Abstract
Background
Life’s Simple 7 (LS7), developed by the American Heart Association, addresses seven key health behaviors and relationship factors. Although LS7 has been studied in relation to various chronic diseases, its association with arthritis remains unclear. This study seeks to investigate the association between LS7 and arthritis, with particular emphasis on the mediating role of body fat percentage (BFP).
Methods
Data from the 2011–2018 National Health and Nutrition Examination Survey (NHANES), including 16,332 adult participants, were analyzed. The connection between LS7 and arthritis was evaluated using multivariable logistic regression, smooth curve fitting, and subgroup analysis. Mediation analysis assessed the role of BFP in this relationship. Additionally, ROC curve analysis was used to assess the predictive performance of the model, and the Boruta algorithm identified the influential factors associated with arthritis.
Results
After adjusting for relevant covariables, each standard deviation increase in LS7 was linked to a 13% lower likelihood of arthritis [OR = 0.87, 95% CI: 0.84, 0.89]. Participants in the highest LS7 tertile (T3) exhibited a 50% reduced likelihood of developing arthritis compared to those in the lowest tertile (T1) [OR = 0.50, 95% CI: 0.43, 0.60]. Mediation analysis confirmed that BFP significantly mediated the LS7-arthritis relationship. Furthermore, the Boruta algorithm identified LS7 and BFP as key variables associated with arthritis.
Conclusion
Elevated LS7 scores were associated with a lower likelihood of arthritis, with BFP serving as a mediating factor. Improving LS7 scores and managing body fat may help prevent arthritis. Due to the study’s cross-sectional design, causality cannot be confirmed. Future research should use longitudinal studies to verify these findings and target high-risk groups.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12944-024-02392-7.
Keywords: Life’s simple 7, Arthritis, Body fat percentage, Mediation analysis, NHANES
Introduction
Arthritis, a chronic inflammatory disorder, affects millions globally, particularly the elderly, causing considerable disability and severely impairing daily life quality [1–3]. The increasing prevalence of arthritis, driven by an aging population, poses substantial challenges for public health systems [4]. Approximately 3% of the global population is affected by inflammatory arthritis, with rheumatoid arthritis impacting 460 out of every 100,000 individuals worldwide [5, 6]. 2017, osteoarthritis accounted for 118.8 disability-adjusted life years per 100,000, adjusted for age, reflecting a 9.6% increase since 1990 [7]. Patients commonly experience joint pain, stiffness, and swelling, which worsen over time and affect mobility. Furthermore, arthritis frequently coexists with other chronic conditions like cardiovascular disease and diabetes, amplifying the health and economic burdens on both individuals and healthcare systems [8, 9]. Despite a range of treatments—including medications, physical therapy, and surgery—effectively managing and preventing arthritis remains a challenge, highlighting the need for preventive strategies focused on lifestyle changes.
Life’s Simple 7 (LS7), developed by the American Heart Association, is a comprehensive tool for assessing cardiovascular health by evaluating seven key factors: smoking, Body Mass Index (BMI, is an indicator that assesses weight in relation to height (kg/m²) and is utilized to categorize weight status among adults), physical activity, diet, blood pressure, blood glucose, and cholesterol levels [10]. While primarily designed for cardiovascular assessment, recent research has connected LS7 to various chronic conditions, including diabetes and obesity [11, 12]. However, the link between LS7 and arthritis has not been extensively studied. This study seeks to investigate the link between LS7 and arthritis, particularly through the mediating effect of Body Fat Percentage (BFP).
BFP serves as a vital indicator of body fat content and is a well-established marker of obesity [13]. Research indicates that obesity is a significant contributor to the development of arthritis, particularly knee osteoarthritis, due to the increased mechanical stress it places on the joints [14]. Moreover, obesity is associated with chronic inflammation, a key mechanism in arthritis progression [15]. Although BMI is frequently utilized as an indicator of obesity; however, its effectiveness in accurately depicting obesity is still a subject of discussion [37, 38]. On the other hand, body fat percentage (BFP), which was defined as the proportion of body fat relative to total body weight, is viewed as a more accurate measure of body fat composition and provides a more accurate assessment of obesity [17, 18]. Therefore, BFP may play a mediating role between LS7 and arthritis, affecting the condition through its influence on body fat levels. Understanding BFP’s role could help shed light on the mechanisms underlying arthritis and know potential pathways for lifestyle interventions.
This research utilizes data from the National Health and Nutrition Examination Survey (NHANES) to perform a cross-sectional analysis of the link between LS7 and arthritis, and investigated the mediating role of BFP. We hypothesize that higher LS7 scores are associated with a reduced likelihood of arthritis, with BFP serving as a partial mediator in this relationship. This research’s novelty lies in identifying BFP as a mediating factor in arthritis development, offering valuable theoretical insights and recommendations for personalized preventive strategies.
Methods
Study population
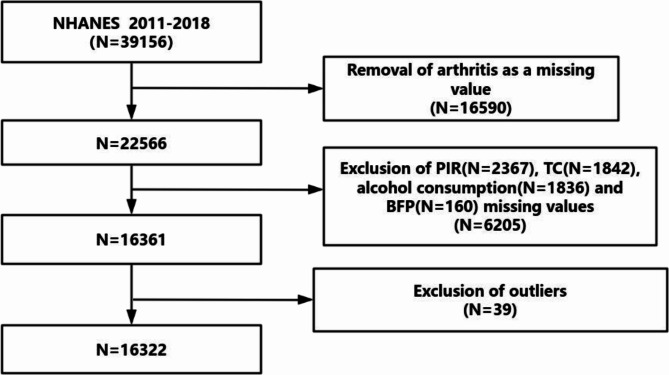
This study made use of data from the National Health and Nutrition Examination Survey (NHANES), offering a representative cross-sectional snapshot of the health and nutritional conditions of the U.S. population. Information was gathered through interviews and physical exams at mobile centers. We analyzed NHANES data from 2011 to 2018, encompassing four survey cycles (n = 39,156). Participants were excluded if they did not have arthritis diagnosis data (n = 16,590), were missing key variable values (total n = 6,205, including PIR (n = 2,367), TC (n = 1,842), alcohol consumption (n = 1,836), and BFP (n = 160)), or were classified as outliers (n = 39). This led to a final pool of 16,322 adults (Fig. 1). Informed consent was obtained from all participants, and the de-identified data is available to the public.
