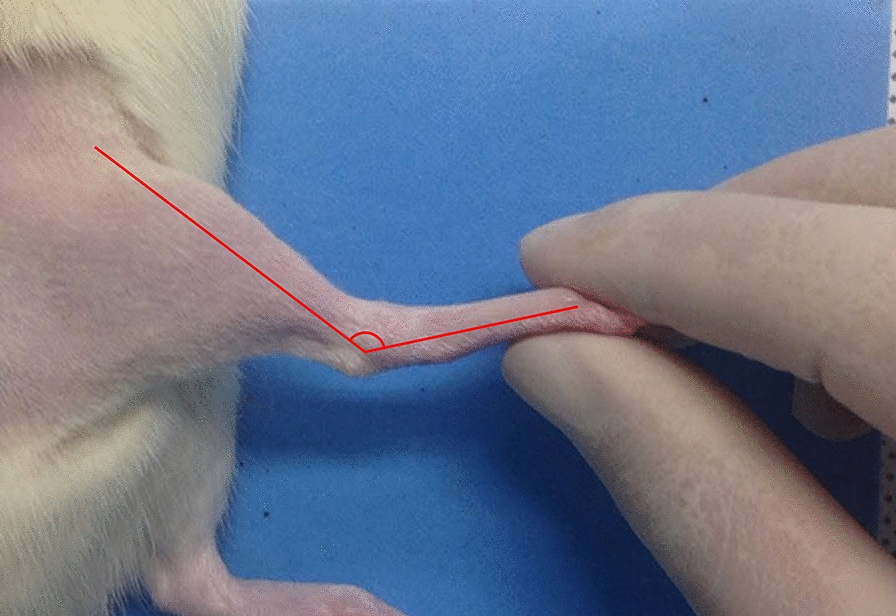
Measurement of the ankle contracture
Electrophysiology
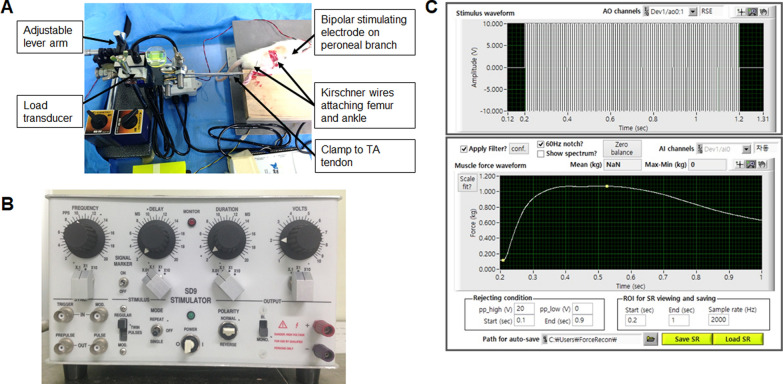
After the sciatic nerve on the experimental side was fully exposed, electrical stimulation was applied using a miniature bipolar stimulating electrode (Harvard Apparatus, Holliston, MA, USA) clamped proximal to the graft site. The stimulation mode was set to pulse (5 mA stimulus intensity, 1 Hz frequency, 1 ms duration). The active surface electrode was then placed in the gastrocnemius muscle belly on the experimental side, the reference surface electrode was placed near the distal tendon, and the ground electrode was placed in the tail. Using a data acquisition system (Powerlab 8/35; AD Instruments Inc., Colorado Springs, CO, USA) and Labchart 7 software (AD Instruments Inc., Colorado Springs, CO, USA) connected to a Bio-amplifier (Bioamp; AD Instruments Inc., Colorado Springs, CO, USA), the peak amplitude of the compound motor action potential (CMAP) was measured. This exposure and measurement procedure was then repeated for the contralateral side, and the CMAP results were normalized using data from the contralateral side (Fig. 3). See the Additional file for more details.
Fig. 3.
Experimental setup for maximal isometric tetanic force testing (A) and the stimulator (B). C The maximal isometric tetanic force was measured using LabView software (National Instruments, Austin, TX), applying stimulation with a frequency from 125 to 175 Hz at 10 V and 0.4 ms of duration under an optimal preload
Maximum isometric tetanic force (MITF)
MITF measurements of the anterior tibial muscles were performed, as previously described [24]. In brief, the tibialis anterior tendon was exposed through an anterior skin incision on the ankle and rigidly fixed to a platform by transfixation using a Kirschner wire. The tibialis anterior tendon was the oriented horizontally and connected to a force transducer (FS05; Inelta Inc., München, Germany), which was mounted on an adjustable lever arm using a custom clamp to regulate muscle tension during isometric contractions. Electrical stimulation was applied using a miniature bipolar stimulating electrode (Harvard Apparatus, Holliston, MA) clamped to the peroneal nerve, and the signal acquired from the force transducer was processed using LabView software (National Instruments, Austin, TX, USA). With an optimal preload, the MITF was measured by applying stimulation with a frequency from 125 to 175 Hz at 10 V and a 0.4 ms duration. The contralateral side was measured in an identical fashion after the skin was closed (Fig. 3; See the Additional file).
Wet muscle weight
After muscle force testing, the animals were euthanized using an overdose of pentobarbital. The tibialis anterior muscles were carefully dissected, and the wet muscles were weighed in grams. See the Additional file.
Histomorphometry of the peroneal nerve
After harvesting of the tibialis anterior muscles, all of the animals were deeply anesthetized, transcardially perfused with saline, and fixed in 4% paraformaldehyde. The sciatic nerve graft segment, including the proximal and distal repair sites, and peroneal nerve segments were harvested.
The peroneal nerve sections were stored in a solution of 2.5% purified glutaraldehyde and 0.5% saccharose, washed in phosphate buffer, and postfixed in 2% osmium tetroxide [25]. The tissue samples were subsequently dehydrated with a graded series of alcohol and embedded in resin, followed by polymerization in a 60 ℃ oven. Finally, the samples were cut into 1-μm sections and stained with toluidine blue (Fisher Scientific, Pittsburgh, Pennsylvania). Histomorphometric analysis was performed using i-Solution software (i-Solution DT: i-Solution Inc., Vancouver, BC, Canada), after digitizing the microscopic images of the nerve sections at 200 × magnification. The morphometric parameters measured included the total nerve area, myelinated fiber area, total number of axons, N-ratio, and nerve fiber density. The N-ratio was calculated as the total myelinated fiber area, divided by the total tissue cable area, which provided information on the number of sprouting events in the regenerating nerve [26]. The nerve fiber density was calculated by dividing the total number of axons by the total nerve area. All histomorphometric measurements were repeated and the results were averaged.
