Abstract
Context
Since the outbreak of COVID-19 in late 2019, the transmission dynamics and clinical presentation patterns of influenza A (Flu A) virus have undergone changes.
Objectives
This article conducted a comparative analysis in clinical characteristics and laboratory results of pediatric patients with Flu A before, during, and after the COVID-19 pandemic.
Methods
The medical records of 885 children hospitalized with Flu A virus infection at a tertiary hospital in Guangdong Province, China, were retrospectively analyzed. Flu A was confirmed in these cases using a direct immunofluorescence antigen assay. The clinical data for this study span from January 1, 2018, to May 31, 2023.
Results
In our study, we observed a total of 340 cases before the COVID-19 pandemic, 196 cases during the pandemic, and 349 cases after the pandemic. Patients after the pandemic had a higher median age on admission (5.66 years, range 3.41–7.70) and exhibited more respiratory symptoms such as cough, sore throat, and nasal stuffiness. The length of hospital stay was longer, and there was a higher percentage of patients with fever duration ≥ 5 days among Flu A patients during the pandemic. Compared to before and during the COVID-19 pandemic, Flu A patients after the pandemic showed significantly reduced white blood cell (WBC) and platelet (PLT) counts (P < 0.001), along with elevated levels of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) in laboratory indexes (P < 0.001). Furthermore, more hospitalized children after the pandemic were diagnosed with benign acute childhood myositis (BACM).
Conclusion
Our research results indicates a significant decrease in Flu A cases during the COVID-19 pandemic, and hospitalized children with Flu A have more severe clinical symptoms after the COVID-19 pandemic. These findings have implications for public health policy and clinical management of Flu A cases.
Clinical trial number
Not applicable.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12887-024-05285-x.
Keywords: Influenza A (flu A), COVID-19 pandemic, Pediatric patients, Clinical feature, Laboratory indexes, Benign acute childhood myositis (BACM)
Introduction
The Flu A virus, also known as H1N1 and belonging to the Orthomyxovirus family, exhibits high variability and can be transmitted through the air, droplets, and direct contact, infecting both humans and other animals. Since its initial emergence in the early 20th century, the H1N1 virus has been responsible for several global pandemics. The 1918 Spanish flu pandemic resulted in a significant number of deaths worldwide, while the 2009 H1N1 flu pandemic marked the first global flu pandemic caused by a swine-origin H1N1 virus [1, 2]. The population at risk for Flu A includes individuals of all age groups, with particular vulnerability observed in children, the elderly, individuals with chronic illnesses, and those with compromised immune systems, who are at higher risk of infection and developing severe illness.
The COVID-19 pandemic, which originated in late 2019, swiftly escalated into a global public health crisis with profound implications for the global economy. This pandemic has also influenced the epidemiology of various respiratory viruses worldwide, including the Flu A virus [3–5]. Between January 2020 and December 2022, the preventive and control measures implemented for COVID-19 effectively curbed the transmission of other respiratory viruses in China, including the flu to some extent. However, by early 2023, with the normalization of population movement and social activities in China, flu activity, predominantly of the H1N1 subtype, experienced a rapid resurgence, leading to an influenza epidemic peak in numerous regions across China [6].
Yangjiang, covering an area of approximately 7,813.4 square kilometers and with a population of 2.7 million, consists of two counties and two districts. It is located in the west of Guangdong Province in southern China. At the end of 2022, Yangjiang experienced an outbreak of SARS-CoV-2 omicron infections [7]. Subsequently, there was a surge in Flu A, and many children were infected. As a result, some schools were forced to close [8].
While there have been numerous reports on respiratory virus infections, such as Flu A and COVID-19, in China [9, 10], few studies have conducted comprehensive analyses spanning before, during, and after the COVID-19 pandemic. This study is designed to investigate the epidemiological characteristics and clinical manifestations of Flu A in hospitalized children across these time periods. By analyzing the impact of the pandemic on the spread of Flu A, we aim to establish a scientific foundation for future influenza prevention and control efforts.