Fig. 1.
A flow diagram of eligible participant selection in the National Health and Nutrition Examination Survey. Abbreviation: PIR, Ratio of family income to poverty; TC, Total cholesterol; BFP, body.fat.percentage
Construction and utilization of tertiles for LS7 and BFP
Tertiles for LS7 and BFP were constructed based on the 33rd and 66th percentiles, calculated using survey-weighted quantiles from the NHANES design. This approach facilitated precise classification within the intricate sampling framework, segmenting participants into three categories (T1, T2, T3) for each variable. Subsequently, these tertiles were utilized as categorical independent variables in multivariable survey-weighted logistic regression models to examine their relationships with arthritis.
Description of life’s simple 7 (LS7) and body fat percentage (BFP)
The primary exposure variable was the LS7 score, established by the American Heart Association to evaluate cardiovascular health using seven criteria: physical activity, tobacco use, BMI, nutritional habits, blood glucose levels, blood pressure, and total cholesterol. Each component receives a score from 0 (indicating poor health) to 2 (indicating ideal health), resulting in a total possible score that ranges from 0 to 14. Increased scores indicate better health outcomes [16] (see Supplementary Table S1).
BFP was determined by applying the formula:
BF%=−44.988+(0.503×age)+(10.689×sex)+(3.172×BMI)−(0.026×BMI2)+(0.181×BMI×sex)−(0.02×BMI×age)−(0.005×BMI2×sex)+(0.00021×BMI2×age).
where age is expressed in years and sex is represented as men = 0 and women = 1 [34].
Definition of arthritis
Arthritis was diagnosed based on self-reported answers to the NHANES inquiry: “Have you ever been told by a doctor or other healthcare provider that you have arthritis?”This self-reported approach is widely accepted in the NHANES study [19, 20].
Covariables
We controlled for several covariables to address potential confounding variables: age, gender, ethnicity, educational attainment, marital status, poverty income ratio (PIR), smoking status, alcohol consumption, diabetes, hypertension, and total cholesterol (see Supplementary Table S2). These covariables were selected based on existing literature that associates these factors with arthritis [21, 26].
Statistical analysis
All analyses were conducted with R software (version 4.3.2). The assessment of normality for continuous variables was conducted through the use of Q-Q plots and histograms (see the Supplementary Material Figure S1) [36]. Variables that exhibit a normal distribution are expressed as mean ± standard deviation (SD), those that do not follow a normal distribution are represented by median and interquartile range (IQR), whereas categorical variables are shown as frequencies and percentages. Given NHANES’s complex multistage probability sampling design representing the U.S. civilian non-institutionalized population, we incorporated sampling weights, clusters, and strata into all analyses. Continuous variables that followed a normal distribution were evaluated using weighted t-tests, whereas those that were not normally distributed were analyzed through the weighted Wilcoxon rank-sum test. For categorical variables, differences among LS7, BFP, and arthritis were examined using weighted chi-square tests.
To assess the relationship between LS7, BFP, and arthritis, we employed multivariable logistic regression models. Three models were constructed: (1) unadjusted, (2) controlled for age, gender, education, marital status, PIR, and race, and (3) additionally accounted for smoking, alcohol use, hypertension, and diabetes. Smooth curve fitting was applied to investigate possible non-linear relationships involving LS7 and arthritis. Specifically, for the Restricted Cubic Splines (RCS) used to examine non-linear associations, we employed the lrm function from the rms package to fit a logistic regression model with RCS terms. We selected 3 knots based on model fit criteria to allow flexibility in capturing non-linear relationships. Subgroup analyses examined associations within different population strata. To confirm the robustness of our findings, machine learning techniques, including ROC curve analysis, were used to evaluate model performance. The area beneath the curve (AUC) indicated the models’ ability to predict outcomes, with greater AUC values reflecting superior performance.
Furthermore, we implemented the Boruta algorithm, a machine learning-based feature selection method, to identify the significant factors of arthritis. The Boruta algorithm, built on the random forest framework, enhances model interpretation by comparing the importance of original variables to randomly shuffled variables, referred to as “shadow features.” Variables with importance scores significantly higher than those of shadow features are considered meaningful and retained in the model, while those with lesser importance are excluded. This ensures that only factor with true significance for arthritis are included. Provide valuable insights for targeted interventions and prevention strategies.
To investigate BFP’s intermediary role in the association between LS7 and arthritis, we utilized the “mediation” package. The analysis involved two stages: Modeling the relationship between LS7 and BFP: A linear regression model was fitted to assess the association between LS7 scores and body fat percentage, adjusting for covariables. Modeling the relationship between BFP, LS7, and arthritis: A probit regression model was used to examine the effect of body fat percentage and LS7 scores on arthritis, while adjusting for the same covariables. We estimated indirect, direct, and total effects using the bootstrap resampling method (1,000 iterations) and calculated mediation proportions to assess BFP’s contribution to the relationship. The mediation proportion was computed as:
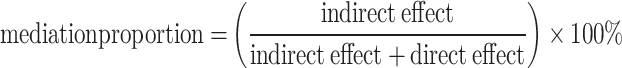 |
Given the reliance of specific components in the BFP formula on BMI, we conducted a deeper examination of the association between BMI and BFP through Pearson association analysis and linear regression analysis.
Results
Baseline characteristics
This study analyzed data from 16,332 participants, representing approximately 185 million adults in the U.S. Among these, 4,467 were diagnosed with arthritis, while 11,685 did not have the condition. The mean LS7 score was recorded at 8.29 (SD 2.43), and the average body fat percentage (BFP) was 35% (SD 10). Initial findings indicated that individuals with arthritis were generally older, exhibited higher total cholesterol levels, had lower LS7 scores, and presented with higher BFP compared to their non-arthritis counterparts. Furthermore, the prevalence of arthritis was notably greater among women, Non-Hispanic Whites, individuals with higher education, smokers, drinkers, and those suffering from hypertension. Detailed participant traits are presented in Table 1.
Table 1.
