Abstract
Rickettsia africae is a re-emerging tick-borne pathogen causing African tick bite fever (ATBF) in humans. Amblyomma variegatum is the principal vector in most sub-Sahara African countries, whereas in South Africa it is A. hebraeum. Reports of high genetic heterogeneity among R. africae isolates in southern Africa have prompted the need for molecular investigations of isolates form South Africa. Therefore, this study aimed to determine the prevalence and genetic diversity of R. africae in A. hebraeum collected from cattle, grazing pasture, as well as from blood of cattle in the Eastern Cape Province of South Africa. Amblyomma hebraeum and blood from cattle were screened by PCR and the gltA, ompA, ompB, sca4, and 17kDa genes were sequenced for R. africae from samples collected from Caquba in Port St. Johns along the coastal region in the Eastern Cape province of South Africa. The overall proportion of adult A. hebraeum that were positive for the gltA and ompA genes was 0.63 (108/180). The overall proportion of nymphs positive for the gltA and ompA genes was 0.62 (23/37) and 0.22 (20/90) from cattle blood. A positive R. africae infection was inferred by analysis of 26 sequences of the ompA, gltA, ompB, 17kDa and sca4 genes. Neighbour-joining and Maximum Likelihood analysis revealed that the study isolates were closely related to R. africae isolates from South Africa deposited in GenBank, forming a clade that was separate from north, east and west African strains. This study provides new information on the epidemiology and phylogeny of R. africae isolated from A. hebraeum ticks in the Eastern Cape province of South Africa. The heterogeneity observed between R. africae isolates from South Africa deposited in GenBank and R. africae isolates from Africa retrieved from Genbank highlight the importance of differentiation and tracking of the genetic movement among R. africae isolates in southern Africa for the better characterisation of ATBF cases, especially in rural communities and travellers visiting the region.
Keywords: African tick-bite fever, Amblyomma hebraeum, Rickettsia africae, Spotted fever, Travel medicine
Introduction
Rickettsia africae is an obligate, intracellular bacterium belonging to the spotted fever group Rickettsiae (Raoult 1997). It is the etiological agent of African tick bite fever (ATBF), an emerging infectious disease endemic to sub-Saharan Africa (Freedman et al. 2006). Since the first case report in 1992 in a patient from Zimbabwe, ATBF has been detected throughout eastern, western and central Africa, and several countries in the Caribbean region and linked to its main tick vector, Amblyomma variegatum (Mutai et al. 2013; Maina et al. 2014; Yssouf et al. 2014). In South Africa, the main tick vector is A. hebraeum, and this tick determines the geographical distribution of R. africae in the country.
Reports suggest that ATBF is not uncommon in international travellers; however, it is less documented in resource-poor livestock farmers in South Africa and other southern African countries. Most cases which have been reported present non-specific signs and symptoms that resemble influenza or malaria resulting in case misdiagnosis and delays in the appropriate treatment (Jensenius et al. 2003; Chandler et al. 2008). In addition, livestock and farming practices play a major role in the transmission dynamics of R. africae to humans as seen in reports from ticks and cattle with a prevalence of up to 92.6% (Mutai et al. 2013; Yssouf et al. 2014).
The ecological plasticity of the vectors A. variegatum and A. hebraeum has been shown to influence the distribution of ATBF. The typical landscape in which A. hebraeum is found are wooded and bushed grasslands with a preference for semi-arid and humid areas but this tick cannot able survive in open grasslands (Petney et al. 1987; Nyangiwe et al. 2011; Yawa et al. 2018).
Genetic diversity studies of R. africae have focused mainly on isolates from A. variegatum, with little to no information on isolates from A. hebraeum from most localities (Maina et al. 2014). In addition, single genes have been used to determine the genetic diversity of the isolates not allowing discrimination at the genus and species level (Thu et al. 2019). As a result, the multiple genes approach has been recently recommended using the citrate synthase (gltA), outer membrane protein A (ompA), outer membrane protein B (ompB) and surface cell antigen 4 (sca4) genes (Fournier et al. 2003). The ompA and gltA genes were used for initial screening as they had a higher heterogeneity and sensitivity compared to the 16S rDNA gene as reported by previous studies (Robinson et al. 2009). Thereafter the sca4, ompB and 17kDa genes were used to further classify the Rickettsia spp. detected. This study determined the genetic diversity of R. africae in A. hebraeum and in R. africae-infected cattle blood.
Materials and methods
Study sites
Sampling of ticks was conducted at two sites in the Eastern Cape Province of South Africa. The first site was situated in Lucingweni in Mthatha (GQW4 + 97; 31°27′14.7° S, 28°45′20.5° E) and the second site was situated in Caquba in Port St. Johns (9FG7 + WF; 31°37′21.7° S, 29°27′49.3° E) (Fig. 1). Lucingweni is located inland with very sparse vegetation cover, whereas Caquba consisted of thick vegetation including shrubs. Caquba is located on the coast with a livestock-wildlife interface having the ideal conditions to sustain A. hebraeum populations. The climate at each site consisted of mild and tropical conditions with an average temperature of 17.5 and 20.3 °C at Lucingweni and Caquba, respectively. At both sites, extensive rearing of cattle, sheep and goats is practiced, and farmers are frequently in direct contact with their animals as well as with the dense pastures grazed by livestock.