Materials and methods
Study population and design
In this retrospective, single-center study, we conducted a review of the electronic medical records of 885 pediatric patients with Flu A at the People’s Hospital of Yangjiang, Guangdong province, China, spanning from January 1, 2018, to May 31, 2023. The patients included in the study were categorized into three groups: 340 cases before the COVID-19 pandemic (January 1, 2018, to December 31, 2019), 196 cases during the pandemic (January 1, 2020, to December 31, 2022), and 340 cases after the pandemic (January 1, 2023, to May 31, 2023).
The diagnostic criteria for children with Flu A were established based on the expert consensus on the diagnosis and treatment of influenza in children (2020 edition) issued by the National Center for Respiratory Disease Clinical Medical Research and the Chinese Medical Association Pediatrics Branch Respiratory Group [11]. This study adhered to the ethical guidelines outlined in the Declaration of Helsinki and was approved by the Institutional Review Board of People’s Hospital of Yangjiang (No. 20230003) and Chaozhou Central Hospital (No. 2023009). Given the retrospective nature of the study, signed informed consent from participants or their guardians was waived.
Clinical data collection and definitions
In this study, electronic medical records of all patients were thoroughly reviewed. The data collected encompassed demographic information, medical history, underlying comorbidities, symptoms and signs, laboratory results, radiology examinations, treatment protocols, and any occurrences of adverse events. The inclusion criteria for children with Flu A were as follows: (1) aged between 1 month and 14 years; (2) diagnosed with Flu A based on antibody immunofluorescence assay results from nasal swab and/or oropharynx swab; (3) exhibited relevant clinical manifestations. The onset of the disease was defined as the day when symptoms first appeared. Additionally, pneumonia was characterized by the presence of symptoms or signs such as cough, abnormal lung auscultation findings, and pulmonary infiltrates on chest imaging. The diagnostic criteria for BACM included children displaying sudden gait-related abnormalities or refusal to bear weight following a viral illness, with normal neurological findings and elevated creatinine kinase levels. With conservative treatment, this condition typically resolves spontaneously within a week without residual effects [12]. The presence of liver injury was defined as an elevation of ALT levels (> 3×upper limit of normal, ULN) or at least moderate elevation of ALP or total bilirubin levels (> 2×ULN) during hospital stay [13].
Given our focus on patients infected with the Flu A virus, data on co-infections with other viruses were excluded from this study.
Diagnostic sampling and methodology
Clinical and laboratory results were analyzed and compared for hospitalized children with Flu A infection before, during, and after the COVID-19 pandemic. Nasal swabs and/or oropharyngeal swabs of patients were collected by trained healthcare professionals following standard operating procedures within 24 h of admission. Respiratory viruses were identified using direct immunofluorescence antigen assay with the D3 Ultra DFA virus identification reagent from Diagnostic Hybrids, Inc., USA, which includes Flu A, Flu B, adenovirus (ADV), Respiratory syncytial virus (RSV), Parainfluenza virus 1 (PIV1), PIV2, and PIV3. The assay was performed in accordance with the manufacturer’s instructions.
Statistical analysis
All statistical analyses were conducted using IBM SPSS statistical software version 20 for Windows (IBM Corp., Armonk, New York, USA). Categorical variables were presented as frequencies and percentages, while continuous variables were expressed as mean ± standard error. Variations in continuous variables between groups were assessed using non-parametric tests. Disparities in categorical variables between groups were evaluated using the Chi-square test or Fisher’s exact test as deemed appropriate, with a significance level set at P < 0.05.