Immunohistochemistry
Immunohistochemical analysis of the nerve specimens was performed as previously described [27]. In brief, the sciatic nerve segments were post-fixed in 4% paraformaldehyde and immersed for 3 days in 30% sucrose solution. The tissues were subsequently embedded in M1 compound (Thermo Fisher Scientific Inc.), and sectioned sagittally on a cryostat at 16 μm. Sections were then treated with 0.2% Triton X-100 in 2% BSA/PBS solution, and blocked with 10% normal serum. Samples were then incubated in primary antibodies (mouse SMI312 monoclonal antibody, 1:1000, Covance, Princeton, NJ, USA; rabbit S-100 polyclonal antibody, 1:1000, Dako Cytomation, Carpinteria, CA, USA) overnight at 4 ℃, and secondary antibodies (FITC-conjugated goat anti-mouse IgG, 1:200, and Rhodamine conjugated goat anti-rabbit IgG, 1:200, both from Jackson ImmunoResearch Labs Inc.) for 2 h at room temperature. Sections were treated with PBS containing DAPI, and coverslipped with Vectashield (Vector Laboratories). Whole SMI312-positive axons at the distal stump (1 mm from the distal end of the scaffold) were observed using confocal microscopy (Carl Zeiss Inc., Oberkochen, Germany) to evaluate the patterns of SMI312-positive axons crossing the allograft and S100-positive Schwann cells along the axon.
Statistical analysis
All results are expressed as a percentage of the value on the contralateral side, to minimize the effect of normal biological variability between animals. The number of rats required in each group was determined based on the results of previous studies on the primary outcome, i.e., the isometric tetanic force of the muscle [24, 28]. It was estimated that 11 rats in each group would provide an 80% power to detect a 10% difference in the mean isometric tetanic muscle force between the groups (α = 0.05, two-sided). To prevent potential attrition, the sample size was increased to 13 rats per group. The groups were compared with respect to weight gain, ankle contracture, CMAP, maximum isometric tetanic force, wet muscle weight, and nerve histomorphometry. All results are expressed as a percentage of the contralateral side to reduce the effect of normal biological variability between animals. One-way analysis of variance (ANOVA) corrected with the Bonferroni post-hoc test was applied to determine statistical differences between groups. All results are reported as the mean ± standard deviation and the level of significance was set at α < 0.05. Analysis was performed using SPSS ver. 23.0 (SPSS Inc., Chicago, IL, USA).
Results
Of the sixty-five rats, five were excluded from the analysis, including one from Group I, two from Group II, and two from Group V. These animals died under anesthesia during either the survival or sacrifice procedure. Apart from these five cases, no complications, such as wound infection or separation at the nerve coaptation site, were noted in the remaining animals. The results are summarized in Tables 1 and 2.
Table 1.
Summary of motor function recovery at 20 weeks
| Group I | Group II | Group III | Group IV | Group V | p-value | |
|---|---|---|---|---|---|---|
| Medication after allograft | autograft(control) | Cryopreserved allograft | Cold preserved allograft | Cryopreserved allograft + FK506 (0.1 mg/kg) | Cold preserved allograft + FK506 (0.1 mg/kg) | |
| No. of animals tested | 12 | 11 | 13 | 13 | 11 | |
| Animal weight | ||||||
| Initial weight (g) | 341 ± 13.35 | 339 ± 26.4 | 327 ± 11.1 | 329 ± 17.6 | 338 ± 25.1 | p = 0.132 |
| Final weight (g) | 521 ± 28.4 | 543 ± 29.1 | 537 ± 19.2 | 486 ± 25.1 | 488 ± 19.1 | p = 0.011 |
| Ankle angle | ||||||
| Maximum passive plantar flexion angle (°) | 127 ± 13.0 | 128 ± 8.5 | 134 ± 12.7 | 138 ± 6.5 | 137 ± 9.8 | p = 0.070 |
| % of contralateral side | 86.1 ± 8.67 | 87.2 ± 6.23 | 90.3 ± 5.91 | 96.5 ± 7.26 | 96.2 ± 5.32 | p < 0.001 |
| CMAP | ||||||
| Amplitude (mV) | 16.0 ± 5.24 | 15.7 ± 5.51 | 18.9 ± 5.66 | 14.9 ± 4.08 | 18.9 ± 5.07 | p = 0.064 |
| % of contralateral side | 63.3 ± 23.67 | 59.9 ± 17.75 | 55.3 ± 14.89 | 61.6 ± 17.75 | 61.0 ± 15.24 | p = 0.082 |
| MITF | ||||||
| Tibialis anterior muscle force (g) | 691.9 ± 115.11 | 609.0 ± 64.57 | 719.0 ± 111.16 | 690.8 ± 111.16 | 682.0 ± 174.59 | p = 0.020 |
| % of contralateral side | 60.9 ± 17.91 | 60.4 ± 16.68 | 60.2 ± 21.29 | 63.7 ± 19.21 | 67.1 ± 19.77 | p = 0.148 |
| Wet muscle weight | ||||||
| Tibialis anterior muscle force (g) | 0.62 ± 0.063 | 0.62 ± 0.050 | 0.597 ± 0.058 | 0.66 ± 0.037 | 0.593 ± 0.064 | p = 0.022 |
| % of contralateral side | 72.1 ± 6.13 | 72.5 ± 6.24 | 69.3 ± 6.53 | 80.5 ± 6.58 | 79.0 ± 8.75 | p = 0.001 |
Table 2.