Fig. 1.
Location of sample collection sites (Mthatha and Port St Johns) in the Eastern Cape province, South Africa
Blood collection
Whole blood was collected from randomly selected cattle of mixed breeds at each location during routine acaricide application from at least 15 out of a total of 40 cattle monthly for a period of 7 months. Whole blood samples were collected in 10-mL ethylenediamine tetraacetic acid (EDTA) treated tubes from the tail vein whilst the animal was restrained in a cattle crush. All blood samples were transported on ice and kept in a refrigerator at 4 °C in the School of Life Sciences Parasitology Laboratory (University of KwaZulu-Natal, Durban, South Africa) until further analysis.
Tick collection
Ticks were collected manually from cattle from predilection sites (dewlap, udder/scrotum, fore/hind legs, perineum, and tail) using blunt-nose forceps. Questing ticks were collected at two quadrants grazed frequently by cattle using the drag sampling method (Daniels et al. 1998). Ticks were preserved in McCartney bottles with 70% ethanol until morphological and molecular analyses.
All ticks were identified morphologically under a stereomicroscope based on keys as described by Walker et al. (2003) and only A. hebraeum ticks were used for further analyses. Individual ticks were sterilized on the surface with 70% ethanol and rehydrated in distilled water. Ticks were dissected longitudinally with a sterile blade into two equal parts. One half of the sample was placed into a microcentrifuge tube and homogenized using glass microbeads in a Disruptor Gene bead-beater (Scientific Industries, Bohemia, NY, USA) to facilitate the release of pathogens and protein digestion and nucleic acid extractions. The other half of the sample was stored at 4 °C until further analysis.
Isolation of DNA from ticks and blood
Genomic DNA was extracted from 100 μL of blood following a previously described protocol (Bereczky et al. 2005) and from half the dissected ticks using the standard protocol for the ZYMO Quick-DNA Miniprep Plus Kit. The standard protocol was modified by incubating ticks overnight at 56 °C to allow complete deproteinization. Purified DNA was stored at 4 °C until further use.
Confirmation of tick DNA
To confirm that the Rickettsia detected in the DNA extracts was that from ticks and not from cattle from which the tick was collected, the tick-specific 16S rRNA gene was amplified as described previously (Macaluso et al. 2003).
Polymerase chain reaction (PCR)
A single-step PCR assay targeting a 1,234 bp and 540 bp fragment of the gltA and ompA genes was carried out to detect SFG Rickettsia (Table 1). PCRs were carried out in a final volume of 25 μL comprising of 12.5 μL of OneTaq Quick-Load 2X Master Mix with standard buffer (NEB, Hitchin, UK), 5 μL of template DNA, 2 μL of forward and reverse primer (100 μM), and 4.5 μL of sterile water in an Applied Biosystems Veriti 96-Well Thermal Cycler (Applied Biosystems, Foster City, CA, USA). Samples that were positive for the gltA and ompA genes were further characterised by amplifying the ompB, 17kDa, sca4, and 16S rRNA genes (Table 1). To determine the expected fragment size, the PCR product was separated by electrophoresis on a 1.5% agarose gel stained with ethidium bromide.
Table 1.
Primers used for the characterisation of Rickettsia spp
| Gene | Primer name | PCR | Size (bp) | Sequences (5′–3′) |
|---|---|---|---|---|
| gltA | gltAF | Single | 1234 | ACCTATACTTAAAGCAAGTATYGGT |
| gltAR | TCTAGGTCTGCTGATTTTTTGTTCA | |||
| ompA | ompAF | Single | 540 | ATGGCGAATATTTCTCCAAAA |
| ompAR | GTTCCGTTAATGGCAGCATCT | |||
| 17kDa | 17kDaF | Semi-nested | 450 | AATGAGTTTTATACTTTACAAAATTCTAAAAACCA |
| 17kDaR | CATTGTTCGTCAGGTTGGCG | |||
| 17kDaF2 | GCTCTTGCAACTTCTATGTT | |||
| 16S rRNA | 16S1 | Single | 1482 | TAAGGAGGTAATCCAGCC |
| 16S-1 | CCTGGCTCAGAACGAA | |||
| Sca4 | Sca4R | Semi-nested | 2700 | ATGAGTAAAGACGGTAACCT |
| Sca4F | TCAGCGTTGTGGAGGGGAAG | |||
| Sca4R | TTCAGTAGAAGATTTAGT | |||
| ompB | ompBF | Semi-nested | 444 | ACATKGTTATACARAGTGYTAATGC |
| ompBR | CCGTCATTTCCAATAACTAACTC | |||
| ompBR2 | SGTTAACTTKACCGYTTATAACTGT |
Sequencing
PCR product purification and sequencing were performed on an ABI 3500XL genetic analyser at Inqaba Biotechnical Industries (Pretoria, South Africa) using the ABI PRISM BigDye Terminator Cycle Sequencing Ready Reaction Kit (Applied Biosystems) according to the supplier’s recommendations.