Baseline characteristics of all participants were stratified by arthritis
| Characteristic | N 1 | Overall N = 185,247,6492 |
Non-arthritis N = 135,818,8162 |
Arthritis N = 49,428,8332 |
p-value3 |
|---|---|---|---|---|---|
| Age | 16,332 | 48 (33, 61) | 42 (30, 55) | 61 (52, 70) | < 0.001 |
| Gender | 16,332 | < 0.001 | |||
| male | 8081 (49%) | 6273 (52%) | 1808 (40%) | ||
| female | 8251 (51%) | 5592 (48%) | 2659 (60%) | ||
| Race | 16,332 | < 0.001 | |||
| Mexican American | 2163 (8.1%) | 1731 (9.6%) | 432 (4.2%) | ||
| Non-Hispanic White | 6593 (68%) | 4351 (65%) | 2242 (77%) | ||
| Non-Hispanic Black | 3527 (10%) | 2526 (11%) | 1001 (9.3%) | ||
| Other Hispanic | 4049 (14%) | 3257 (15%) | 792 (9.8%) | ||
| Education level | 16,332 | 0.01 | |||
| Below high school | 6959 (35%) | 4870 (35%) | 2089 (38%) | ||
| High School or above | 9373 (65%) | 6995 (65%) | 2378 (62%) | ||
| Married/live with partner | 16,332 | 0.7 | |||
| Yes | 9617 (63%) | 7147 (63%) | 2470 (63%) | ||
| No | 6715 (37%) | 4718 (37%) | 1997 (37%) | ||
| PIR | 16,332 | 2.99 (1.48, 5.00) | 3.00 (1.49, 5.00) | 2.96 (1.46, 5.00) | 0.6 |
| TC | 16,332 | 192± (42) | 191± (41) | 194± (44) | 0.003 |
| Smoking | 16,332 | < 0.001 | |||
| Yes | 7240 (45%) | 4817 (41%) | 2423 (54%) | ||
| No | 9092 (55%) | 7048 (59%) | 2044 (46%) | ||
| Drinking | 16,332 | < 0.001 | |||
| Yes | 10,939 (73%) | 8153 (74%) | 2786 (69%) | ||
| No | 5393 (27%) | 3712 (26%) | 1681 (31%) | ||
| DM | 16,332 | < 0.001 | |||
| Yes | 3096 (14%) | 1730 (11%) | 1366 (24%) | ||
| No | 13,236 (86%) | 10,135 (89%) | 3101 (76%) | ||
| HP | 16,332 | < 0.001 | |||
| Yes | 7587 (42%) | 4474 (33%) | 3113 (64%) | ||
| No | 8745 (58%) | 7391 (67%) | 1354 (36%) | ||
| LS7 | 16,332 | 8.29± (2.43) | 8.72± (2.36) | 7.13± (2.24) | < 0.001 |
| LS7 | 16,332 | < 0.001 | |||
| T1 | 6870 (37%) | 4077 (30%) | 2793 (56%) | ||
| T2 | 4752 (30%) | 3612 (30%) | 1140 (29%) | ||
| T3 | 4710 (33%) | 4176 (40%) | 534 (15%) | ||
| BFP | 16,332 | 35± (10) | 34± (10) | 40± (9) | < 0.001 |
| T1 | 5399 (33%) | 4628 (39%) | 771 (18%) | ||
| T2 | 5127 (33%) | 3789 (34%) | 1338 (31%) | ||
| T3 | 5806 (34%) | 3448 (28%) | 2358 (51%) | ||
|
1 N not Missing (unweighted) 2 n (unweighted) (%); Mean± (SD); Median (p25, p75) 3 Pearson’s X^2: Rao & Scott adjustment; Design-based KruskalWallis test | |||||
Continuous variables that followed a normal distribution were evaluated using weighted t-tests, whereas those that were not normally distributed were analyzed through the weighted Wilcoxon rank-sum test
Percentages (weighted N, %) for categorical variables: the P value was calculated by the weighted chi-square test
Abbreviation: PIR, Ratio of family income to poverty; TC, Total cholesterol; DM, Diabetes; HP, hypertension; LS7, Life’s Simple 7; BFP, Body Fat Percentage
Association between LS7 and arthritis
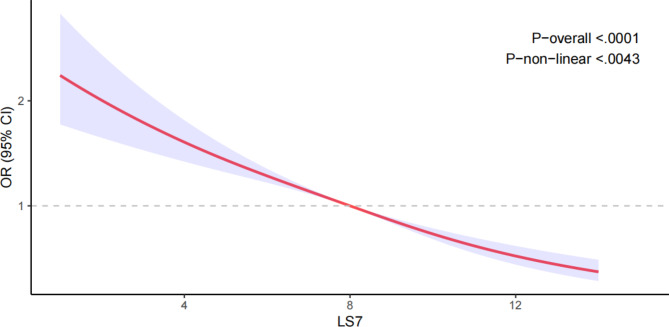
Table 2 illustrates the findings from three models assessing the link between LS7 and arthritis. In Model 3, following the adjustment for all covariables, every one standard deviation increase in LS7 was associated with a 13% reduction in the likelihood of arthritis [OR = 0.87 (95% CI: 0.84, 0.89)]. Moreover, participants in the top tertile of LS7 (T3) demonstrated a 50% lower likelihood of developing arthritis when compared to individuals in the bottom tertile (T1) [OR = 0.50 (95% CI: 0.43, 0.60)]. As LS7 scores increased from T1 to T3, the odds ratios (OR) correspondingly decreased, with a statistically significant trend (p < 0.001). The restricted cubic spline (RCS) analysis (Fig. 2) further demonstrated a non-linear negative relationship between LS7 and arthritis (nonlinearity p = 0.0043).
Table 2.