Morphological analysis of the peroneal nerve at 20 weeks
| Group I | Group II | Group III | Group IV | Group V | p-value | |
|---|---|---|---|---|---|---|
| Medication after allograft | Autograft (control) | Cryopreserved allograft | Cold preserved allograft | Cryopreserved allograft + FK506 (0.2 mg/kg) | Cold preserved allograft + FK506 (0.2 mg/kg) | |
| No. of animals tested | 12 | 11 | 13 | 13 | 11 | |
| Total nerve area (µm2) | 880183 ± 268850 | 872014 ± 123240 | 826854 ± 165708 | 849256 ± 155056 | 812159 ± 169737 | p = 0.920 |
| % of contralateral | 109.48 ± 38.2 | 88.90 ± 18.42 | 96.91 ± 28.44 | 111.22 ± 43.41 | 93.16 ± 21.51 | p = 0.336 |
| Myelinated axon count | 3529 ± 1001.04 | 3866 ± 860.92 | 4776 ± 977.45 | 3580 ± 773.11 | 4532 ± 953.92 | p = 0.003 |
| % of contralateral side | 148.92 ± 59.70 | 174.31 ± 46.10 | 140.76 ± 47.13 | 134.45 ± 48.18 | 148.43 ± 42.55 | p = 0.369 |
| Myeline fiber area (µm2) | 265942 ± 73308 | 214999 ± 49393 | 272209 ± 53276 | 246158 ± 41720 | 289754 ± 64044 | p = 0.037 |
| % of contralateral side | 71.32 ± 17.40 | 59.06 ± 17.44 | 79.77 ± 18.23 | 77.58 ± 20.47 | 87.56 ± 20.57 | p = 0.013 |
| N ratio | 0.3136 ± 0.0371 | 0.2473 ± 0.0470 | 0.3379 ± 0.0515 | 0.2972 ± 0.0370 | 0.3542 ± 0.0872 | p < 0.001 |
| % of contralateral side | 64.80 ± 7.38 | 58.21 ± 10.85 | 68.93 ± 13.48 | 76.28 ± 11.74 | 79.09 ± 8.26 | p < 0.001 |
| Nerve fiber density | 4106 ± 903 | 4457 ± 902 | 5919 ± 1436 | 4314 ± 1005 | 5599 ± 896 | p < 0.001 |
| % of contralateral side | 141.86 ± 58.10 | 198.83 ± 54.07 | 161.46 ± 87.51 | 129.64 ± 87.51 | 161.48 ± 37.83 | p = 0.072 |
| Myelinated axon Diameter (µm) | 22.77 ± 5.07 | 22.45 ± 5.58 | 19.59 ± 6.84 | 24.32 ± 4.98 | 19.00 ± 3.32 | p = 0.123 |
| % of contralateral side | 33.65 ± 12.28 | 27.20 ± 9.59 | 39.51 ± 17.49 | 40.26 ± 12.45 | 38.24 ± 8.77 | p = 0.085 |
Animal weight
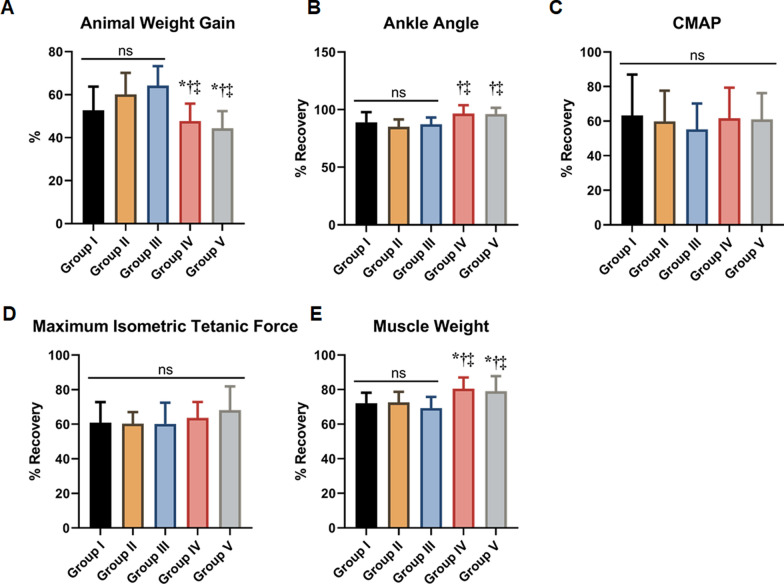
At twenty weeks, the mean percentage of animal weight gain at sacrifice was 52.7 ± 11.7% for Group I, 60.2 ± 10.2% for Group II, 64.2 ± 9.1% for Group III, 47.7 ± 8.1% for Group IV, and 44.4 ± 8.0% for Group V. Group IV and V, which received FK 506 following nerve allograft, showed significantly lower weight gain compared to the other groups (all comparisons, p < 0.001); however, there were no differences between Groups IV and V (p = 0.997; Fig. 4A).
Fig. 4.
Motor regeneration was assessed at 20 weeks. A Weight gain of rats at sacrifice compared to in the survival procedure. B Group comparison for recovery of the ankle angle compared to the contralateral side. C Group comparison for recovery of the compound muscle action potential (CMAP) compared to the contralateral side. D Group comparison for recovery of the Maximum Isomeetric Tetanic Force (MITF), compared to contralateral side. E Group comparison for recovery of muscle weight of the tibialis anterior compared to the contralateral side. *p < 0.05 (vs Group I); †p < 0.05 (vs Group II); ‡p < 0.05 (vs Group III). ns, not significant
Ankle contracture
The normalized ankle plantar flexion angle recovery on the experimental side, expressed as a percentage of the control side, was 86.1% ± 8.7% for Group I, 87.2% ± 6.2% for Group II, 90.3% ± 5.9% for Group III, 96.5% ± 7.3% for Group IV, and 96.2 ± 5.3% for Group V. Group IV and V, which received a low dose of FK506, demonstrated superior recovery of the ankle angle compared to the other groups (all comparisons, p < 0.05); however, there was no significant difference between Groups IV and V (p = 0.853; Fig. 4B).
Compound muscle action potential (CMAP)
The normalized CMAP amplitudes were 63.3 ± 5.2% for Group I, 59.9 ± 17.8% for Group II, 55.3 ± 14.9% for Group III, 61.6 ± 17.8% for Group IV, and 61.0 ± 15.2% for Group V. Group I showed a higher CMAP amplitude compared to the other groups; however, no significant differences were found among the groups (all comparisons, p > 0.05; Fig. 4C).
Maximum isometric tetanic force (MITF)
The mean normalized isometric tetanic force was 60.9 ± 17.9% for Group I, 60.4 ± 6.7% for Group II, 60.2 ± 21.3% for Group III, 63.7 ± 9.2% for Group IV, and 65.1 ± 19.8% for Group V. Groups IV and V showed significantly improved recovery of MITF compared to Groups I, II, and III; however, these differences were not statistically significant (all comparisons, p > 0.05; Fig. 4D).
Muscle weight
The tibialis anterior muscle weight was 72.1 ± 6.1% in Group I, 72.5 ± 6.2% in Group II, 69.3 ± 6.5% in Group III, 80.5 ± 6.6% in Group IV, and 79.0 ± 8.8% in Group V, compared to the contralateral side. Group IV and V, which received a low dose of FK506 after surgery, demonstrated significantly superior recovery of the wet muscle weight compared to the other groups (all comparisons, p < 0.05), while there was no significant difference between group IV and V (p = 0.899; Fig. 4E).