Phylogenetic analysis
Sequences obtained were aligned using the Clustal W algorithm and edited and trimmed using BioEdit v.7.0.9.0 (Hall 1999). The data set was converted into amino acids using MEGA v.7 to check for stop codons in the reading frame of the sequences (Kumar et al. 2016), the protein sequences were then used to guide the nucleotide alignment. The sequences were deposited into GenBank (Altschul et al. 1990) and the relevant accession numbers were retrieved (Table S1) (Benson et al. 2015).
Phylogenetic trees were constructed using the Neighbor-joining method in MEGA v.7 (Kumar et al. 2016) to elucidate the phylogenetic relationships between isolates from this study and the closest matches identified by homology searches downloaded from GenBank (Altschul et al. 1990). MEGA v.7 software was used to estimate the best substitution model for the Maximum Likelihood (ML) method. The ML tree was constructed using the Tamura-3-parameter model (T92) and ML tree values were transferred onto the Neighbor-joining tree.
Statistical analysis
Descriptive statistics were performed to summarize the tick and parasite prevalence data using Microsoft Excel (2016). Parameters included: number infected (NI) and total number of ticks (N) from cattle and pastures. Prevalence (%) of tick species collected was calculated using the formula adapted from Thrusfield (1995): (total no. A. hebraeum infected with R. africae/total no. A. hebraeum screened for R. africae) × 100.
Results
Prevalence of tick species
Ticks were detected from two genera, Amblyomma and Rhipicephalus. In the Amblyomma genus, A. hebraeum was the only species detected. Prevalence in cattle was 56.5% (217/384) and from pastures it was 45.5% (293/645). In the Rhipicephalus genus, R. micro-plus and other unidentified Rhipicephalus spp. were detected. In cattle, a prevalence of 11.3% (43/384) was detected (Table S2) and 54.5% (352/645) from pastures. Cattle from Caquba were infested with adult and nymphal stages of A. hebraeum throughout the study period (Table 2).
Table 2.
Number of Ambyomma hebraeum nymphs and/or adults (from cattle and pasture) and cattle blood samples screened (N) and infected (NI) by PCR using the gltA and ompA genes for Rickettsiae africae in Caquba in the Eastern Cape province of South Africa
| Month of collection | Prevalence in cattle blood |
Prevalence in A. hebraeum from cattle |
Prevalence in A. hebraeum from pastures |
|
|---|---|---|---|---|
| N (NI) | Nymphs N (NI) |
Adults N (NI) |
Nymphs N (NI) |
|
| July 2018 | 21 (4) | 8 (6) | 17 (8) | 0 |
| Aug 2018 | 12 (0) | 20 (11) | 43 (26) | 0 |
| Sept 2018 | 14 (3) | 2 (0) | 25 (23) | 7 (2) |
| Oct 2018 | 9 (3) | 0 | 35 (10) | 5 (3) |
| Jan 2019 | 15 (0) | 0 | 26 (15) | 0 |
| March 2019 | 7 (2) | 6 (5) | 29 (21) | 0 |
| June 2019 | 12 (8) | 1 (1) | 5 (4) | 0 |
| Total | 90 (20) | 37 (23) | 180 (107) | 12 (5) |
Prevalence of Rickettsia africae
There was variation in the number of ticks screened for the presence of R. africae from both cattle and pasture which was dependent on the availability of ticks at the time of collection (Table 2). Prevalence in cattle blood was highest in the month of June 2019 (8/12; 66.7%). In August 2018 and January 2019 none of the blood samples screened were positive for infection. Of the 180 adult A. hebraeum collected from cattle, 107 (59.4%) were positive for R. africae, and out of a total of 37 nymphs collected from the same cattle, 23 (62.2%) were positive (Table 2). Nymphs were mostly abundant on cattle in August 2019 (a total of 20) and none was found during October 2018 and January 2019. From 12 nymphs collected from pastures, 5 (41.7%) were positive (Table 2.
Genetic characterization of isolates
In total 26 sequences were obtained and final sequence alignment lengths were trimmed for ompA (n = 9, 563 bp), gltA (n = 3, 946 bp), ompB (n = 5, 786 bp), sca4 (n = 3, 1012 bp), 17kDa (n = 3, 479 bp) and 16S rRNA (n = 3, 1004 bp) genes (Table S3). No stop codons were observed, and nucleotide sequences were subjected to a BLAST analysis for preliminary verification of their identity. All searches showed study sequences to be similar to those deposited in GenBank (Table 3) with sequence percent similarity in accordance with the guidelines of Fournier et al. (2003).
Table 3.
