Abstract
Background:
Conflicting data exist around oral contraceptive exposure and subsequent multiple sclerosis (MS).
Objective:
To use routinely collected primary healthcare data to explore the potential association between oral contraceptive exposure and subsequent MS in females at population level.
Methods:
We performed a nested case–control study using electronic primary care data, with complete electronic ascertainment from 1990. Logistic regression was used to evaluate associations between contraceptive exposure and MS, without and with adjusting for age, ethnicity and deprivation.
Results:
A total of 4455 females were included: 891 cases and 3564 controls. No association was seen between oral contraceptive exposure and subsequent MS, or between any contraceptive, combined oral contraceptive pill (COCP) or progesterone-only pill (POP) use 0–2, 2–5 or >5 years prior to MS. Conclusions: In the largest population-based study to date, we find no evidence of an association between oral contraceptive exposure and subsequent MS diagnosis.
Keywords: Epidemiology, multiple sclerosis
Introduction
Multiple sclerosis (MS) is an inflammatory disease of the central nervous system. It is more common in females than males, and symptom onset is most commonly in early to mid-adulthood. 1 Given the sex ratio in both incidence and prevalence, 2 sex differences in clinical manifestations 3 and changes in the natural history of the disease around pregnancy,4,5 the hypothesis that sex hormones play a role in the development of MS is well established.
Conflicting data exist around hormonal oral contraceptive (OC) exposure and subsequent MS. Secondary care-based case–control studies have associated OC use with higher risk of MS; 6 however, primary care record studies have previously associated ‘ever’ use with lower MS risk. 7 Some have suggested that long-term use of OC is not associated with a significant change in MS risk.8,9 A further source of complexity is that prescription practices have been shown to change during the MS prodrome, 10 supporting a secondary behavioural influence on these observations.
As retrospective studies are confounded by recall bias, and studies based on secondary and tertiary care populations only include a subset of the MS population, leveraging prospectively recorded primary healthcare data offers an ideal opportunity to try to better understand this relationship. Understanding the time-based patterns of contraceptive initiation and hormonal exposures can help with causal inference, and separating out OC type enables the evaluation of oestrogen exposure at least partially distinct from contraceptive intent.
In this study, we evaluated the association of OC prescription including oestrogen-containing pills and progesterone-only pills (POPs) with the subsequent diagnosis of MS in a routinely acquired primary care dataset from East London.
Methods
The current study was based on East London electronic primary care data, collected up to February 2018.11,12 Briefly, primary care data were compiled from Egton Medical Information Systems (EMIS) electronic health care records for the Secure Health Analysis and Research in East London project. Anonymised individual-level records from four clinical commissioning groups (CCGs) in East London (Hackney and City of London, Newham, Tower Hamlets and Waltham Forest) were included, incorporating clinical diagnoses and prescriptions entered directly into the EMIS system from 1990 with paper records prior to this manually transcribed at GP practice level. The database included health records of 1,016,277 patients from general practices. A nested case–control design was performed. Only individuals with sex recorded as female were included in analyses.
All females with a recorded and dated diagnosis of MS were identified as cases. Codes used to identify MS cases were as previously described. 12 Four controls, matched by month and year of birth, were assigned to each case and given a dummy date of MS diagnosis corresponding to the MS diagnosis date of their matched case.
Two types of OCs were included in the study, oestrogen-containing OC, also known as combined oral contraceptive pill (COCP), and progesterone-only contraceptive pills (POP). A prescription associated with a date prior to MS diagnosis was treated as an exposure. Non-exposure was defined as (1) individuals without a prescription of OC at any time or (2) individuals with a prescription only after MS diagnosis. A prescription record with a term of the drug but without a valid date of prescription or vice versa was regarded as ‘Unknown’. To evaluate the date order of prescription and MS diagnosis for controls, each control was assigned a dummy date of MS diagnosis corresponding to the diagnosis date of their matched case. Cases with an MS diagnosis age of <18 years were excluded from analyses.
Logistic regression was used to evaluate associations between contraceptive exposures and subsequent MS diagnosis, without and with adjusting for age, ethnicity and index of multiple deprivation (IMD). Three time periods (0–2, 2–5 and >5 years before diagnosis) were analysed in addition to the entire pre-diagnostic period. Given the need for prescription, OC exposure from 1990 to 2018 was reliably captured in this study. Exposures to any OCs were first evaluated and then COCP and POP were explored separately. All analyses were performed using R, and a p-value < 0.05 was taken as statically significant.