Histomorphometry
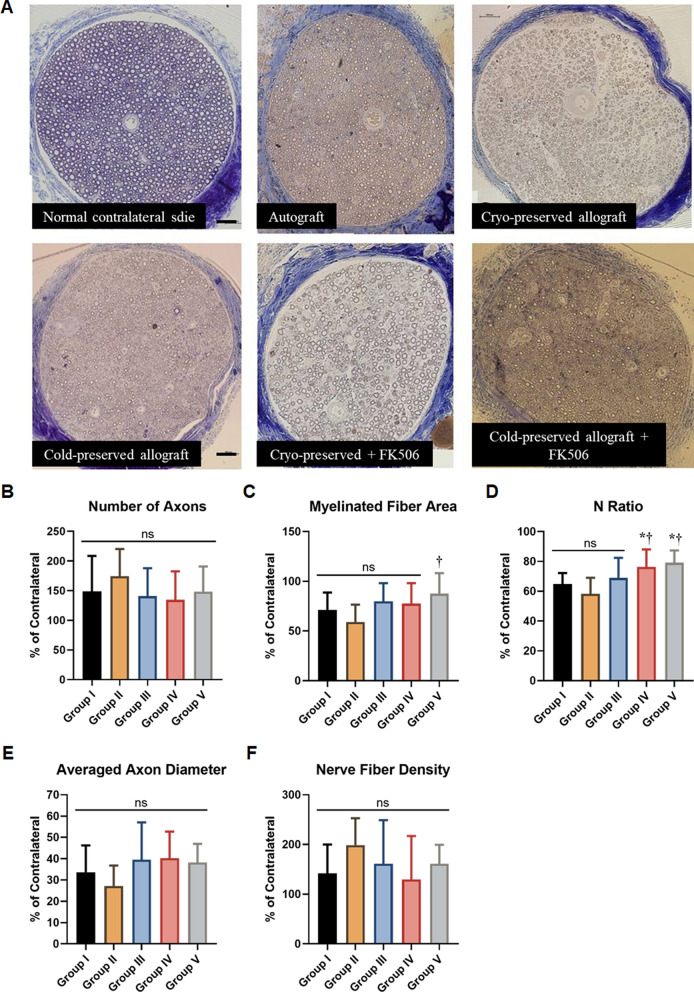
The total area of the peroneal nerve on the allograft-transplanted side showed no significant difference between the groups (p = 0.920); however, the peroneal nerve contained more myelinated axons than the contralateral side. The normalized value of the total number of axons was 148. 9 ± 59.7% for Group I, 174.3 ± 46.1% for Group II, 140.8 ± 47.1% for Group III, 134.5 ± 48.2% for Group IV, and 148.4 ± 42.6% for Group V compared to the contralateral side. None of the groups showed any significant differences (p = 0.369; Fig. 5A and B).
Fig. 5.
Histomorphometry and group comparison for recovery of the peroneal nerve. A Histomorphometry of the peroneal nerve at 20 weeks (Bars = 200 μm). B Group comparison for the total numbers of axon. C Group comparison for myeline fiber area, with respect to the contralateral side. D Group comparison for the N ratio. E Group comparison for the average axon diameter on the operated side. F Group comparison for the nerve fiber density. *p < 0.05 (vs Group I); †p < 0.05 (vs Group II). ns, not significant
The normalized myeline fiber area with respect to the contralateral side was 71.3 ± 17.4% for Group I, 59.1 ± 17.4% for Group II, 79.8 ± 18.2% for Group III, 77.6 ± 20.5% for Group IV, and 87.6 ± 20.6% Group V. Group V exhibited a higher myelinated fiber area than the other groups, with a statistically significant difference compared to Group II (p = 0.007; Fig. 5C).
The N-ratio was 64.8 ± 7.4% for Group I, 58.2 ± 10.9% for Group II, 76.3 ± 11.8% for Group III, 68.9 ± 13.5% for Group IV, and 79.1 ± 8.3% for Group V (p < 0.001). Post hoc analysis revealed that Groups IV and V had higher N ratios than Groups I and II (p = 0.048 and p = 0.002 for group IV; p = 0.019 and p < 0.001 for group V, respectively). However, no significant differences were found between group III and the other groups (p > 0.05; Fig. 5D).
The average axon diameter on the operated side was 22.8 ± 5.1 µm for Group I, 22.5 ± 5.6 μm for Group II, 19.6 ± 6.8 μm for Group III, 24.3 ± 5.0 μm for Group IV, and 19.0 ± 3.3 μm for Group V, corresponding to 33.7 ± 12.3%, 27.2 ± 9.6%, 39.5 ± 17.5%, 40.3 ± 12.5%, and 38.2 ± 8.8% of the contralateral side, respectively. No significant differences were found between the groups with respect to these parameters (p = 0.123 and p = 0.085, respectively; Fig. 5E).
The normalized nerve fiber density was 141.9 ± 58.0% in Group I, 198.8 ± 54.1% in Group II, 161.5 ± 87.5% in Group III, 129. 6 ± 87.5% in Group IV, and 161.5 ± 33.7% in Group V. No significant differences were found between the groups (p = 0.072; Fig. 5F).
Immunohistochemistry
Immunohistochemical analysis of the transplanted sciatic nerve 20 weeks after surgery revealed that axons stained with SMI312 were present across the transplanted allograft in all groups, while the morphology and distribution patterns of the axons were similar in all groups, except for Group II, in which the axons were less densely packed and slightly disorganized (Fig. 6).
Fig. 6.
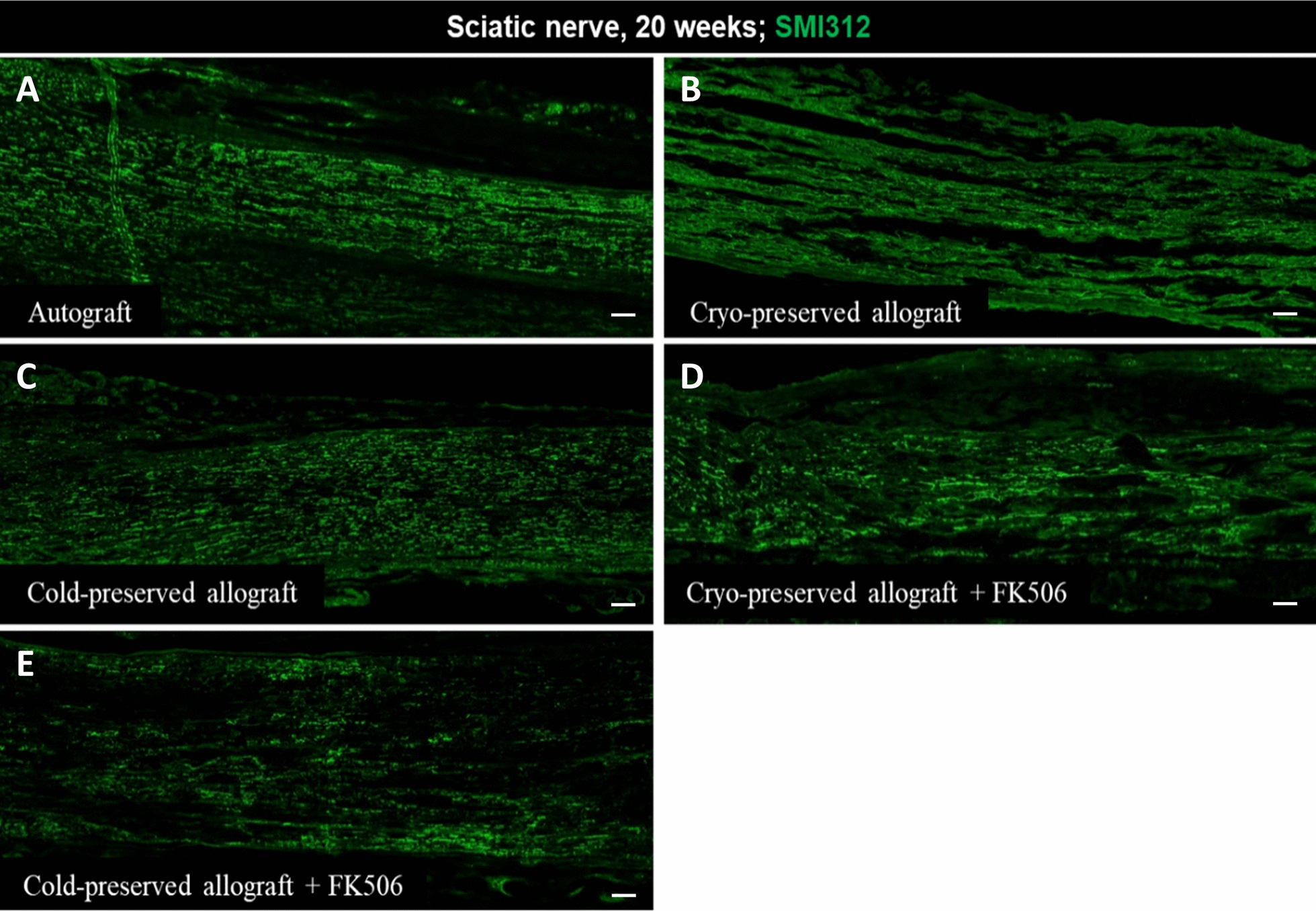
Immunohistochemistry of the transplanted sciatic nerve at 20 weeks. A Autograft. B Cryo-preserved allograft. C Cold-preserved allograft. D Cryo-preserved allograft received FK506. E Cold-presefrved allograft received FK506 (Bars = 200 μm)
Discussion
Owing to the readily supply of nerve tissues of various lengths and diameters, nerve allografts have been extensively investigated in both experimental and clinical research. However, nerve allografts are subject to rejection, necessitating the continuous use of immunosuppressive drugs linked with secondary risks and potentially toxic effects [22, 29–31].
Several animal studies have previously demonstrated that cold- and cryo-preservation techniques decrease the immune response and risk of graft rejection, preserve Schwann cell viability, and maintain the basal lamina of the nerve [17, 32, 33]. These techniques have also been shown to decrease the expression of Class II human leukocyte antigens (HLAs) and intercellular adhesion molecule–1, which are both essential for the immunological recognition of foreign tissues. This reduced expression supports axonal regeneration across the short peripheral nerve gaps. Studies have indicated that cold cryopreservation of nerve allografts harvested from cadaveric donors is an effective surgical strategy to restore the function of damaged peripheral nerves without the need for immunosuppressants.