Association between LS7, BFP, and arthritis
| Characteristics | Model 1 [OR (95% CI)] |
p-value | Model 2 [OR (95% CI)] |
p-value | Model 3 [OR (95% CI)] |
p-value |
|---|---|---|---|---|---|---|
| LS7 - Arthritis | ||||||
| Continuous | 0.75(0.74,0.77) | < 0.001 | 0.84(0.82,0.86) | < 0.001 | 0.87(0.84,0.89) | < 0.001 |
| Tertile | ||||||
| T1 | 1 (ref.) | 1 (ref.) | 1 (ref.) | |||
| T2 | 0.50(0.45,0.56) | < 0.001 | 0.67(0.59,0.76) | < 0.001 | 0.77(0.67,0.88) | < 0.001 |
| T3 | 0.20(0.17,0.23) | < 0.001 | 0.39(0.33,0.46) | < 0.001 | 0.50(0.43,0.60) | < 0.001 |
| P for trend | < 2e-16 | 4.70e-16 | 1.68e-10 | |||
| BFP - Arthritis | ||||||
| Continuous | 1.07(1.06,1.07) | < 0.001 | 1.06(1.05,1.07) | < 0.001 | 1.05(1.05,1.06) | < 0.001 |
| Tertile | ||||||
| T1 | 1 (ref.) | 1 (ref.) | 1 (ref.) | |||
| T2 | 2.05(1.76,2.39) | < 0.001 | 1.46(1.24,1.73) | < 0.001 | 1.37(1.14,1.63) | < 0.001 |
| T3 | 4.08(3.57,4.67) | < 0.001 | 2.86(2.41,3.40) | < 0.001 | 2.47(2.04,3.00) | < 0.001 |
| P for trend | < 2e-16 | < 2e-16 | 1.45e-12 |
Model 1: no covariables were adjusted
Model 2: age, gender, education level, marital, PIR, and race were adjusted
Model 3: age, gender, education level, marital, PIR, race, smoking, drinking, hypertension, diabetes, and total cholesterol were adjusted
Abbreviation: LS7, Life’s Simple 7; BFP, Body Fat Percentage
Fig. 2.
Dose-response relationships between LS7 and arthritis. OR (solid lines) and 95% confidence levels (shaded areas) were adjusted for age, gender, education level, marital, PIR, race, smoking, drinking, hypertension, diabetes, and total cholesterol
Relationship between LS7 and BFP
Linear regression analysis indicated a significant relationship between LS7 and BFP (β = -0.11, 95% CI: -0.12, -0.11, p < 0.001) (refer to Supplementary Table S3).
Subgroup analysis of LS7 and arthritis
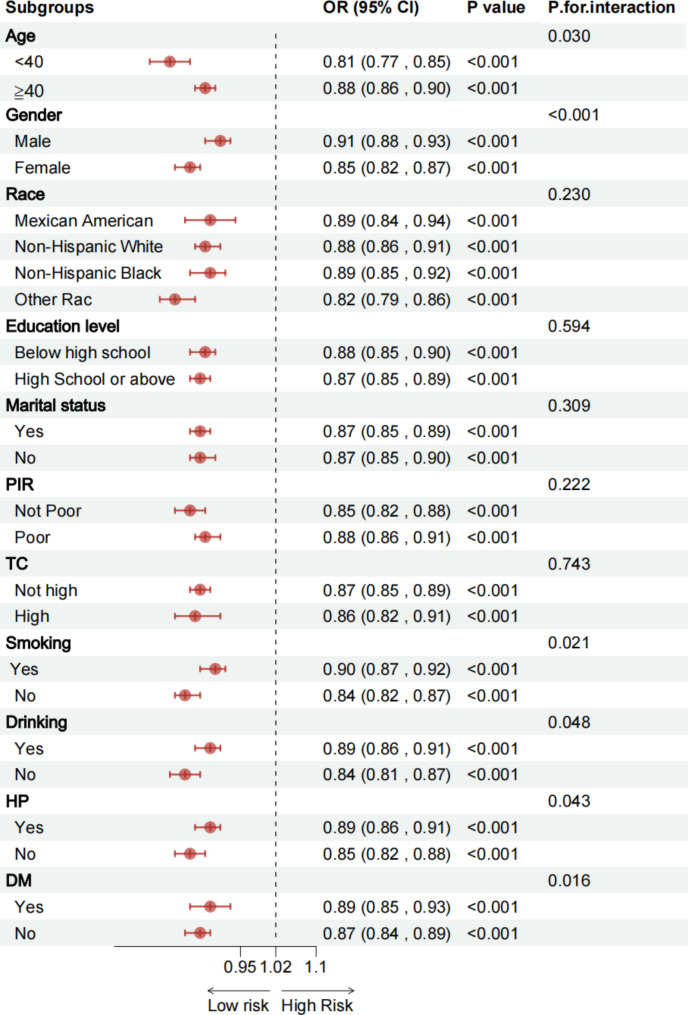
Figure 3 presents the results of the subgroup analysis, examining the relationship between LS7 and arthritis across several covariables, including age, sex, education level, marital status, poverty income ratio (PIR), race, smoking habits, alcohol intake, hypertension, diabetes, and total cholesterol. Results consistently showed a negative association between LS7 and arthritis across subgroups, with significant interactions noted for age, gender, smoking, drinking, HP, and DM (p < 0.05), indicating that the protective effect of LS7 might be stronger in certain populations. Furthermore, the consistently low p-values (< 0.001) observed in most subgroups suggest that the relationship between LS7 and arthritis is robust and stable across different demographic and clinical variables.
Fig. 3.
Subgroup analysis between LS7 and arthritis. Abbreviation: PIR, Ratio of family income to poverty; TC, Total cholesterol; DM, Diabetes; HP, hypertension; LS7, Life’s Simple 7; BFP, Body Fat Percentage
Relationship between BFP and arthritis
Table 2 also outlines the association between BFP and arthritis. In Model 3, after controlling for all covariables, each standard deviation increase in BFP was linked with a 5% increase in the likelihood of developing arthritis [OR = 1.05, 95% CI: 1.05, 1.06]. Further analysis revealed that participants in the highest BFP tertile (T3) faced a 1.47-fold increased likelihood of arthritis when contrasted with individuals in the lowest tertile (T1) [OR = 2.47, 95% CI: 2.04, 3.00]. As BFP rose from T1 to T3, the OR values progressively increased, with a statistically significant trend (p < 0.001), underscoring a positive relationship between BFP and arthritis.