Ethical permissions
The NHS Health Research Authority waived the need for ethical approval when using this anonymised population-level data set.
Results
A total of 4455 females were included in the analyses: 891 cases and 3564 controls. The demographic details of included participants are shown in Table 1. The mean age at MS diagnosis was 36.4 years, with a median age at MS diagnosis of 35.0 years. A total of 213 (23.9%) and 777 (21.8%) of cases and controls, respectively, had been prescribed at least one form of contraceptive prior to MS onset or corresponding dummy date. The pattern of contraceptive prescriptions was similar in cases and controls, with around 45% prescribed COCP only, 26% prescribed POP only and 29% having been prescribed both contraceptives (Table 2).
Table 1.
Demographic information on multiple sclerosis (MS) cases and matched controls in East London primary care data.
| Characteristic | Case (N = 891) | Control (N = 3564) |
|---|---|---|
| Age, mean (SD), years | 47 (14.3) | 47 (14.3) |
| Age of MS, mean (SD), years | 36 (11.3) | NA (NA) |
| Ethnicity | ||
| White | 551 (61.8%) | 1612 (45.2%) |
| Black | 128 (14.4%) | 601 (16.9%) |
| South Asian | 59 (6.6%) | 702 (19.7%) |
| Other | 72 (8.1%) | 403 (11.3%) |
| Unknown | 81 (9.1%) | 246 (6.9%) |
| IMD | ||
| 1-2 (most deprived) | 332 (37.3%) | 1375 (38.6%) |
| 3-4 | 257 (28.8%) | 1121 (31.5%) |
| 5-6 | 59 (6.6%) | 160 (4.5%) |
| 7-8 | 14 (1.6%) | 45 (1.3%) |
| 9-10 (least deprived) | 13 (1.5%) | 23 (0.6%) |
| Unknown | 216 (24.2%) | 840 (23.6%) |
| Any contraceptives | 213 (100%) | 777 (100%) |
| COCP prescription only | 95 (44.6%) | 350 (45.0%) |
| POP prescription only | 56 (26.3%) | 198 (25.5%) |
| COCP prescription and POP prescription | 62 (29.1%) | 229 (29.5%) |
SD: standard deviation; MS: multiple sclerosis; NA: not applicable; IMD: index of multiple deprivation; COCP: combined oral contraceptive pill; POP: progesterone-only pill.
Table 2.
Odds ratios of the association between (a) any oral contraceptive pill exposure and subsequent multiple sclerosis, (b) combined oral contraceptive pill exposure and subsequent multiple sclerosis and (c) progesterone-only oral contraceptive exposure and subsequent multiple sclerosis.
| All | <2 years before diagnosis | 2-5 years before diagnosis | >5 years before diagnosis | |
|---|---|---|---|---|
| Any oral contraceptive pill exposure and subsequent multiple sclerosis | ||||
| Odds ratio (95% CI) for any OC from unadjusted model | 1.13 (0.95, 1.34) | 1.03 (0.70, 1.48) | 1.32 (0.97, 1.77) | 1.07 (0.86, 1.34) |
| Odds ratio (95% CI) for any OC from adjusted model a | 1.13 (0.94, 1.35) | 1.04 (0.70, 1.51) | 1.34 (0.98, 1.83) | 1.06 (0.84, 1.33) |