According to one study by Konofaos et al. [34], peripheral nerves preserved in cold storage for at least 3 weeks have a lower proportion of Schwann cells and a reduced number of viable cells compared to freshly harvested nerves. However, cold preservation for extended periods can diminish lymphocytic infiltration, and delay or prevent the typical biphasic increase in lymphocyte output observed in fresh allografts [35]. Furthermore, cold preservation maintains the intricate basal lamina microstructure and laminin, which is an important extracellular membrane component [17, 33]. Based on previous studies showing that 7 weeks of cold preservation reduces the cellular immune response more effectively than 1 or 4 weeks, we used allografts preserved in UW solution for 7 weeks [32]. Consequently, we confirmed that cold-preserved nerve allografts provided comparable motor function recovery and nerve regeneration to autografts, indicating that prolonged cold preservation could be an effective method for producing unlimited graft material for peripheral nerve reconstruction [32]. Additionally, Mackinnon et al. [4] demonstrated the successful recovery of motor and sensory functions in seven patients with an average nerve gap of 23 cm using several allograft cables followed by immunosuppression.
Schwann cells, which produce neurotrophic factors and basal lamina components essential for supporting regenerating axons, are sensitive to freezing [7, 36, 37]. Recent research by Fasna et al. [37] reported good axonal regeneration without any impairment at 6 weeks after surgery, utilizing DMSO as a cryoprotectant in a rat model, although the results were not as favorable as with fresh autografts. In their study, the basal lamina of Schwann cells and the perineurium remained largely intact after six weeks of cryopreservation. However, in our study, we found no significant differences in histomorphometric parameters between the cryopreservation and autograft groups at 20 weeks post-operatively. Immunohistochemistry of the transplanted sciatic nerve in the cryopreservation group revealed a similar number of SM312-stained axons crossing the transplanted allograft, although their organization was less structured than that of the autograft group. Furthermore, functional recovery measures, including ankle contracture, CMAP, MITF, and wet muscle weight of the tibialis anterior were comparable with those in the autograft group. These findings indicate that cryopreserved nerve allografts can achieve sufficient axonal regeneration, both histologically and clinically, potentially serving as an alternative to autografts without requiring immunosuppressive treatments. Squintani et al. [16] previously reported promising results in 10 clinical cases in which peripheral nerve reconstruction was performed using cryopreserved allografts ranging from 4 to 10 cm. All patients had regained motor function at the 2-year follow-up.
Although several pharmacological agents have been investigated for their potential neuroregenerative properties, most studies have focused on the immunosuppressive agent FK506 [22, 23, 38–41], which exerts neuroregenerative properties linked to its potent T-cell inhibitory effects. The pathways and mediators involved in these effects include FKBP 52, GAP 43, and HSP 56 [18–21]. In fact, continuous FK506 administration over extended time periods has produced functional recovery levels comparable to those achieved with autografts in animal studies involving sciatic nerve reconstruction using allografts [40–42].
Previous studies have addressed the dose-dependent effects of FK506 on nerve regeneration in composite tissue allografts. According to Wang et al. [43], 5 mg/kg is the most effective dose for promoting axonal regeneration in a rat model of sciatic nerve crush. Yang et al. [23] further determined that a dose of 2.0 mg/kg FK506 significantly increased the nerve percentage, density, fiber width, and total number of fibers. However, they also found notable neuroregenerative effects at low doses of 0.5 or 1.0 mg/kg FK506 per day, although these doses were insufficient for immunosuppression in composite tissue allografts. In contrast, Rustemeyer et al. [22] reported that 0.2 mg/kg FK506 was less effective than 0.1 mg/kg for nerve regeneration. This discrepancy may be due to the factors that influence FK506’s affinity for FKBP, as their study involved slightly mismatched strains (Lewis and Dark Agouti strains).