Results
Clinical manifestations
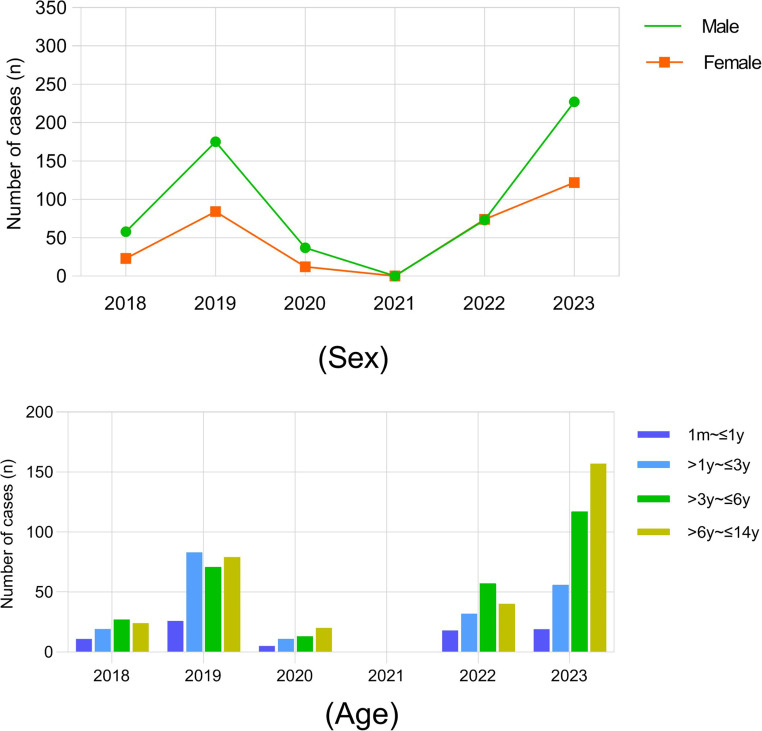
In this study, we conducted an analysis of 885 hospitalized children with Flu A virus infection from January 2018 to May 2023. The data presented in Table 1; Fig. 1 revealed that the number of Flu A cases during the COVID-19 pandemic (from January 1, 2020, to December 31, 2022) was significantly lower at 196 cases compared to before the pandemic (January 1, 2018, to December 31, 2019, with 340 cases) and after the pandemic (January 1, 2020, to December 31, 2022, with 349 cases). Especially, it seems that transmission was interrupted in 2021 and no positive cases were found.
Table 1.
Clinical characteristics of pediatric patients with influenza A infection before, during, and after COVID-19 pandemic
| Characteristics | Before the pandemic (n = 340) | During the pandemic (n = 196) | After the pandemic (n = 349) | P-value |
|---|---|---|---|---|
| Age on admission (median, IQR) | 3.96 (1.83 ~ 6.56) | 4.38 (2.35 ~ 6.88) | 5.66 (3.41 ~ 7.70) | < 0.001 |
| Gender | 0.015 | |||
| male | 233 (68.5) | 110 (56.1) | 227 (65.0) | |
| female | 107 (31.5) | 86 (43.9) | 122 (35.0) | |
| Length of hospital stay (day, mean ± SD)* | 3.34 ± 1.39 | 3.94 ± 1.64 | 3.80 ± 1.76 | < 0.001 |
| Clinical symptoms | ||||
| Fever | 337 (99.1) | 192 (98.0) | 347 (99.4) | 0.300 |
| peak temperature ≥ 39℃ | 307 (90.3) | 167 (85.2) | 301 (86.2) | 0.144 |
| fever duration ≥ 5d | 24 (7.1) | 60 (30.6) | 31 (8.8) | < 0.001 |
| Respiratory symptoms | ||||
| cough | 281 (82.6) | 146 (74.5) | 308 (88.3) | < 0.001 |
| sore throat | 17 (5.0) | 19 (9.7) | 74 (21.2) | < 0.001 |
| nasal stuffiness | 37 (10.9) | 38 (19.4) | 78 (22.3) | < 0.001 |
| rhinorrhea | 53 (15.6) | 75 (38.3) | 113 (32.4) | < 0.001 |
| dyspnea | 5 (1.5) | 6 (3.1) | 11 (3.2) | 0.339 |
| Gastrointestinal symptoms | ||||
| vomiting | 41 (12.1) | 33 (16.8) | 93 (26.6) | < 0.001 |
| diarrhea | 17 (5.0) | 8 (4.1) | 14 (4.0) | 0.203 |
| poor appetite | 117 (34.4) | 62 (31.6) | 69 (19.7) | < 0.001 |
| Pneumonia (chest CT imaging) | 30/103 (29.1) | 20/80 (25.0) | 50/146 (34.2) | 0.341 |
| bilateral pneumonia | 27/103 (26.2) | 17/80 (21.2) | 43/146 (29.4) | 0.423 |
| unilateral pneumonia | 3/103 (2.9) | 3/80 (3.8) | 7/146 (4.8) | 0.774 |
| Bacterial co-infection △ | 6/239 (2.5) | 3/164 (1.8) | 4/234 (1.7) | 0.830 |
| Clinical diagnosis | ||||
| febrile convulsion | 70 (20.6) | 62 (36.7) | 79 (22.6) | 0.012 |
| acute tonsillitis | 135 (39.7) | 166 (84.7) | 305 (87.4) | < 0.001 |
| benign acute childhood myositis | 0 (0.0) | 1 (3.5) | 14 (4.0) | < 0.001 |
| liver function damage | 8 (2.3) | 3 (1.5) | 10 (2.9) | 0.617 |
| underlaying disease▲ | 25 (7.4) | 26 (15.4) | 33 (9.7) | 0.078 |
| Medical treatment | ||||
| antiviral (oseltamivir) | 337 (99.1) | 190 (96.9) | 337 (96.6) | 0.068 |
| antibiotics | 321 (94.4) | 150 (76.5) | 173 (49.6) | < 0.001 |