Mediation analysis
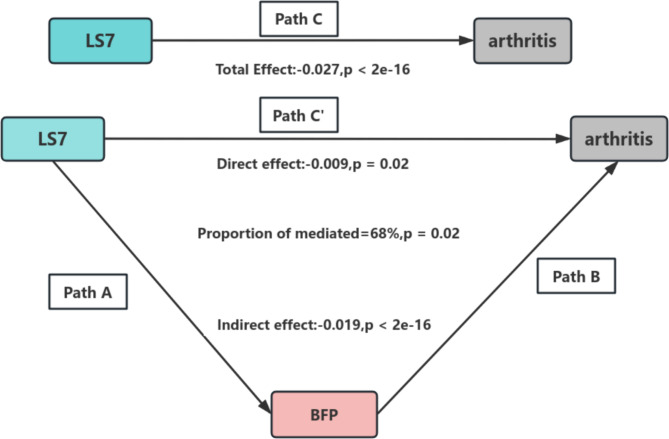
The mediation analysis indicated an indirect impact of -0.019 (95% CI: -0.0214 to -0.02, p < 2e-16), reflected indirect effect of the mediator on the relationship between LS7 and arthritis. The direct effect was measured at -0.009 (p = 0.02), suggesting a remaining portion of the direct effect. The total effect was calculated as -0.027 (p < 2e-16), with approximately 68% of the effect being mediated by the mediator variable. Therefore, BFP is identified as a mediator in the association between LS7 and the occurrence of arthritis. The mediation pathways (Path A, Path B, and Path C) and their effects are illustrated in Fig. 4.
Fig. 4.
Schematic diagram of the mediation effect analysis. Path C (Total Effect): This represents the overall relationship between LS7 and arthritis, combining both direct and indirect effects. Path C′ (Direct Effect): This refers to the effect of LS7 on arthritis that is not mediated by BFP, represent the independent influence of LS7. Path A : This pathway shows the effect of LS7 on the BFP. Path B : This pathway shows the effect of BFP on the arthritis. The indirect effect is estimated as the multiplication of paths A and B (path A*B). The mediated proportion is calculated as indirect effect/ (indirect effect + direct effect) × 100%. Abbreviation: LS7, Life’s Simple 7; BFP, Body Fat Percentage
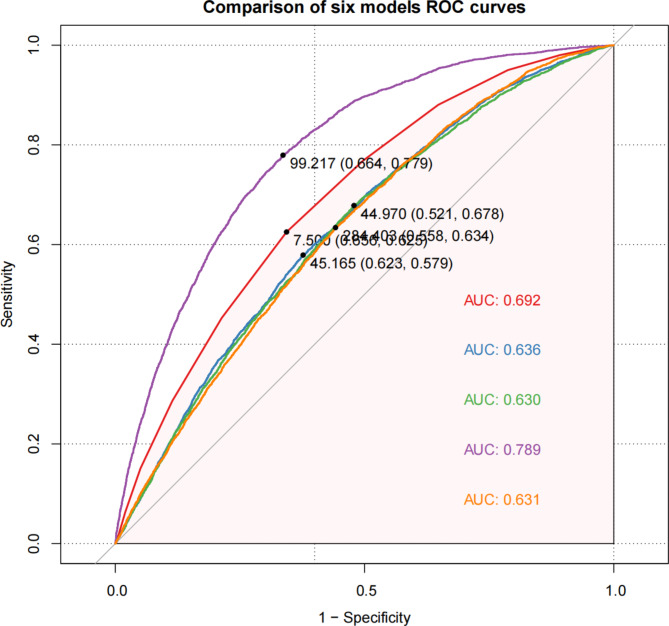
Comparison of ROC curves across five models
Figure 5 compares the ROC curves of five models used to classify arthritis versus non-arthritis cases. Model D demonstrated the best performance, achieving an AUC (area under the curve) of 0.789, signifying excellent classification capability. With a sensitivity of 0.664, the specificity was 0.779, indicating strong generalizability for Model D in this study. Model A presented an AUC of 0.692, slightly lower than Model D but still showing commendable classification performance, especially with a sensitivity of 0.656 and specificity of 0.625.
Fig. 5.
Evaluating Predictive Power of Variables Using ROC Curves. Each of the five models is added in order LS7, BFP, PIR, AGE and TC. (A: AUC: LS7;B: AUC: LS7 + BFP; C:AUC: LS7 + BFP + PIR; D:AUC: LS7 + BFP + PIR + AGE; E:AUC: LS7 + BFP + PIR + AGE + TC)
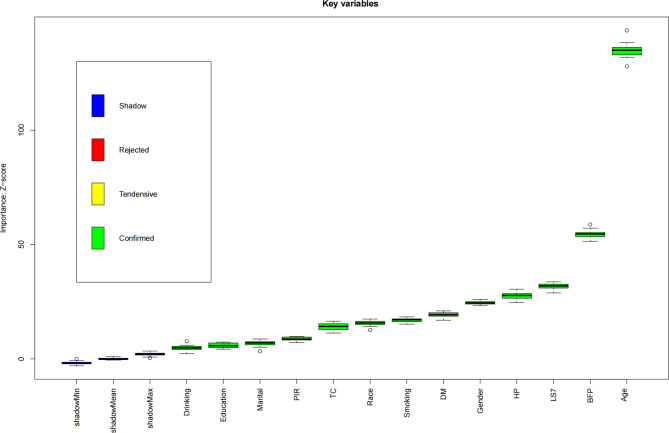
Boruta algorithm for feature selection in arthritis
Figure 6 illustrates the outcomes of the Boruta algorithm, which identified critical features related to arthritis. Variables highlighted in the green box were confirmed as significant feature. BFP and LS7 ranked second and third in importance, emphasizing their vital roles in arthritis assessment. Additional key variables included drinking, education, marital status, PIR, total cholesterol, race, smoking, diabetes, gender, hypertension, and age. The Boruta algorithm effectively identified these factors as significant contributors to arthritis.
Fig. 6.