In this study, which used strongly mismatched major histocompatibility complex rats as a model, allograft groups treated with 0.1 mg/kg FK506 showed superior recovery in some functional and histological parameters compared to the allograft group without FK506 administration, demonstrating the neuroregeneration effects of a low dose of FK506 [44, 45]. The results of this and prior studies could be explained by Udina et al. [46] and Rustemeyer et al. [22], who suggested that a low dose of FK506 induces neuroregenerative effects by activating the immunuophilin FKBP-52 without significantly activating FKBP-12, which mediates immunosuppressive effects. Conversely, at higher doses, FK506 preferentially bound to FKBP-12, leading to enhanced immunosuppression and reduced neuroregenerative effects. However, factors influencing the affinity of FK506 for FKBPs depending on concentration still remain unclear.
Previous studies have indicated that cold- or cryopreserved allografts may delay revascularization, resulting in inferior early axonal regeneration [17, 47, 48]. Zhang et al. [49] suggested that cryopreservation of allografts might compromise endoneurial revascularization [37]. However, as noted in our study, immunohistochemical analysis of the cryopreservation group without FK506 administration revealed less densely packed, and somewhat disorganized axons compared with the cryopreservation group treated with FK506, despite no significant differences in motor function and histomorphometry of the nerve distal to the repair site between the cryopreservation group without FK506 and the autograft group. The decreased survival of resident Schwann cells in cryopreserved allografts may further adversely affect regeneration due to the lack of neurotrophic and neurotropic factors, as well as adhesion molecules typically provided by Schwann cells during early regeneration [37]. This further suggests that axonal regeneration in the cryopreserved allograft may primarily depend not on the migration ability of host Schwann cells, but rather on resident Schwann cells in early regeneration [50]. Therefore, cryopreservation alone may not sufficiently support axonal regeneration in patients with long-segment peripheral nerve defects.
This study is limited by the use of only a single dose of 0.1 mg/kg FK506 and a single endpoint (20 weeks), which may not be optimal for observing motor recovery. Further, we did not select an FK506 dose based on clinically relevant scenarios. Evaluating multiple time points before sacrifice would provide a better understanding of the progress of nerve regeneration; however, this approach would be challenging because of the potential for attrition. Additionally, mechanical testing, such as tensile strength measurements, and functional assessments, like the Sciatic Functional Index (SFI), were not performed in this study. Including these evaluations would have provided a more comprehensive understanding of the structural and functional outcomes of nerve regeneration. Their omission represents a limitation of this study, and future research should consider these assessments to further enhance the evaluation of nerve reconstruction techniques [51]. One noteworthy finding in this study was the lower weight gain observed in the groups treated with FK506 (Groups IV and V) compared to the other groups. FK506 is known to induce unexpected weight loss, which may be attributed to gastrointestinal side effects such as nausea, vomiting, or diarrhea, as well as metabolic alterations or immunosuppression. Although the exact mechanisms remain unclear, some studies have reported significant weight loss associated with FK506 administration [52–54]. This association highlights the importance of monitoring systemic effects in future studies involving FK506 treatment.
Conclusion
Overall, the results of this study indicate that pretreatment of allograft nerves could be an effective alternative for reconstructing peripheral nerve defects. The administration of a low dose of FK506 accelerated motor recovery after nerve reconstruction using cold or cryopreserved allografts. Based on this rat model, using cold- or cryopreserved allograft nerves from cadaveric donors appears to be a valid surgical strategy to restore motor function in damaged peripheral nerves without the need for immunosuppression.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
JPK and SCH contributed equally to this work. Both JHL and KWK are co-corresponding authors for this study.
Abbreviations
- ANOVA
Analysis of variance
- CMAP
Compound muscle action potential
- FKBP 52
FK binding protein 52
- GAP 43
Growth-associated protein 43
- HLAs
Human leukocyte antigens
- HSP 56
Heat shock protein 56
- MITF
Maximal isometric tetanic force
- TA
Tibialis anterior
Authors’ contributions
JPK, SCH and DHL processed the experimental data, performed the analysis, drafted the manuscript and designed the figures. HWK, IYP aided in interpreting the results and worked on the manuscript. JSB, YKS and SHS performed the measurements, JHL and KWK were involved in planning and supervised the work.
Funding
This research was supported by the Bio&Medical Technology Development Program of the National Research Foundation (NRF) funded by the Korean government (MSIT) (RS-2-00220408) and by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2022R1I1A3069471).
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethical approval and consent to participate
All procedures involving animals were reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) of the DanKook University (DKU-16-039).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jong Pil Kim and Soon Chul Heo have contributed equally to this work.
Contributor Information
Jun Hee Lee, Email: junheelee@dankook.ac.kr.
Kyung Wook Kim, Email: scanlip@naver.com.
References
- 1.Griffin JW, Hogan MV, Chhabra AB, Deal DN. Peripheral nerve repair and reconstruction. J Bone Joint Surg Am. 2013;95:2144–51. 10.2106/JBJS.L.00704. [DOI] [PubMed] [Google Scholar]
- 2.Shin RH, Friedrich PF, Crum BA, Bishop AT, Shin AY. Treatment of a segmental nerve defect in the rat with use of bioabsorbable synthetic nerve conduits: a comparison of commercially available conduits. J Bone Joint Surg Am. 2009;91:2194–204. 10.2106/JBJS.H.01301. [DOI] [PubMed] [Google Scholar]