Characterisation of Rickettsia spp. isolates from Ambyomma hebraeum and blood from cattle, plus guidelines for minimum similarity (%) by Fournier et al. (2003)
| Sample ID | % identity to closest Rickettsia spp. (i.e., R. africae in all instances) by BLAST search (GenBank accession number) | ||||
|---|---|---|---|---|---|
| gltA | ompA | ompB | Sca4 | 17kDa | |
| Minimum % similarity (≥) | 99.9 | 98.8 | 99.2 | 99.3 | No guidelines for this gene |
| C1 | 99.20 (CP001612.1) | 99.82 (MH751466.1) | 98.69 (KU721071.1) | 99.62 (AF151724.2) | 100 (CP001612.1) |
| C3 | 99.02 (CP001612.1) | 100 (MH751466.1) | 99.52 (KU721071.1) | 98.31 (CP001612.1) | 100 (CP001612.1) |
| C9 | 98.58 (CP001612.1) | 100 (MH751466.1) | 99.52 (KU721071.1) | 98.31 (CP001612.1) | 100 (CP001612.1) |
| BC1 | – | 99.81 (MH751466.1) | – | – | – |
| C12 | – | 99.49 (MH751466.1) | – | – | – |
| C19 | – | 99.66 (MH751466.1) | – | – | – |
| C20 | – | 99.16 (MH751466.1) | – | – | – |
| C21 | – | 98.99 (MH751466.1) | – | – | – |
| R1 | – | – | 99.75 (KU721071.1) | – | – |
| R3 | – | – | 99.75 (KU721071) | – | – |
‘–’ No sequencing results were obtained for this sample
For the 16S rRNA gene, study samples collected from A. hebraeum adults from cattle (C1, C3 and C9) were found to be the most similar to a R. conorii isolate retrieved from GenBank with a similarity of 98.3%. This was the lowest similarity when compared to all other genes. For all other genes, isolates collected from A. hebraeum adults from cattle (C1, C3 and C9) were most similar to R. africae isolates retrieved from GenBank, with sca4 having the lowest similarity (98.3–99.3%), followed by gltA (98.6–99.2%), ompB (98.7–99.8%), ompA (99.2–100%) and 17kDa with 100% similarity across all sequences (Table 3). Based on the consensus across five genes (gltA, ompA, ompB, sca4 and 17kDa), sequences were identified as R. africae and all other positive samples were inferred to be R. africae.
Phylogenetic relationships between isolates
Rickettsia africae isolates from South Africa deposited in GenBank (C1, C3, C9, C12, C19, C20, C21 and BC1) formed a clade that was well supported by ML (65%) with R. africae isolates from the KwaZulu-Natal (MH751466.1) and Limpopo provinces (MG953294.1) retrieved from GenBank (Fig. 2). Rickettsia africae isolates from Kenya (AF548342.1) and Benin (KT633261.1) formed a strongly supported clade (94%), whereas R. africae isolates from the Carribean (GU247115.1), Madagascar (KJ645931.1) and east Africa (U83436.2) were found on the node of the tree with poor support. All isolates from the above regions except South Africa were isolated from A. variegatum. Rickettsia africae isolates from Egypt (HQ335137.1) and Lebanon (KY233233.1) formed a strongly supported clade (86%), and these were isolated from Hyalomma spp. From the gltA phylogenetic tree (Fig. S1), study isolates C1 and C9 from A. hebraeum formed a group that was supported by ML (65%) whereas sample C3 formed a monophyletic group with R. africae isolates from Africa that was poorly supported (Fig. 2). In the ompB tree (Fig. S2), study isolates C1 and R3 isolated from A. hebraeum and the rodent Otomys irroratus formed a group that was supported by ML (64%). Rickettsia africae isolates from Kenya (KF660532.1) and Sao Tome (MF667453.1) formed a group that was supported (55%); however, all other R. africae isolates from South Africa and Africa were situated on the node with no support.
Fig. 2.
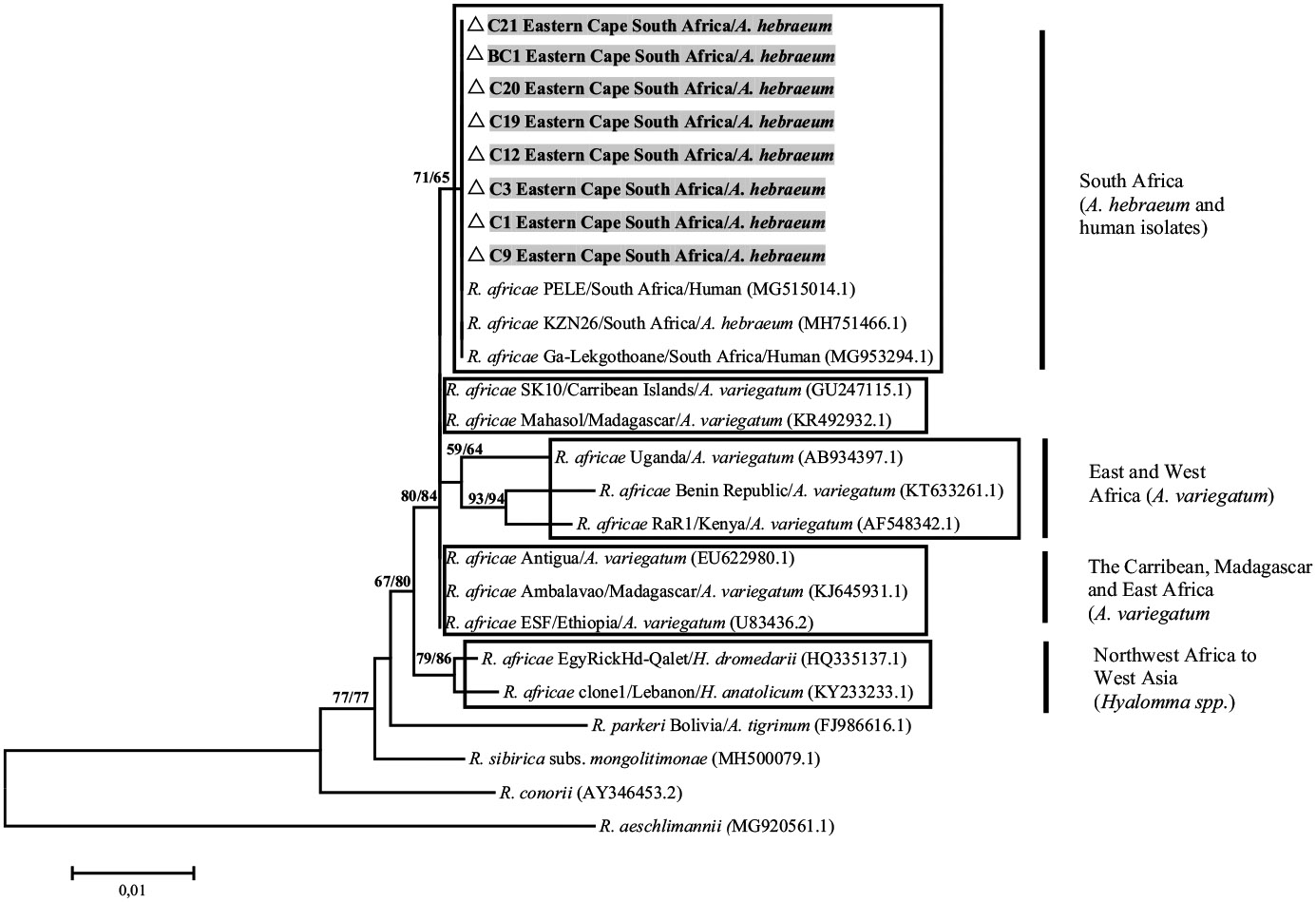
Neighbour joining phylogenetic tree based on the ompA gene (563 bp) of Rickettsia africae strains from Genbank and study samples from the Eastern Cape, South Africa (T92 + G model. Bootstrap values indicated show NJ/ML values. Taxon labels show the species name strain/origin/source of isolate. Isolates shaded in grey are from this study