| Number of cases (% of N) | 891 (20.0%) | 715 (19.6%) | 739 (19.9%) | 793 (19.7%) |
| Number of cases with non-exposure (%) | 678 (76.1%) | 678 (94.8%) | 678 (91.7%) | 678 (85.5%) |
| Number of cases with OC exposure (%) | 213 (23.9%) | 37 (5.2%) | 61 (8.3%) | 115 (14.5%) |
| Number of controls (% of N) | 3564 (80.0%) | 2933 (80.4%) | 2976 (80.1%) | 3226 (80.3%) |
| Number of controls with non-exposure (%) | 2786 (78.2%) | 2786 (95.0%) | 2786 (93.6%) | 2786 (86.4%) |
| Number of controls with OC exposure (%) | 777 (21.8%) | 147 (5.0%) | 190 (6.4%) | 440 (13.6%) |
| Combined oral contraceptive pill exposure and subsequent multiple sclerosis | ||||
| Odds ratio (95% CI) for COCP from unadjusted model | 1.10 (0.91, 1.33) | 0.95 (0.59, 1.48) | 1.25 (0.85, 1.79) | 1.09 (0.85, 1.38) |
| Odds ratio (95% CI) for COCP from adjusted model a | 1.08 (0.88, 1.32) | 0.93 (0.57, 1.48) | 1.25 (0.84, 1.81) | 1.06 (0.83, 1.35) |
| Number of cases (% of N) | 891 (20.0%) | 757 (19.7%) | 773 (19.9%) | 829 (19.9%) |
| Number of cases with non-COCP (%) | 734 (82.4%) | 734 (97.0%) | 734 (95.0%) | 734 (88.5%) |
| Number of cases with COCP exposure (%) | 157 (17.6%) | 23 (3.0%) | 39 (5.0%) | 95 (11.5%) |
| Number of controls (% of N) | 3564 (80.0%) | 3081 (80.3%) | 3110 (80.1%) | 3337 (80.1%) |
| Number of controls with non-COCP (%) | 2983 (83.7%) | 2983 (96.8%) | 2983 (95.9%) | 2983 (89.4%) |
| Number of controls with COCP exposure (%) | 579 (16.2%) | 98 (3.2%) | 127 (4.1%) | 354 (10.6%) |
| Progesterone-only oral contraceptive exposure and subsequent multiple sclerosis | ||||
| Odds ratio (95% CI) for POP from unadjusted model | 1.12 (0.90, 1.39) | 1.35 (0.88, 2.02) | 1.19 (0.80, 1.72) | 0.98 (0.71, 1.33) |
| Odds ratio (95% CI) for POP from adjusted model a | 1.13 (0.90, 1.41) | 1.35 (0.87, 2.04) | 1.18 (0.79, 1.73) | 0.99 (0.71, 1.36) |
| Number of cases (% of N) | 891 (20.0%) | 804 (19.9%) | 809 (19.9%) | 824 (19.8%) |
| Number of cases with non-POP (%) | 773 (86.8%) | 773 (96.1%) | 773 (95.6%) | 773 (93.8%) |
| Number of cases with POP exposure (%) | 118 (13.2%) | 31 (3.9%) | 36 (4.4%) | 51 (6.2%) |
| Number of controls (% of N) | 3564 (80.0%) | 3230 (80.1%) | 3260 (80.1%) | 3348 (80.2%) |
| Number of controls with non-POP (%) | 3137 (88.0%) | 3137 (97.1%) | 3137 (96.2%) | 3137 (93.7%) |
| Number of controls with POP exposure (%) | 427 (12.0%) | 93 (2.9%) | 123 (3.8%) | 221 (6.3%) |
CI: confidence interval; OC: oral contraceptives; IMD: index of multiple deprivation; COCP: combined oral contraceptive pill; POP: progesterone-only pill.
Adjusted for age, ethnicity and IMD level.