- 3.Sahakyants T, Lee JY, Friedrich PF, Bishop AT, Shin AY. Return of motor function after repair of a 3-cm gap in a rabbit peroneal nerve: a comparison of autograft, collagen conduit, and conduit filled with collagen-GAG matrix. J Bone Joint Surg Am. 2013;95:1952–8. 10.2106/JBJS.M.00215. [DOI] [PubMed] [Google Scholar]
- 4.Mackinnon SE, Doolabh VB, Novak CB, Trulock EP. Clinical outcome following nerve allograft transplantation. Plast Reconstr Surg. 2001;107:1419–29. 10.1097/00006534-200105000-00016. [DOI] [PubMed] [Google Scholar]
- 5.Moore AM, Ray WZ, Chenard KE, Tung T, Mackinnon SE. Nerve allotransplantation as it pertains to composite tissue transplantation. Hand (N Y). 2009;4:239–44. 10.1007/s11552-009-9183-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Isaacs J, Browne T. Overcoming short gaps in peripheral nerve repair: conduits and human acellular nerve allograft. Hand (N Y). 2014;9:131–7. 10.1007/s11552-014-9601-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rinker B, Vyas KS. Clinical applications of autografts, conduits, and allografts in repair of nerve defects in the hand: current guidelines. Clin Plast Surg. 2014;41:533–50. 10.1016/j.cps.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 8.Karabekmez FE, Duymaz A, Moran SL. Early clinical outcomes with the use of decellularized nerve allograft for repair of sensory defects within the hand. Hand (N Y). 2009;4:245–9. 10.1007/s11552-009-9195-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhong H, Chen B, Lu S, Zhao M, Guo Y, Hou S. Nerve regeneration and functional recovery after a sciatic nerve gap is repaired by an acellular nerve allograft made through chemical extraction in canines. J Reconstr Microsurg. 2007;23:479–87. 10.1055/s-2007-992340. [DOI] [PubMed] [Google Scholar]
- 10.Gilpin A, Yang Y. Decellularization strategies for regenerative medicine: from processing techniques to applications. BioMed Res Int. 2017;2017:9831534. 10.1155/2017/9831534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brooks DN, Weber RV, Chao JD, Rinker BD, Zoldos J, Robichaux MR, et al. Processed nerve allografts for peripheral nerve reconstruction: a multicenter study of utilization and outcomes in sensory, mixed, and motor nerve reconstructions. Microsurgery. 2012;32:1–14. 10.1002/micr.20975. [DOI] [PubMed] [Google Scholar]
- 12.Cho MS, Rinker BD, Weber RV, Chao JD, Ingari JV, Brooks D, et al. Functional outcome following nerve repair in the upper extremity using processed nerve allograft. J Hand Surg Am. 2012;37:2340–9. 10.1016/j.jhsa.2012.08.028. [DOI] [PubMed] [Google Scholar]
- 13.Jessen K, Mirsky R. The repair Schwann cell and its function in regenerating nerves. J Physiol. 2016;594:3521–31. 10.1113/JP270874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hess JR, Brenner MJ, Fox IK, Nichols CM, Myckatyn TM, Hunter DA, et al. Use of cold-preserved allografts seeded with autologous Schwann cells in the treatment of a long-gap peripheral nerve injury. Plast Reconstr Surg. 2007;119:246–59. 10.1097/01.prs.0000245341.71666.97. [DOI] [PubMed] [Google Scholar]
- 15.Brenner MJ, Lowe JB 3rd, Fox IK, Mackinnon SE, Hunter DA, Darcy MD, et al. Effects of Schwann cells and donor antigen on long-nerve allograft regeneration. Microsurgery. 2005;25:61–70. 10.1002/micr.20083. [DOI] [PubMed] [Google Scholar]
- 16.Squintani G, Bonetti B, Paolin A, Vici D, Cogliati E, Murer B, et al. Nerve regeneration across cryopreserved allografts from cadaveric donors: a novel approach for peripheral nerve reconstruction. J Neurosurg. 2013;119:907–13. 10.3171/2013.6.JNS121801. [DOI] [PubMed] [Google Scholar]
- 17.Evans PJ, Mackinnon SE, Levi AD, Wade JA, Hunter DA, Nakao Y, et al. Cold preserved nerve allografts: changes in basement membrane, viability, immunogenicity, and regeneration. Muscle Nerve. 1998;21:1507–22. 10.1002/(sici)1097-4598(199811)21:11%3c1507::aid-mus21%3e3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 18.Goslin K, Schreyer DJ, Skene JH, Banker G. Development of neuronal polarity: GAP-43 distinguishes axonal from dendritic growth cones. Nature. 1988;336:672–4. 10.1038/336672a0. [DOI] [PubMed] [Google Scholar]
- 19.Gold BG, Densmore V, Shou W, Matzuk MM, Gordon HS. Immunophilin FK506-binding protein 52 (not FK506-binding protein 12) mediates the neurotrophic action of FK506. J Pharmacol Exp Ther. 1999;289:1202–10. [PubMed] [Google Scholar]
- 20.Sanchez ER. Hsp56: a novel heat shock protein associated with untransformed steroid receptor complexes. J Biol Chem. 1990;265:22067–70. 10.1016/S0021-9258(18)45667-0. [PubMed] [Google Scholar]
- 21.Lyons WE, Steiner JP, Snyder SH, Dawson TM. Neuronal regeneration enhances the expression of the immunophilin FKBP-12. J Neurosci. 1995;15:2985–94. 10.1523/JNEUROSCI.15-04-02985.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rustemeyer J, Dicke U. Allografting combined with systemic FK506 produces greater functional recovery than conduit implantation in a rat model of sciatic nerve injury. J Reconstr Microsurg. 2010;26:123–9. 10.1055/s-0029-1243297. [DOI] [PubMed] [Google Scholar]
- 23.Yang RK, Lowe JB 3rd, Sobol JB, Sen SK, Hunter DA, Mackinnon SE. Dose-dependent effects of FK506 on neuroregeneration in a rat model. Plast Reconstr Surg. 2003;112:1832–40. 10.1097/01.PRS.0000091167.27303.18. [DOI] [PubMed] [Google Scholar]
- 24.Shin RH, Vathana T, Giessler GA, Friedrich PF, Bishop AT, Shin AY. Isometric tetanic force measurement method of the tibialis anterior in the rat. Microsurgery. 2008;28:452–7. 10.1002/micr.20520. [DOI] [PubMed] [Google Scholar]
- 25.Di Scipio F, Raimondo S, Tos P, Geuna S. A simple protocol for paraffin-embedded myelin sheath staining with osmium tetroxide for light microscope observation. Microsc Res Tech. 2008;71:497–502. 10.1002/jemt.20577. [DOI] [PubMed] [Google Scholar]
- 26.Itoh S, Takakuda K, Ichinose S, Kikuchi M, Schinomiya K. A study of induction of nerve regeneration using bioabsorbable tubes. J Reconstr Microsurg. 2001;17:115–23. 10.1055/s-2001-12700. [DOI] [PubMed] [Google Scholar]
- 27.Ahn HS, Hwang JY, Kim MS, Lee JY, Kim JW, Kim HS, et al. Carbon-nanotube-interfaced glass fiber scaffold for regeneration of transected sciatic nerve. Acta Biomater. 2015;13:324–34. 10.1016/j.actbio.2014.11.026. [DOI] [PubMed] [Google Scholar]
- 28.Lee JY, Giusti G, Friedrich PF, Archibald SJ, Kemnitzer JE, Patel J, et al. The effect of collagen nerve conduits filled with collagen-glycosaminoglycan matrix on peripheral motor nerve regeneration in a rat model. J Bone Joint Surg Am. 2012;94:2084–91. 10.2106/JBJS.K.00658. [DOI] [PubMed] [Google Scholar]