Discussion
The presence of A. hebraeum in this study in the Eastern Cape province of South Africa lies within its reported geographical range, where it is present in the northern and eastern coastal regions of South Africa (Spickett 2013). Adult ticks were present throughout the study period in Caquba, and this was in accordance with previous studies where no clearly defined seasonal patterns were observed for the life cycle of A. hebraeum (Norval et al. 1991). However, the duration of this survey was too short to draw any inferences regarding the seasonality of A. hebraeum ticks from cattle and pastures.
The low nymph count from vegetation in our study is in accordance with previous studies that reported low counts of A. variegatum nymphs by drag sampling (Horak et al. 2011; Spickett et al. 1991). This is due to the difference in strategies of Amblyomma spp. from other ixodid ticks in which unfed nymphs prefer to quest from leaf litter in response to stimuli from hosts instead of questing on vegetation (Bryson et al. 2000; Horak et al. 2007).
This study detected a high prevalence of R. africae infection in A. hebraeum adults and nymphs from cattle in Caquba. Results are in agreement with those of other studies which have reported infection rates up to 92.6% in A. variegatum ticks from elsewhere in Africa (Mutai et al. 2013; Yssouf et al. 2014). Contrary to these findings, a previous study reported 20% prevalence of R. africae infection in A. hebraeum ticks, with Rhiphichephalus spp. having 30% prevalence (Mtshali et al. 2016). However, due to the scarcity of surveillance studies of Rickettsia spp. infection in A. hebraeum in South Africa, it is difficult to infer the significance of R. africae infections in our study to other regions of South Africa.
Prevalence of R. africae infection reported in this study was higher in A. hebraeum tissue samples than in blood from cattle. The low prevalence reported in this study could be an indication that the infections were in the chronic phase where the rickettsemia was low (hence, low circulation/absence of the parasite in blood) or there could have been inhibitors which interfered with DNA extraction resulting in false negatives (Palmer et al. 1986; Tondella et al. 2002). A seroprevalence of 64.4% for R. africae from cattle was reported by Eisawi et al. (2017). This correlated with reports from the French West Indies where a seroprevalence of 55.3% for R. africae was reported in cattle (Parola et al. 1999). The wide distribution of R. africae with their respective tick vectors, mainly A. variegatum, is mainly attributed to the movement of the cattle host (Barré et al. 1995) and that immune cattle may serve as reservoirs of infection (Allsopp et al. 2004).
Phylogenetic analyses in this study showed that the ompA gene provided a strong intraspecific resolution among the various R. africae isolates compared to all other genes (Fig. 2). The formation of four major R. africae groups provides further support for the geographical heterogeneity within the R. africae lineages as previously reported (Kimita et al. 2016). A distinct cluster comprising of R. africae isolates from South Africa deposited in GenBank and R. africae isolates from South Africa retrieved from Genbank was observed which was separate from R. africae clusters from northern and western Africa, Antigua, Caribbean Islands and Madagascar retrieved from Genbank. This corresponds with the sequence alignment of ompA where a thymine (T) base pair substitution of R. africae isolates from Africa retrieved from GenBank had a cytosine (C) base pair at position 574 bp suggesting divergence of R. africae isolates from South Africa deposited in GenBank and R. africae isolates from Africa retrieved from GenBank (Table S3).