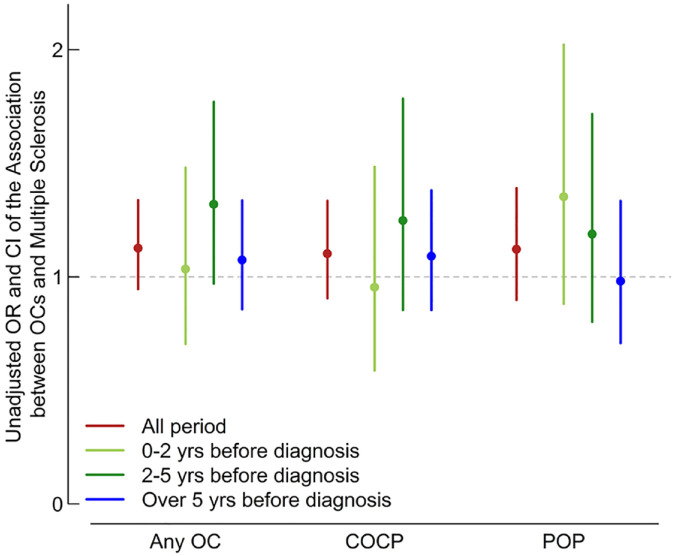
No association was seen between ever use of OC and subsequent MS (unadjusted odds ratio, (uOR) = 1.13, 95% confidence interval (CI) = 0.95–1.34; adjusted OR (aOR) = 1.13, 95% CI = 0.94–1.35; Table 2). No effect was seen when restricting to ever COCP (uOR = 1.10, 95% CI = 0.91–1.33; aOR = 1.08, 95% CI = 0.88–1.32; Table 2) or ever POP (uOR = 1.12, 95% CI = 0.90–1.39; aOR = 1.13, 95% CI = 0.90–1.41; Table 2). No association was seen between any OC, COCP or POP initiation at 0–2, 2–5 or >5 years prior to MS and subsequent MS. No clear sequential change in odds of overall contraceptive or COCP use was seen during the time epochs studied (Table 2 and Figure 1). The point estimates for OR of POP initiation increased with proximity to MS diagnosis; however, differences between time epochs were statistically non-significant (Table 2 and Figure 1).
Figure 1.
Relationship between oral contraceptive exposure and subsequent multiple sclerosis.
Discussion
The results from this population-based study were consistent with previous epidemiological studies conducted prior to 2000, that no epidemiologic evidence on association between OC use and subsequent MS was seen.8,9,13,14 While the point estimates for POP initiation increased with proximity to MS diagnosis, the confidence intervals overlapped with each other and the null throughout, and this was not seen with combined or all contraceptive exposure. This makes a causal relationship unlikely. The increase in POP initiation in the years immediately prior to MS diagnosis, during the period of MS prodrome, hints at a non-statistically significant impact of reverse causation in our population.
Strengths of our study include the prospective recording of data, in some cases for many years prior to MS onset, and the demographic heterogeneity of our population. In contrast to many prior studies, we were able to separate oestrogen-containing and non-oestrogen-containing OCs, in order to attempt to understand specific hormonal exposure as opposed to contraceptive effect. While we did not uncover any time-based patterns of initiation relating to MS diagnosis date, we had the ability to do so. The social and ethnic diversity of the population in which this study was sited represents an additional strength. While groups were not matched in terms of ethnic background, with additional over-representation of non-deprived individuals in the MS cohort, previous work by our group and others has not supported differential magnitude of effect of MS risk factors by ethnicity.12,15,16 Furthermore, the robustness of our point estimates to adjustment for these factors indicates no significant impact of these demographic features on this null relationship.
However, this work was not without limitations. Given variations in prescription data, we were only able to reliably estimate the first prescription date of OC within the EMIS system. It was therefore not feasible to estimate the duration of exposure or intake of OC, which restrained the study from further analysis. Further to this, we recognise that receiving a prescription is not the same as taking the medication; however, we have had to make this assumption and recognise that the assumption holds for the majority of the population. We were unable to accurately ascertain OC exposure prior to 1990; however, this was equally true for MS cases and matched controls. Finally, a potential confounder of exposure to OCs is pregnancy and pregnancy desire; importantly, pregnancy has previously been associated with later MS diagnosis. 5 We were unable to adequately account for this confounder due to concerns around incomplete data within the primary care record with respect to pregnancies and outcomes, particularly in the context of pregnancy losses.
In this large population-based study of OC use prior to MS, we find no evidence to support a relationship between either oestrogen-containing or progesterone-only OC and subsequent MS. It remains unclear whether there is the potential for OCs to exert a disease-modifying effect once inflammatory disease activity is established, and this requires further study.
Footnotes
Data Availability Statement: These individual line patient data are not publicly available.
The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship and/or publication of this article: This work was performed in the Centre for Preventive Neurology, which is partly funded by Barts Charity.
ORCID iD: Ruth Dobson  https://orcid.org/0000-0002-2993-585X
https://orcid.org/0000-0002-2993-585X
Contributor Information
Qiqi Zhang, Centre for Preventive Neurology, Wolfson Institute of Population Health, Queen Mary University of London, London, UK.
Alastair J Noyce, Centre for Preventive Neurology, Wolfson Institute of Population Health, Queen Mary University of London, London, UK; Department of Neurology, Royal London Hospital, Barts Health NHS Trust, London, UK.