Important characteristic variables identified as associated with arthritis by the Boruta algorithm. Abbreviation: PIR, Ratio of family income to poverty; TC, Total cholesterol; DM, Diabetes; HP, hypertension; LS7, Life’s Simple 7; BFP, Body Fat Percentage
Relationship between BMI and BFP
The analysis using Pearson relationship indicates a robust positive association (Pearson r = 0.86) between BFP and BMI, suggesting that as BMI rises, BFP also experiences a significant increase (Refer to Supplementary Figure S2). Furthermore, the linear regression analysis reinforces this connection, exhibiting a slope of 1.586. This indicates that for every unit increase in BMI, BFP tends to rise by approximately 1.586 units on average. The goodness-of-fit of the model (R² = 0.740) implies that roughly 74% of the variability observed in BFP can be accounted for by BMI. Nonetheless, the residuals from the regression model exhibit some fluctuations, ranging from a minimum of -76.39 to a maximum of 18.80, which suggests that the relationship between BFP and BMI is not entirely linear. Therefore, while there is a strong relationship between BMI and BFP, they are not perfectly equivalent.
Discussion
This study used NHANES data to explore the association between LS7 scores and arthritis, while also investigating the mediating role of BFP. The findings indicate a significant negative association between higher LS7 scores and arthritis, with BFP acting as an important mediator in this relationship. These enhance the understanding of the mechanisms involved in the onset of arthritis and suggest potential preventive measures, especially in terms of lifestyle choices and metabolic health.
Our results reveal a meaningful negative relationship between LS7 scores and arthritis. After controlling for all covariables, each standard deviation increase in LS7 was linked to a 13% decrease in the likelihood of arthritis. Additionally, individuals in the highest LS7 tertile (T3) exhibited a 50% reduced likelihood of developing arthritis when contrasted with those in the lowest tertile (T1). These findings suggest that improving the LS7 scores may reduce the likelihood of arthritis.
The LS7 score, which includes tobacco use, body mass index (BMI), physical activity, dietary habits, blood sugar levels, blood pressure, and cholesterol, reflects key lifestyle factors influencing arthritis. Smoking increases pro-inflammatory cytokines like TNF-α and interleukin-1β, exacerbating joint damage and arthritis progression [28]. Thus, reducing tobacco use may help prevent arthritis by lowering inflammation. Obesity, indicated may by a high BMI, contributes to arthritis, particularly osteoarthritis, by adding mechanical stress to joints and promoting the release of pro-inflammatory adipokines like interleukin-6 and TNF-α, which degrade cartilage [23, 24, 27]. Maintaining a healthy BMI can reduce both mechanical and inflammatory stress on joints. Physical activity strengthens muscles and joints, reducing degeneration, and has anti-inflammatory effects, lowering pro-inflammatory cytokines and improving metabolism [31, 32]. Diets rich in fruits, vegetables, and healthy fats, such as the Mediterranean diet, help reduce inflammation and support joint health by modulating oxidative stress and lipid metabolism [33]. Elevated blood sugar and insulin resistance, common in metabolic syndrome, exacerbate joint inflammation and promote arthritis [29]. Managing blood glucose through diet and exercise may alleviate these effects. Hypertension also increases systemic inflammation, raising pro-inflammatory cytokines that damage joints [30]. Managing blood pressure through lifestyle changes may protect joint health. High cholesterol, like LDL, triggers inflammatory pathways that damage joint tissues and contribute to arthritis [39]. Maintaining healthy cholesterol levels can help modulate these pathways. In conclusion, optimizing LS7 scores by improving lifestyle factors—reducing tobacco use, maintaining a healthy BMI, staying active, eating a balanced diet, and managing blood sugar, blood pressure, and cholesterol—may help reduce arthritis development and progression through effects on inflammation and joint health.
The mediation analysis we conducted reveals that BFP plays a significant role in mediating the association between LS7 and arthritis. Serving as an indicator of obesity, BFP represents an individual’s fat content. Obesity not only imposes mechanical stress on joints but also fosters chronic inflammation. Adipocytes release a variety of cytokines, including interleukin-6, resistin, and tumor necrosis factor-alpha, all of which can intensify joint inflammation [22–25]. Furthermore, studies show that children who are obese have an increased likelihood of developing arthritis, with this risk escalating as they age and being more common among females [35]. Our findings suggest that improving LS7 scores to reduce BFP levels may help lowering the likelihood of arthritis. This reflects the mediating role of BFP in the LS7-arthritis relationship and may provide strategies for the prevention of arthritis in the future.
The ROC analysis offers further confirmation of the findings from the study. Through the comparison of predictive models, it was shown that integrating LS7, BFP, and additional covariables (such as PIR, age, and TC) improved the classification ability between arthritis and non-arthritis cases, with Model D attaining the peak AUC of 0.789. This underscores the importance of incorporating BFP along with LS7 and other factors, reinforcing its significance in detecting individuals with arthritis.
In our subgroup analysis, the inverse association between LS7 and arthritis exhibited significant interactions in specific groups, particularly concerning age, gender, smoking habits, alcohol consumption, HP and DM. The results indicated that older adults, females, smokers, and individuals with hypertension or diabetes may experience greater benefits from higher LS7 scores, suggesting that the impact of healthy lifestyles may vary across different populations. Moreover, the consistently low p-values (< 0.001) across most subgroups underscore the robustness and stability of the association between LS7 and arthritis, lending greater credibility to these findings. Therefore, personalized lifestyle interventions should be designed to address the specific characteristics and needs of the target populations to achieve optimal prevention outcomes.
The relationship between BMI and BFP shows a strong positive relationship, But not exactly the same. While BMI serves as a straightforward and practical indicator, BFP offers a more thorough measurement that includes additional elements like age, sex, and their interplay with BMI. Consequently, BMI may be more suited for regular preliminary screening, BFP can deliver a more detailed, accurate, and holistic evaluation of body fat.
While this study highlights important relationships between LS7, BFP, and arthritis, several limitations should be noted. First, the cross-sectional design of this study prevents the establishment of causality. Although the mediating role of BFP provides insights into the potential pathways linking LS7 to arthritis, further research, such as prospective cohort studies, randomized controlled trials, or experimental studies, is required to confirm these mechanisms and establish causative relationships. Second, the reliance on self-reported arthritis diagnoses in NHANES data may introduce information bias. Participants may underreport or misclassify their condition due to recall inaccuracies or lack of medical confirmation, potentially affecting the reliability of the results. Using physician-confirmed diagnoses or integrating biomarkers in future studies could improve diagnostic accuracy. Third, although we adjusted for a broad range of covariables to enhance the robustness of our findings, the inherent limitations of the NHANES dataset mean that residual confounding cannot be fully excluded. Unmeasured factors, such as genetic predispositions or environmental influences, may still influence the observed associations. Finally, the findings are based on data from the NHANES population, which, while representative of the U.S., may not fully generalize to other populations with differing demographic, lifestyle, or health profiles. Given these limitations, the results of this study should be interpreted cautiously and objectively. Further research, particularly longitudinal and experimental studies, is essential to validate these findings and explore the underlying mechanisms in greater depth.
