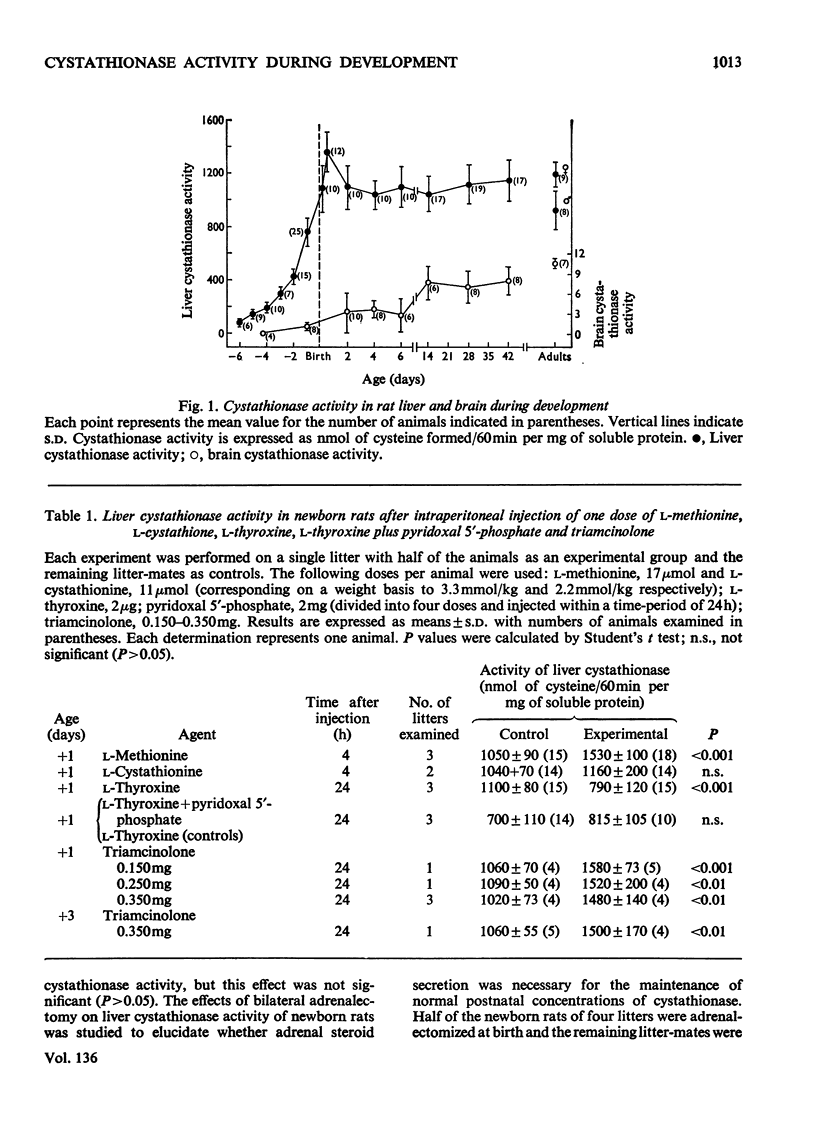
Abstract
High activity of cystathionase was present in rat liver but only low amounts of activity in rat brain during development. Triamcinolone had no effect on liver cystathionase activity in foetuses but increased the enzyme activity significantly in postnatal rats. l-Thyroxine decreased liver cystathionase activity significantly in newborn rats; administration of pyridoxal 5′-phosphate did not prevent this effect. l-Methionine significantly increased liver cystathionase activity in newborn rats.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CHATAGNER F., JOLLES-BERGERET B., TRAUTMANN O. [Thyroid hormones and desulfuration enzymes of L-cysteine in the rat liver]. Biochim Biophys Acta. 1962 Jun 4;59:744–746. doi: 10.1016/0006-3002(62)90667-4. [DOI] [PubMed] [Google Scholar]
- CHATAGNER F., TRAUTMANN O. EFFECT OF DL-ETHIONINE ON THE LEVEL OF CYSTATHIONASE IN RAT LIVER. Nature. 1963 Oct 5;200:75–75. doi: 10.1038/200075a0. [DOI] [PubMed] [Google Scholar]
- CLELAND W. W. DITHIOTHREITOL, A NEW PROTECTIVE REAGENT FOR SH GROUPS. Biochemistry. 1964 Apr;3:480–482. doi: 10.1021/bi00892a002. [DOI] [PubMed] [Google Scholar]
- Chase P. H., Volpe J. J., Laster L. Transsulfuration in mammals: fetal and early development of methionine-activating enzyme and its relation to hormonal influences. J Clin Invest. 1968 Sep;47(9):2099–2108. doi: 10.1172/JCI105895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatagner F., Durieu-Trautmann O., Rain M. C. Effects of puromycin and actinomycin D on the increase of cystathionase and cysteine sulphinic acid decarboxylase activities in the liver of thyroidectomized rat. Nature. 1967 Apr 1;214(5083):88–90. doi: 10.1038/214088a0. [DOI] [PubMed] [Google Scholar]
- Frimpter G. W., George W. F., Andelman R. J. Cystathioninuria and B6 dependency. Ann N Y Acad Sci. 1969 Sep 30;166(1):109–115. doi: 10.1111/j.1749-6632.1969.tb54261.x. [DOI] [PubMed] [Google Scholar]
- Frimpter G. W., Greenberg A. J. Renal clearance of cystathionine in homozygous and heterozygous cystathioninuria, cystinuria, and the normal state. J Clin Invest. 1967 Jun;46(6):975–982. doi: 10.1172/JCI105604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaitonde M. K. A spectrophotometric method for the direct determination of cysteine in the presence of other naturally occurring amino acids. Biochem J. 1967 Aug;104(2):627–633. doi: 10.1042/bj1040627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greengard O., Dewey H. K. The developmental formation of liver glucose 6-phosphatase and reduced nicotinamide adenine dinucleotide phosphate dehydrogenase in fetal rats treated with thyroxine. J Biol Chem. 1968 May 25;243(10):2745–2749. [PubMed] [Google Scholar]