John Robson, Centre for Primary Care, Wolfson Institute of Population Health, Queen Mary University of London, London, UK.
Gavin Giovannoni, Department of Neurology, Royal London Hospital, Barts Health NHS Trust, London, UK; Centre for Neuroscience, Surgery and Trauma, Blizard Institute, Queen Mary University of London, London, UK.
Charles R Marshall, Centre for Preventive Neurology, Wolfson Institute of Population Health, Queen Mary University of London, London, UK; Department of Neurology, Royal London Hospital, Barts Health NHS Trust, London, UK.
Ruth Dobson, Centre for Preventive Neurology, Wolfson Institute of Population Health, Queen Mary University of London, London, UK; Department of Neurology, Royal London Hospital, Barts Health NHS Trust, London, UK.
References
- 1. Multiple Sclerosis Society. MS in the UK, https://www.mssociety.org.uk/what-we-do/our-work/our-evidence/ms-in-the-uk
- 2. Trojano M, Lucchese G, Graziano G, et al. Geographical variations in sex ratio trends over time in multiple sclerosis. PLoS One 2012; 7: e48078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kalincik T, Vivek V, Jokubaitis V, et al. Sex as a determinant of relapse incidence and progressive course of multiple sclerosis. Brain 2013; 136: 3609–3617. [DOI] [PubMed] [Google Scholar]
- 4. Confavreux C, Hutchinson M, Hours MM, et al. Rate of pregnancy-related relapse in multiple sclerosis. N Engl J Med 1998; 339: 285–291. [DOI] [PubMed] [Google Scholar]
- 5. Nguyen A-L, Vodehnalova K, Kalincik T, et al. Association of pregnancy with the onset of clinically isolated syndrome. JAMA Neurol 2020; 77: 1496–1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hellwig K, Chen LH, Stancyzk FZ, et al. Oral contraceptives and multiple sclerosis/clinically isolated syndrome susceptibility. PLoS One 2016; 11: e0149094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Alonso Á, Jick SS, Olek MJ, et al. Recent use of oral contraceptives and the risk of multiple sclerosis. Arch Neurol 2005; 62: 1362–1365. [DOI] [PubMed] [Google Scholar]
- 8. Alonso A, Clark CJ. Oral contraceptives and the risk of multiple sclerosis: A review of the epidemiologic evidence. J Neurol Sci 2009; 286: 73–75. [DOI] [PubMed] [Google Scholar]
- 9. Hernán MA, Hohol MJ, Olek MJ, et al. Oral contraceptives and the incidence of multiple sclerosis. Neurology 2000; 55: 848–854. [DOI] [PubMed] [Google Scholar]
- 10. Zhao Y, Wijnands JMA, Högg T, et al. Interrogation of the multiple sclerosis prodrome using high-dimensional health data. Neuroepidemiology 2020; 54: 140–147. [DOI] [PubMed] [Google Scholar]
- 11. Simonet C, Bestwick J, Jitlal M, et al. Assessment of risk factors and early presentations of Parkinson disease in primary care in a diverse UK population. JAMA Neurol 2022; 79: 359–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dobson R, Jitlal M, Marshall CR, et al. Ethnic and socioeconomic associations with multiple sclerosis risk. Ann Neurol 2020; 87: 599–608. [DOI] [PubMed] [Google Scholar]
- 13. Villard-Mackintosh L, Vessey MP. Oral contraceptives and reproductive factors in multiple sclerosis incidence. Contraception 1993; 47: 161–168. [DOI] [PubMed] [Google Scholar]
- 14. Thorogood M, Hannaford PC. The influence of oral contraceptives on the risk of multiple sclerosis. Br J Obstet Gynaecol 1998; 105: 1296–1299. [DOI] [PubMed] [Google Scholar]
- 15. Jacobs BM, Tank P, Bestwick JP, et al. Modifiable risk factors for multiple sclerosis have consistent directions of effect across diverse ethnic backgrounds: A nested case-control study in an English population-based cohort. J Neurol 2024; 271: 241–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Langer-Gould A, Wu J, Lucas R, et al. Epstein-Barr virus, cytomegalovirus, and multiple sclerosis susceptibility. Neurology 2017; 89: 1330–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]