| ambroxol or budesonide via aerosol | 230 (67.6) | 108 (55.1) | 179 (51.3) | < 0.001 |
| Oxygen therapy | 0.347 | |||
| nasal catheter oxygen inhalation | 79 (23.2) | 48 (28.4) | 82 (23.5) | |
| noninvasive mechanical ventilation | 0 (0.0) | 0 (0.0) | 2 (0.6) | |
| Outcome | > 0.999 | |||
| cure/discharge | 340 (100.0) | 169 (100.0) | 348 (99.7) | |
| death (in-hospital mortality) | 0 (0.0) | 0 (0.0) | 1 (0.3) |
Note: Data are n (%)
* Excluding two patients who were transferred to higher-level hospital for treatment
△Before the pandemic group: staphylococcus aureus (six cases, sputum culture). During the pandemic group: includes staphylococcus aureus (one case, sputum culture), streptococcus pneumoniae (one case, sputum culture) and salmonella (one case, blood culture). After the pandemic group: includes staphylococcus aureus (one case, sputum cluture), pseudomonas aeruginosa (one case, sputum culture) and streptococcus pneumoniae (two cases, sputum culture)
▲Before the epidemic: includes G6PD deficiency (sixteen cases), epilepsy (five cases), thalassemia (two cases), congenital heart disease (one case), hepatoblastoma (one case), thrombocytopenic purpura (one case) and asthma (one case); During the epidemic: includes G6PD deficiency (thirteen cases), thalassemia (eight cases), nephrotic syndrome (three cases), epilepsy (one case), and thrombocytopenic purpura (one case); After the epidemic: includes G6PD deficiency (eight cases), thalassemia (eight cases), epilepsy (eight cases), Kawasaki disease (three cases), nephrotic syndrome (two cases), acute lymphoblastic leukemia (one case), Congenital adrenal cortical hyperplasia (one case), acute infectious mononucleosis (one case), and asthma (one case)
Fig. 1.
Sex and age distribution of influenza A cases from 2018 to 2023
The median ages of patients were 3.96 years (IQR, 1.83–6.56) before the COVID-19 pandemic, 4.38 years (IQR, 2.35–6.88) during the pandemic, and 5.66 years (IQR, 3.41–7.70) after COVID-19. Notably, children affected with Flu A virus after the COVID-19 pandemic tended to be older than those before and during the pandemic (Fig. 1). The sex ratio of males to females ranged from 1.28 to 2.17 in the three groups. Fever and cough were identified as the most common symptoms, with the highest number of patients experiencing fever duration ≥ 5 days (60 cases, 30.6%) and had longer hospital stays (3.94 ± 1.64 days) during the COVID-19 pandemic. However, hospitalized children with Flu A virus after the pandemic exhibited more upper respiratory tract infection symptoms, including cough, sore throat, and nasal stuffiness, occurring in 88.3%, 21.2%, and 22.3% of cases, respectively. The clinical characteristics of pediatric patients with Flu A infection before, during, and after the COVID-19 pandemic were compared, similarities and differences were presented in Table 1.