Sporadic reports in the Mediterranean showed a 4% prevalence of R. africae in A. variegatum from passerine birds further supporting this observation (Wallménius et al. 2014). In addition, the presence of isolates in St Kitts and Nevis and Antigua Islands is likely due to the translocation of infected cattle and/or ticks from Africa (Parola et al. 2005).
Phylogenetic trees based on the gltA and ompB genes resolved intraspecific variation among study samples (C1, C3 and C9) better than when based on ompA. In the gltA tree (Fig. S1), samples C1 and C9 from A. hebraeum ticks formed a clade that was supported (65%) in ML. In the ompB tree, R. africae isolate (R1) from O. irroratus tissue from this study formed a clade with R. africae sample C1 (64%) suggesting these samples are more closely related to each other than to R. africae sample C3. The observation of homogeneity among R. africae isolates from South Africa in this study and heterogeneity when compared to R. africae strains from Africa suggest a limited geographic range of R. africae in South African isolates. Results were not comparable across all genes as illustrated in the trees due to the paucity of R. africae isolates from Africa from GenBank and short sequence reads. As reported previously, PCR failure is common among the variable genes (ompA, ompB and sca4) for rickettsial classification (Nakao et al. 2013). This is attributed to nucleotide mismatches in the primer annealing sites and this results in poor sequence reads which result in incorrect phylogenetic inferences being made on isolates (Thu et al. 2019).
Conclusion
This study demonstrates that the use of a multiple gene approach to characterize Rickettsia spp. is necessary and that single gene trees can confound the true relationship between isolates. A 62% prevalence of R. africae infection provides one of the first epidemiological surveillance studies of R. africae from A. hebraeum from cattle in South Africa. The significance of these findings is important in the differentiation of R. africae strains, to allow the tracking of origin of strains and provide targeted treatments. This, paralleled with the growth in tourist visits to game reserves in South Africa, has led to R. africae being regarded as the most widely distributed of all human pathogenic SFG rickettsiae. Therefore, it is recommended that extensive epidemiological surveillance of R. africae is conducted in South Africa.
Additionally, the low genetic diversity shown by the ompA, ompB and sca4 genes amongst study isolates indicates the need for new primers to be designed to cover longer regions of sequences to further characterise R. africae isolates throughout South Africa. Furthermore, it is recommended that patients displaying febrile illnesses should be routinely screened to accurately assess the incidence of R. africae in humans. Finally, studies should combine each member of the host, vector, and pathogen system to understand the combined effect of the evolutionary processes linking different species. This includes the screening of domestic and wild rodents for R. africae. Consideration of A. hebraeum dispersal and population dynamics in future population genetic studies will provide insights into the evolutionary forces driving species distribution, gene flow and host adaptations.
Supplementary Material
Acknowledgements
We acknowledge and thank the farmers from Caquba and Lucingweni who allowed us to sample their cattle and the laboratory technician Elma for her assistance with blood collections.
Funding
This work was funded in part by NIH grant 1R01AI136035 as part of the joint NIH-NSF-USDA Ecology and Evolution of Infectious Diseases programme and the National Research Foundation of South Africa.
Footnotes
Electronic supplementary material The online version of this article (https://doi.org/10.1007/s10493-020-00555-6) contains supplementary material, which is available to authorized users.
Conflict of interests The authors declare that they have no competing interests.
Ethical approval Ethical considerations were fulfilled by obtaining approval of the study from the Animal Research Ethics Committee, University of KwaZulu Natal (Ref: AREC/056/017).
Data availability
The datasets supporting the findings of this article are included within the article and its additional files. The sequences generated in this study for R.africae for the 16SrRNA gene were deposited in the GenBank database under the accession numbers MN988998- MN989000. The sequences for Rickettsia africae for the ompA gene were deposited under the accession numbers MN972462, MT009455 and MT009349-MT009354. The sequences for Rickettsia africae for the ompB gene were deposited under the accession numbers MT150897-MT150899. The sequences for Rickettsia africae for the gltA gene were deposited under the accession numbers MT150894-MT150896. The sequences for Rickettsia africae for the sca4 gene were deposited under the accession numbers MT150900-MT150902. The sequences for Rickettsia africae for the 17kDa gene were deposited under the accession numbers MT150903-MT150905.