- LEVITZ M., CONDON G. P., DANCIS J. Sulfurylation of estrogens by the human fetus. Endocrinology. 1961 May;68:825–830. doi: 10.1210/endo-68-5-825. [DOI] [PubMed] [Google Scholar]
- LIN E. C., KNOX W. E. Specificity of the adaptive response to tyrosine-alpha-ketoglutarate transaminase in the rat. J Biol Chem. 1958 Nov;233(5):1186–1189. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MASCITELLI-CORIANDOLI E., BOLDRINI R. Pyridoxine and pyridoxal phosphate concentrations in the liver and heart of thyroxinized animals. Experientia. 1959 Jun 15;15(6):229–230. doi: 10.1007/BF02158122. [DOI] [PubMed] [Google Scholar]
- MASON M., GULLEKSON E. H. Estrogen-enzyme interactions: Inhibition and protection of kynurenine transaminase by the sulfate esters of diethylstilbestrol, estradiol, and estrone. J Biol Chem. 1960 May;235:1312–1316. [PubMed] [Google Scholar]
- Mudd S. H., Finkelstein J. D., Irreverre F., Laster L. Transsulfuration in mammals. Microassays and tissue distributions of three enzymes of the pathway. J Biol Chem. 1965 Nov;240(11):4382–4392. [PubMed] [Google Scholar]
- Räihä N. C., Suihkonen J. Factors influencing the development of urea-synthesizing enzymes in rat liver. Biochem J. 1968 May;107(6):793–797. doi: 10.1042/bj1070793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturman J. A., Gaull G., Raiha N. C. Absence of cystathionase in human fetal liver: is cystine essential? Science. 1970 Jul 3;169(3940):74–76. doi: 10.1126/science.169.3940.74. [DOI] [PubMed] [Google Scholar]
- Volpe J. J., Laster L. Trans-sulphuration in primate brain: regional distribution of s stages of development. J Neurochem. 1970 Mar;17(3):425–437. doi: 10.1111/j.1471-4159.1970.tb02229.x. [DOI] [PubMed] [Google Scholar]
- Volpe J. J., Laster L. Transsulfuration in fetal and postnatal mammalian liver and brain. Cystathionine synthase, its relation to hormonal influences, and cystathionine. Biol Neonate. 1972;20(5):385–403. doi: 10.1159/000240481. [DOI] [PubMed] [Google Scholar]
- Yeung D., Stanley R. S., Oliver I. T. Development of gluconeogenesis in neonatal rat liver. Effect of triamcinolone. Biochem J. 1967 Dec;105(3):1219–1227. doi: 10.1042/bj1051219. [DOI] [PMC free article] [PubMed] [Google Scholar]