Conclusion
In summary, this study identified an association between higher LS7 scores and a lower likelihood of arthritis, with BFP acting as a mediating factor in this relationship. The findings suggest that lifestyle factors and body fat levels may play a role in arthritis. While these results highlight the potential importance of promoting healthy lifestyle behaviors and managing body fat, the study’s cross-sectional design limits causal interpretation. Future research should employ longitudinal studies to further explore these associations and to develop tailored strategies for high-risk populations to better address the burden of arthritis.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We sincerely thank the NHANES database for supplying the data used in this research.
Abbreviations
- BFP
Body Fat Percentage
- LS7
Life’s Simple 7
- BMI
Body Mass Index
- NCHS
National Center for Health Statistics
- NHANES
National Health and Nutrition Examination Survey
- OR
Odds Ratio
- CI
Confidence Interval
- PIR
Ratio of family income to poverty
- TC
Total cholesterol
- DM
Diabetes
- HP
Hypertension
Author contributions
Huan Chen conceptualized and drafted the manuscript. Chan Kang provided the study design and critical feedback, while other contributors offered supervision and additional guidance.
Funding
The authors declare that no financial support was received for the research, writing, or publication of this paper.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
Approval for utilizing human participant data was granted by the National Center for Health Statistics (NCHS) Research Ethics Review Board. This study adhered to institutional guidelines and relevant local regulations. Written informed consent was obtained from all participants before their inclusion in the study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sun X, Li R, Cai Y, et al. Clinical remission of rheumatoid arthritis in a multicenter real-world study in Asia-Pacific region. Lancet Reg Health West Pac. 2021;15:100240. 10.1016/j.lanwpc.2021.100240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cheleschi S, Giordano N, Volpi N, et al. A complex relationship between Visfatin and Resistin and microRNA: an in Vitro Study on Human chondrocyte cultures. Int J Mol Sci. 2018;19(12):null. 10.3390/ijms19123909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van der Heijde D, Mease PJ, Landewé RBM, et al. Secukinumab provides sustained low rates of radiographic progression in psoriatic arthritis: 52-week results from a phase 3 study, FUTURE 5. Rheumatology (Oxford). 2020;59(6):1325–34. 10.1093/rheumatology/kez420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Felson DT, Neogi T. Emerging treatment models in Rheumatology: challenges for Osteoarthritis trials. Arthritis Rheumatol. 2018;70(8):1175–81. 10.1002/art.40515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iwaszko M, Wielińska J,Świerkot J, et al. IL-33 gene polymorphisms as potential biomarkers of Disease susceptibility and response to TNF inhibitors in Rheumatoid Arthritis, Ankylosing Spondylitis, and psoriatic arthritis patients. Front Immunol. 2021;12:631603. 10.3389/fimmu.2021.631603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khalid Almutairi,Jiri Nossent, Preen DB, et al. The global prevalence of rheumatoid arthritis: a meta-analysis based on a systematic review. Rheumatol Int. 2020;41(5):863–77. 10.1007/s00296-020-04731-0. [DOI] [PubMed] [Google Scholar]
- 7.Saeid SA-AK, Damian Hoy, et al. Global, regional and national burden of osteoarthritis 1990–2017: a systematic analysis of the global burden of Disease Study 2017. Ann Rheum Dis. 2020;79(6):819–28. 10.1136/annrheumdis-2019-216515. [DOI] [PubMed] [Google Scholar]
- 8.Li L, Feng D, Zeng J, et al. Association between rheumatoid factor and metabolic syndrome in general population. Diabetol Metab Syndr. 2022;14(1):165. 10.1186/s13098-022-00914-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li J, Zhang B, Liu WX, et al. Metformin limits osteoarthritis development and progression through activation of AMPK signalling. Ann Rheum Dis. 2020;79(5):635–45. 10.1136/annrheumdis-2019-216713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.González HM, Tarraf W, Harrison K, et al. Midlife cardiovascular health and 20-year cognitive decline: atherosclerosis risk in communities Study results. Alzheimers Dement. 2018;14(5):579–89. 10.1016/j.jalz.2017.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shen R, Guo X, Zou T, Ma L. Modifiable risk factors and metabolic health in risk of cardiovascular disease among US adults: a nationwide cross-sectional study. Int J Cardiol Cardiovasc Risk Prev. 2024;22:200283. 10.1016/j.ijcrp.2024.200283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sadiq R, Broni EK, Levine LD, Retnakaran R, Echouffo-Tcheugui JB. Association of ideal cardiovascular health and history of gestational diabetes mellitus in NHANES 2007–2018. Diabetes Res Clin Pract. 2024;217:111857. 10.1016/j.diabres.2024.111857. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Du S, Hong X, Yang Y, et al. Association between body fat percentage and H-type hypertension in postmenopausal women. Front Public Health. 2022;10:950805. 10.3389/fpubh.2022.950805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dragan S, Șerban MC, Damian G, et al. Dietary patterns and interventions to Alleviate Chronic Pain. Nutrients. 2020;12(9). 10.3390/nu12092510. [DOI] [PMC free article] [PubMed]
- 15.Bai N, Lu X, Jin L, et al. CLSTN3 gene variant associates with obesity risk and contributes to dysfunction in white adipose tissue. Mol Metab. 2022;63:101531. 10.1016/j.molmet.2022.101531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang L, Chang G, Cai S, Zou X, Qin M, Tan Y. The association of Life’s simple 7 and infertility among U.S. women. Front Endocrinol (Lausanne). 2024;15:1288289. 10.3389/fendo.2024.1288289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xiong Y, Wangsheng F, Wang S, et al. Positive association between body fat percentage and hyperuricemia in patients with hypertension: the China H-type hypertension registry study. Nutr Metab Cardiovasc Dis. 2021;31(11):3076–84. 10.1016/j.numecd.2021.07.002. [DOI] [PubMed] [Google Scholar]