References
- Allsopp BA, Bezuidenhout JD, Prozesky L (2004) Infectious diseases of livestock. Oxford University Press, Cape Town, pp 507–535 [Google Scholar]
- Altschul SF, Gish W, Miller W, Meyers EW, Lipman DJ (1990) Basic Local Alignment Search Tool. J Mol Biol 215:403–410 [DOI] [PubMed] [Google Scholar]
- Barré N, Garris G, Camus E (1995) Propagation of the tick Amblyomma variegatum in the Caribbean. Rev Sci Tech 14:841–855 [DOI] [PubMed] [Google Scholar]
- Benson DA, Clark K, Karsch-Mizrachi I, Lipman DJ, Ostell J, Sayers EW (2015) GenBank. Nucleic Acids Res 43:D30–D35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bereczky S, Mårtensson A, Gil JP, Färnert A (2005) Short report: Rapid DNA extraction from archive blood spots on filter paper for genotyping of Plasmodium falciparum. Am J Trop Med Hyg 72:249–251 [PubMed] [Google Scholar]
- Bryson NR, Horak IG, Venter EH, Yunker CE (2000) Collection of free-living nymphs and adults of Amblyomma hebraeum (Acari: Ixodidae) with pheromone/carbon dioxide traps at 5 different ecological sites in heartwater endemic regions of South Africa. Exp Appl Acarol 24:971–982 [DOI] [PubMed] [Google Scholar]
- Chandler CIR, Chonya S, Boniface G, Juma K, Reyburn H, Whitty CJM (2008) The importance of context in malaria diagnosis and treatment decisions—a quantitative analysis of observed clinical encounters in Tanzania. Trop Med Int Health 13:1131–1142 [DOI] [PubMed] [Google Scholar]
- Daniels TJ, Boccia TM, Varde S, Marcus J, Le J, Bucher DJ, Falco RC, Schwartz I (1998) Geographic risk for lyme disease and human granulocytic ehrlichiosis in southern New York state. Appl Environ Microbiol 64:4663–4669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisawi NM, Hassan DA, Hussien MO, Musa AB, El Hussein ARM (2017) Seroprevalence of spotted fever group (SFG) rickettsiae infection in domestic ruminants in Khartoum State, Sudan. Vet Med 3:91–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier PE, Dumler JS, Greub G, Zhang J, Wu Y, Raoult D (2003) Gene sequence-based criteria for identification of new Rickettsia isolates and description of Rickettsia heilongjiangensis sp. nov. J Clin Microbiol 41:5456–5465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman DO, Weld LH, Kozarsky PE, Fisk T, Robins R, von Sonnenburg F, Keystone JS, Pandey P et al. (2006) Spectrum of disease and relation to place of exposure among Ill returned travelers. N Engl J Med 354:119–130 [DOI] [PubMed] [Google Scholar]
- Hall TA (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Res 41:95–98 [Google Scholar]
- Horak IG, Gallivan GJ, Spickett AM (2011) The dynamics of questing ticks collected for 164 consecutive months off the vegetation of two landscape zones in the Kruger National Park (1988–2002). Part I. Total ticks, Amblyomma hebraeum and Rhipicephalus decoloratus. Onderstepoort J Vet Res 78:1–10 [DOI] [PubMed] [Google Scholar]
- Horak IG, Golezardy H, Uys AC (2007) Ticks associated with the three largest wild ruminant species in southern Africa. Onderstepoort J Vet Res 74:231–242 [DOI] [PubMed] [Google Scholar]
- Jensenius M, Fournier PE, Vene S, Hoel T, Hasle G, Henriksen AZ, Hellum KB, Raoult D, Myrvang B (2003) African tick bite fever in travelers to rural sub-Equatorial Africa. Clin Infect Dis 36:1411–1417 [DOI] [PubMed] [Google Scholar]
- Kimita G, Mutai B, Nyanjom SG, Wamunyokoli F, Waitumbi J (2016) Phylogenetic variants of Rickettsia africae, and incidental identification of "Candidatus Rickettsia moyalensis" in Kenya. PLoS Negl Trop Dis 10:1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Stecher G, Tamura K (2016) MEGA7: Molecular evolutionary genetics analysis version 7.0 for Bigger Datasets. Mol Biol Evol 33:1870–1874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macaluso KR, Davis J, Alam U, Korman A, Rutherford JS, Rosenberg R, Azad AF (2003) Spotted fever group rickettsiae in ticks from the Masai Mara region of Kenya. Am J Trop Med Hyg 68:551–553 [DOI] [PubMed] [Google Scholar]
- Maina AN, Jiang J, Omulo SA, Cutler SJ, Ade F, Ogola E, Feikin DR, Njenga MK, Cleaveland S, Mpoke S, Ng’ang’a Z, Breiman RF, Knobel DL, Richards AL (2014) High prevalence of Rickettsia africae variants in Amblyomma variegatum ticks from domestic mammals in rural Western Kenya: Implications for Human Health. Vector-Borne Zoonot Dis 14:693–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mtshali K, Khumalo Z, Nakao R, Grab DJ, Sugimoto C, Thekisoe O (2016) Molecular detection of zoonotic tick-borne pathogens from ticks collected from ruminants in four South African provinces. J Vet Med Sci 77:1573–1579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutai BK, Wainaina JM, Magiri CG, Nganga JK, Ithondeka PM, Njagi ON, Jiang J, Richards AL, Waitumbi JN (2013) Zoonotic surveillance for Rickettsiae in domestic animals in Kenya. Vector-Borne Zoonot Dis 13:360–366 [DOI] [PubMed] [Google Scholar]