Moreover, Flu A patients after the pandemic were more likely to BACM (14/349, 4.0%). There was a significant male predominance (12/14, 85.7%) with a median age of 7 years. Muscle pain persisted for an average of 3 days, while the median stay in the hospital was 2 days. These patients were treated conservatively, and all of them recovered without any complications (Supplemental Table 1).
Laboratory and imaging findings
In terms of imaging characteristics, no significant differences in CT scan presentations were observed among Flu A patients before, during, and after the COVID-19 pandemic. Bacterial co-infections were confirmed by respiratory sputum culture in twelve cases and blood culture in one case, however, there was no statistical difference between three groups (Table 1).
Regarding hematology and biochemical indicators, as shown in Table 2, the Flu A group post-COVID-19 pandemic exhibited significantly lower white blood cell (WBC) and platelet (PLT) counts, along with elevated serum levels of alanine aminotransferase (ALT) and aspartate aminotransferase (AST), compared to the Flu A groups before and during the COVID-19 pandemic. These variations in laboratory indices were found to be statistically significant (P < 0.01).
Table 2.
Comparison of laboratory findings between pediatric patients infected with influenza A before, during and after COVID-19 epidemic
| Laboratory findings | Before the pandemic (n = 340) | During the pandemic (n = 196) | After the pandemic (n = 349) | P-value |
|---|---|---|---|---|
| WBC (×109/L) | ||||
| Reduced | 41/222 (18.5) | 23/129 (17.8) | 72/204 (35.3) | < 0.001 |
| Elevated | 16/222 (7.2) | 0/129 (0.0) | 13/204 (6.4) | < 0.001 |
| LYM (×109/L) | ||||
| Reduced | 112/222 (50.5) | 40/129 (31.0) | 90/204 (44.1) | 0.002 |
| Hb (g/L) | ||||
| Reduced | 45/222 (20.3) | 24/131 (18.3) | 55/211 (26.1) | 0.182 |
| PLT (×109/L) | ||||
| Reduced | 47/222 (21.2) | 14/129 (10.9) | 54/204 (26.5) | 0.003 |
| CRP (mg/L) | ||||
| Elevated | 80/260 (30.8) | 33/128 (25.8) | 22/103 (21.4) | 0.175 |
| PCT (ng/mL) | ||||
| Elevated | 289/290 (99.7) | 121/123 (98.4) | 187/193 (96.9) | 0.046 |
| ALT (U/L) | ||||
| Elevated | 12/316 (3.8) | 5/145 (3.5) | 29/289 (10.0) | 0.002 |
| AST (U/L) | ||||
| Elevated | 97/322 (30.1) | 34/145 (23.5) | 116/289 (40.1) | 0.001 |
| CK (U/L) | ||||
| Elevated | 52/317 (16.4) | 21/147 (14.3) | 44/287 (15.3) | 0.827 |
| CK-MB (U/L) | ||||
| Elevated | 310/317 (97.7) | 140/147 (95.2) | 271/287 (94.4) | 0.043 |
Note: Data are n (%)
WBC: white blood cell; LYM: lymphocyte; Hb: hemoglobin; PLT: platelet; CRP: C-reactive protein; PCT: procalcitonin; ALT: alanine aminotransferase; AST: aspartate aminotransferase; CK: creatine kinase; CK-MB: creatine kinase-MB
Elevated: exceeding the upper limit of the normal range. Reduced: below the lower limit of the normal range
Treatment and prognosis
During hospitalization, all included patients received supportive care including fluid and electrolyte replacement therapy, as well as oxygen supplementation. Common treatments administered to patients comprised antiviral therapy (oseltamivir), antimicrobial therapy (oral or intravenous antibiotics), and the use of ambroxol or budesonide aerosol. The administration of all medications was at the discretion of the attending physician (Table 1).