- 18.Zeng Q, Dong SY, Sun XN, Xie J, Cui Y. Percent body fat is a better predictor of cardiovascular risk factors than body mass index. Braz J Med Biol Res. 2012;45(7):591–600. 10.1590/s0100-879x2012007500059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang W, Hou S, Wang K, Ling B, Yu H. Association of body roundness index with female infertility: 2013–2018 NHANES. Front Nutr. 2024;11:1416637. 10.3389/fnut.2024.1416637. Published 2024 Oct 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiang Y, Jia M. Association between Non-HDL to HDL cholesterol ratio (NHHR) and psoriasis in adults: a cross-sectional study using 2009–2014 data. Clin Cosmet Investig Dermatol. 2024;17:2523–31. 10.2147/CCID.S492053. Published 2024 Nov 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li X, Huang P, Wang H, et al. Relationship between weight-adjusted waist index (WWI) and osteoarthritis: a cross-sectional study using NHANES data. Sci Rep. 2024;14(1):28554. 10.1038/s41598-024-80151-5. Published 2024 Nov 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Donaubauer AJ, Becker I, Weissmann T, et al. Low dose Radiation Therapy induces long-lasting reduction of Pain and Immune modulations in the Peripheral blood - interim analysis of the IMMO-LDRT01 Trial. Front Immunol. 2021;12:740742. 10.3389/fimmu.2021.740742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Andrea Booth,Aaron Magnuson,Josephine, Fouts K, et al. Adipose tissue, obesity and adipokines: role in cancer promotion. Horm Mol Biol Clin Investig. 2015;21(1):57–74. 10.1515/hmbci-2014-0037. [DOI] [PubMed] [Google Scholar]
- 24.Iwona, Kojta. Marta Chacińska,Agnieszka Błachnio-Zabielska. Obesity, bioactive lipids, and adipose tissue inflammation in insulin resistance. Nutrients. 2020;12(5):1305–1305. 10.3390/nu12051305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao J. Hyaluronic acid-modified and TPCA-1-Loaded Gold Nanocages alleviate inflammation. Pharmaceutics. 2019;11(3). 10.3390/pharmaceutics11030143. [DOI] [PMC free article] [PubMed]
- 26.Zhang Y, Yu H, Fu J, et al. Oxidative balance score and the potential for suffering rheumatoid arthritis: a cross-sectional study. Front Immunol. 2024;15:1454594. 10.3389/fimmu.2024.1454594. Published 2024 Nov 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rohm TV, Fuchs R, Müller RL, et al. Obesity in humans is characterized by gut inflammation as shown by pro-inflammatory intestinal macrophage Accumulation. Front Immunol. 2021;12:668654. 10.3389/fimmu.2021.668654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miyagawa I, Nakayamada S, Ueno M, et al. Precision medicine based on the phenotypic differences in peripheral T helper cells in patients with psoriatic arthritis: one year follow-up outcomes. Front Med (Lausanne). 2022;9:934937. 10.3389/fmed.2022.934937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li L, Feng D, Zeng J, Ye P, Chen Y, Wei D. Association between rheumatoid factor and metabolic syndrome in general population. Diabetol Metab Syndr. 2022;14(1):165. 10.1186/s13098-022-00914-w. Published 2022 Nov 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yokota K, Sato K, Miyazaki T, et al. Characterization and function of Tumor Necrosis factor and interleukin-6-Induced osteoclasts in rheumatoid arthritis. Arthritis Rheumatol. 2021;73(7):1145–54. 10.1002/art.41666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oh GS, Lee JH, Byun K, et al. Effect of intake of leucine-rich protein supplement in parallel with Resistance Exercise on the body composition and function of healthy adults. Nutrients. 2022;14(21). 10.3390/nu14214501. [DOI] [PMC free article] [PubMed]
- 32.Fernandes P, de Mendonça Oliveira L. Physical Exercise induces Immunoregulation of TREG, M2, and pDCs in a lung allergic inflammation model. Front Immunol. 2019;10:854. 10.3389/fimmu.2019.00854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Raad T, George E, Griffin A, Larkin L, Fraser A, Kennedy N, Tierney A. Effects of a telehealth-delivered Mediterranean diet intervention in adults with rheumatoid arthritis (MEDRA): a randomised controlled trial. BMC Musculoskelet Disord. 2024;25(1):631. 10.1186/s12891-024-07742-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gómez-Ambrosi J, Silva C, Catalán V, Rodríguez A, Galofré JC, Escalada J, Valentí V, Rotellar F, Romero S, Ramírez B, Salvador J, Frühbeck G. Clinical usefulness of a new equation for estimating body fat. Diabetes Care. 2012;35(2):383–8. 10.2337/dc11-1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Emmanuel Baah. Obesity and arthritis. Curr Developments Nutr. 2020;4(0). 10.1093/cdn/nzaa063_004. nzaa063_004-nzaa063_004.
- 36.Teitelbaum J, Goudie S, An Open-Label. Pilot trial of HRG80™ red ginseng in chronic fatigue syndrome, Fibromyalgia, and post-viral fatigue. Pharmaceuticals (Basel). 2021;15(1). 10.3390/ph15010043. [DOI] [PMC free article] [PubMed]
- 37.Xiong A, Nie W, Cheng L, et al. Association between Obesity and poor prognosis in patients receiving Anlotinib for Advanced Non-small Cell Lung Cancer. Front Pharmacol. 2022;13:812555. 10.3389/fphar.2022.812555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peng X, Huang J, Liu Y, et al. Influence of changes in obesity indicators on the risk of hypertension: a Cohort Study in Southern China. Ann Nutr Metab. 2021;77(2):100–8. 10.1159/000515059. [DOI] [PubMed] [Google Scholar]
- 39.Schwarz A, Bonaterra GA, Schwarzbach H, et al. Oxidized LDL-induced JAB1 influences NF-κB independent inflammatory signaling in human macrophages during foam cell formation. J Biomed Sci. 2017;24(1):12. 10.1186/s12929-017-0320-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.