- Nakao R, Qiu Y, Igarashi M, Magona JW, Zhou L, Ito K, Sugimoto C (2013) High prevalence of spotted fever group rickettsiae in Amblyomma variegatum from Uganda and their identification using sizes of intergenic spacers. Ticks Tick-Borne Dis 4:506–512 [DOI] [PubMed] [Google Scholar]
- Norval RAI, Andrew HR, Meltzer MI (1991) Seasonal occurrence of the bont tick (Amblyomma hebraeum) in the southern lowveld of Zimbabwe. Exp Appl Acarol 13:81–96 [DOI] [PubMed] [Google Scholar]
- Nyangiwe N, Goni S, Hervé-Claude LP, Ruddat I, Horak IG (2011) Ticks on pastures and on two breeds of cattle in the Eastern Cape province, South Africa. Onderstepoort J Vet Res 78:9. [DOI] [PubMed] [Google Scholar]
- Palmer GH, Barbet AF, Kuttler KL, McGuire TC (1986) Detection of an Anaplasma marginale common surface protein present in all stages of infection. J Clin Microbiol 23:1078–1083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parola P, Paddock CD, Raoult D (2005) Tick-borne rickettsioses around the world: emerging diseases challenging old concepts. Clin Microbiol Rev 18:719–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parola P, Vestris G, Martinez D, Brochier B, Roux V, Raoult D (1999) Tick-borne rickettiosis in Guade-loupe, the French West Indies: Isolation of Rickettsia africae from Amblyomma variegatum ticks and serosurvey in humans, cattle, and goats. Am J Trop Med Hyg 60:888–893 [DOI] [PubMed] [Google Scholar]
- Petney TN, Horak IG, Rechav Y (1987) The ecology of the african vectors of heartwater, with particular reference to Amblyomma hebraeum and Amblyomma variegatum. Onderstepoort J Vet Res 54:381–395 [PubMed] [Google Scholar]
- Raoult D, Roux V (1997) Rickettsioses as paradigms of new or emerging infectious diseases. Clin Microbiol Rev 10:694–719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson JB, Eremeeva ME, Olson PE, Thornton SA, Medina MJ, Sumner JW, Daschi FA (2009) New approaches to detection and identification of Rickettsia africae and Ehrlichia ruminantium in Amblyomma variegatum (Acari: Ixodidae) ticks from the Caribbean. J Med Entomol 46:942–951 [DOI] [PubMed] [Google Scholar]
- Spickett AM (2013) Ixodid Ticks of Major Economic Importance and Their Distribution in South Africa, pp 79–79 [Google Scholar]
- Spickett AM, Horak IG, Braack LE, van Ark H (1991) Drag-sampling of free-living ixodid ticks in the Kruger National Park. Onderstepoort J Vet Res 58:27–32 [PubMed] [Google Scholar]
- Thrusfield M (1995) Veterinary Epidemiology, 2nd edn. Black Wellscience Ltd, London [Google Scholar]
- Thu MJ, Qiu Y, Matsuno K, Kajihara M, Mori-Kajihara A, Omori R, Monma N, Chiba K, Seto J, Gokuden M, Andoh M, Oosako H, Katakura K, Takada A, Sugimoto C, Isoda N, Nakao R (2019) Diversity of spotted fever group rickettsiae and their association with host ticks in Japan. Sci Rep 9:1500–1500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tondella MLC, Talkington DF, Holloway BP, Dowell SF, Cowley K, Soriano-Gabarro M, Elkind MS, Fields BS (2002) Development and evaluation of real-time PCR-based fluorescence assays for detection of Chlamydia pneumoniae. J Clin Microbiol 40:575–583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker AR, Bouattour A, Camicas JL, Estrada-Pena A, Horak IG, Latif AA, Pegram RG, Preston PM (2003) Ticks of domestic animals in Africa: a guide to identification of species. Edinburgh (Scotland) Bioscience Reports. [Google Scholar]
- Wallménius K, Barboutis C, Fransson T, Jaenson TGT, Lindgren PE, Nyström F, Olsen B, Salaneck E, Nilsson K (2014) Spotted fever Rickettsia species in Hyalomma and Ixodes ticks infesting migratory birds in the European Mediterranean area. Parasites Vectors 7:318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yawa M, Nyangiwe N, Muchenje V, Kadzere CT, Mpendulo TC, Marufu MC (2018) Ecological preferences and seasonal dynamics of ticks (Acari: Ixodidae) on and off bovine hosts in the Eastern Cape province, South Africa. Exp Appl Acarol 74:317–328 [DOI] [PubMed] [Google Scholar]
- Yssouf A, Socolovschi C, Kernif T, Temmam S, Lagadec E, Tortosa P, Parola P (2014) First molecular detection of Rickettsia africae in ticks from the Union of the Comoros. Parasites Vectors 7:444–444 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets supporting the findings of this article are included within the article and its additional files. The sequences generated in this study for R.africae for the 16SrRNA gene were deposited in the GenBank database under the accession numbers MN988998- MN989000. The sequences for Rickettsia africae for the ompA gene were deposited under the accession numbers MN972462, MT009455 and MT009349-MT009354. The sequences for Rickettsia africae for the ompB gene were deposited under the accession numbers MT150897-MT150899. The sequences for Rickettsia africae for the gltA gene were deposited under the accession numbers MT150894-MT150896. The sequences for Rickettsia africae for the sca4 gene were deposited under the accession numbers MT150900-MT150902. The sequences for Rickettsia africae for the 17kDa gene were deposited under the accession numbers MT150903-MT150905.