- 29.Doolabh VB, Mackinnon SE. FK506 accelerates functional recovery following nerve grafting in a rat model. Plast Reconstr Surg. 1999;103:1928–36. 10.1097/00006534-199906000-00018. [DOI] [PubMed] [Google Scholar]
- 30.Fansa H, Keilhoff G, Altmann S, Plogmeier K, Wolf G, Schneider W. The effect of the immunosuppressant FK 506 on peripheral nerve regeneration following nerve grafting. J Hand Surg Br. 1999;24:38–42. 10.1016/s0266-7681(99)90021-9. [DOI] [PubMed] [Google Scholar]
- 31.Udina E, Gold BG, Navarro X. Comparison of continuous and discontinuous FK506 administration on autograft or allograft repair of sciatic nerve resection. Muscle Nerve. 2004;29:812–22. 10.1002/mus.20029. [DOI] [PubMed] [Google Scholar]
- 32.Fox IK, Jaramillo A, Hunter DA, Rickman SR, Mohanakumar T, Mackinnon SE. Prolonged cold-preservation of nerve allografts. Muscle Nerve. 2005;31:59–69. 10.1002/mus.20231. [DOI] [PubMed] [Google Scholar]
- 33.Atchabahian A, Mackinnon SE, Hunter DA. Cold preservation of nerve grafts decreases expression of ICAM-1 and class II MHC antigens. J Reconstr Microsurg. 1999;15:307–11. 10.1055/s-2007-1000107. [DOI] [PubMed] [Google Scholar]
- 34.Konofaos P, Burns J, Terzis JK. Effect of low-dose FK506 after contralateral C7 transfer to the musculocutaneous nerve: a study in rats. J Reconstr Microsurg. 2010;26:225–33. 10.1055/s-0030-1248230. [DOI] [PubMed] [Google Scholar]
- 35.Evans PJ, MacKinnon SE, Midha R, Wade JA, Hunter DA, Nakao Y, et al. Regeneration across cold preserved peripheral nerve allografts. Microsurgery. 1999;19:115–27. 10.1002/(sici)1098-2752(1999)19:3%3c115::aid-micr1%3e3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 36.Houschyar KS, Momeni A, Pyles MN, Cha JY, Maan ZN, Duscher D, et al. The role of current techniques and concepts in peripheral nerve repair. Plast Surg Int. 2016;2016:4175293. 10.1155/2016/4175293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fansa H, Lassner F, Kook PH, Keilhoff G, Schneider W. Cryopreservation of peripheral nerve grafts. Muscle Nerve. 2000;23:1227–33. 10.1002/1097-4598(200008)23:8%3c1227::aid-mus11%3e3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 38.Azizi S, Mohammadi R, Amini K, Fallah R. Effects of topically administered FK506 on sciatic nerve regeneration and reinnervation after vein graft repair of short nerve gaps. Neurosurg Focus. 2012;32:E5. 10.3171/2012.1.FOCUS11320. [DOI] [PubMed] [Google Scholar]
- 39.Gold BG, Katoh K, Storm-Dickerson T. The immunosuppressant FK506 increases the rate of axonal regeneration in rat sciatic nerve. J Neurosci. 1995;15:7509–16. 10.1523/JNEUROSCI.15-11-07509.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jensen JN, Brenner MJ, Tung TH, Hunter DA, Mackinnon SE. Effect of FK506 on peripheral nerve regeneration through long grafts in inbred swine. Ann Plast Surg. 2005;54:420–7. 10.1097/01.sap.0000151461.60911.c0. [DOI] [PubMed] [Google Scholar]
- 41.Udina E, Voda J, Gold BG, Navarro X. Comparative dose-dependence study of FK506 on transected mouse sciatic nerve repaired by allograft or xenograft. J Peripher Nerv Syst. 2003;8:145–54. 10.1046/j.1529-8027.2003.03020.x. [DOI] [PubMed] [Google Scholar]
- 42.Cottrell BL, Perez-Abadia G, Onifer SM, Magnuson DS, Burke DA, Grossi FV, et al. Neuroregeneration in composite tissue allografts: effect of low-dose FK506 and mycophenolate mofetil immunotherapy. Plast Reconstr Surg. 2006;118:615–23. 10.1097/01.prs.0000233029.57397.4a. [DOI] [PubMed] [Google Scholar]
- 43.Wang MS, Zeleny-Pooley M, Gold BG. Comparative dose-dependence study of FK506 and cyclosporin A on the rate of axonal regeneration in the rat sciatic nerve. J Pharmacol Exp Ther. 1997;282:1084–93. [PubMed] [Google Scholar]
- 44.Lin H, Wei RQ, Bolling SF. Tumor necrosis factor-alpha and interferon-gamma modulation of nitric oxide and allograft survival. J Surg Res. 1995;59:103–10. 10.1006/jsre.1995.1139. [DOI] [PubMed] [Google Scholar]
- 45.Zamfirescu DG, Owen E, Lascar I, Molitor M, Zegrea I, Popescu M, et al. Sentinel skin allograft-a reliable marker for monitoring of composite tissue transplant rejection. Transpl Proc. 2009;41:503–8. 10.1016/j.transproceed.2009.01.023. [DOI] [PubMed] [Google Scholar]
- 46.Udina E, Ceballos D, Verdú E, Gold BG, Navarro X. Bimodal dose-dependence of FK506 on the rate of axonal regeneration in mouse peripheral nerve. Muscle Nerve. 2002;26:348–55. 10.1002/mus.10195. [DOI] [PubMed] [Google Scholar]
- 47.Hirasé Y, Kojima T, Uchida M, Takeishi M. Cryopreserved allogeneic vessel and nerve grafts: hind-limb replantation model in the rat. J Reconstr Microsurg. 1992;8:437–43. 10.1055/s-2007-1006728. [DOI] [PubMed] [Google Scholar]
- 48.Zalewski AA, Fahy GM, Azzam NA, Azzam RN. The fate of cryopreserved nerve isografts and allografts in normal and immunosuppressed rats. J Comp Neurol. 1993;331:134–47. 10.1002/cne.903310109. [DOI] [PubMed] [Google Scholar]
- 49.Zhang F, Attkiss KJ, Walker M, Buncke HJ. Effect of cryopreservation on survival of composite tissue grafts. J Reconstr Microsurg. 1998;14:559–64. 10.1055/s-2008-1040776. [DOI] [PubMed] [Google Scholar]
- 50.Trumble TE, Parvin D. Cell viability and migration in nerve isografts and allografts. J Reconstr Microsurg. 1994;10:27–34. 10.1055/s-2007-1006568. [DOI] [PubMed] [Google Scholar]
- 51.Mohammadi R, Azizi S, Delirezh N, Hobbenaghi R, Amini K. Functional, histomorphometrical and immunohistochemical assessment of sciatic nerve regeneration through inside-out vein graft in rat. Iran J Vet Surg. 2008;03:39–50. [Google Scholar]
- 52.Hwang HP, Yu HC, Chung BH, et al. Severe anorexia and weight loss induced by tacrolimus in kidney transplant recipients. Korean J Transpl. 2021;35:108–11. 10.4285/kjt.2021.35.2.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Francke M, Visser W, Severs D, de Mik-van EA, Hesselink D, De Winter B. Body composition is associated with tacrolimus pharmacokinetics in kidney transplant recipients. Eur J Clin Pharmacol. 2022;78:1273–87. 10.1007/s00228-022-03323-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kolonko A, Pokora P, Słabiak-Błaż N, et al. The relationship between initial tacrolimus metabolism rate and recipients body composition in kidney transplantation. J Clin Med. 2021;10:5793. 10.3390/jcm10245793. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.