In total, the majority of patients in the study exhibited favorable clinical outcomes, with only one patient succumbing to multiple organ dysfunction syndrome and acute necrotizing encephalopathy. Additionally, a 4-year-old boy experienced multiple organ dysfunction. Both patients were admitted to the hospital after the onset of the COVID-19 pandemic, and their cases are summarized in Table 3.
Table 3.
The clinical manifestations of two critically hospitalized children
| Case | Sex | Age (Y) | Duration of Hospitalization | Complaints and Past Medical History | Physical Examination upon Admission | CT scan | Laboratory Results | Treatment | Diagnosis | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Male | 11 | 5 days |
Increased bowel movements; Fever for one day; The highest recorded temperature was 39 °C, accompanied by nausea, vomiting, and abdominal pain. Past medical history not reported. |
Body temperature of 40.5 °C; pulse rate of 168 beats per minute; Blood pressure of 105/49 mmHg; Respiratory rate of 25 breaths per minute. |
Minor inflammation in the lower lobe of the left lung; Head CT revealed brain swelling with decreased density in both cerebral hemispheres and the brainstem. |
Troponin T at 963 ng/L (Reference < 100 ng/L); ALT 210.1 U/L, AST 520.9 U/L, CK-MB 129.5 U/L, IL-6 at 8132.61 pg/ml (Reference 0-5.3pg/ml). |
Oxygen therapy; Intravenous fluids; Mechanical ventilation. |
Flu A-associated acute necrotizing encephalopathy and multi-organ failure | Death |
| 2 | Male | 4 | 14 days | 4-day history of fever and cough. Received cefotaxime treatment at an outside hospital for 2 days, no significant improvement. Past medical history of cerebral palsy and epilepsy, with a highest recorded temperature of 39 °C. | Body temperature of 38.3℃. Respiratory rate of 20 breaths/min, Height 86 cm, Weight 11.5 kg. Conscious and alert, with average mental state and signs of malnutrition. Tonsils enlarged. Coarse breath sounds in both lungs, symmetrical, with occasional rales. Heart rate 120 beats/min, regular rhythm. Increased muscle tension in all four limbs. Hands are clenched and flexed, lower limbs are stiff. | No CT scan |
ALT 810.6 U/L, AST 2249.2 U/L, LDH 3799 U/L, CK 52,436 U/L, CK-MB 510.9 U/L; Respiratory sputum culture: pseudomonas aeruginosa. |
Anti-inflammatory treatment |
Multiple organ dysfunction syndrome (liver injury, myocarditis); Spastic cerebral palsy; Bronchopneumonia; Flu A. |
Condition improved, discharged |
Discussion
Influenza poses a significant public health challenge globally, with children being particularly vulnerable to contracting the virus and experiencing severe complications. A thorough understanding of the epidemiology and clinical characteristics of Flu A is essential for effective prevention, control, and treatment strategies. In our study, we comprehensively and comparatively analyzed the clinical features of hospitalized children with Flu A before, during, and after the COVID-19 pandemic. Our data revealed a significant decrease in Flu A cases among children during the pandemic, which aligning with previous research findings [14, 15]. This suggests that stringent public health measures and heightened awareness of respiratory hygiene during the COVID-19 pandemic have had a positive impact on reducing the spread and prevalence of Flu A. However, following the gradual relaxation of epidemic control measures in late 2022, we observed a resurgence of Flu A cases and a delayed peak of winter influenza appeared between January to May 2023. This phenomenon has been observed in different countries worldwide [16]. It could potentially be attributed to the paucity of protective immunity arising from extended periods of low exposure to a given pathogen, leaving a greater proportion of the population susceptible to the disease. Another reason may be the impact of the interruption of vaccination, routine vaccination coverage of the national planned immunization declined during the COVID-19 pandemic, which inevitably led to an increase in the susceptible population of vaccine preventable diseases. It is crucial to monitor the evolving trends of Flu A closely in the future to inform appropriate public health responses.
Overall, males have a higher ratio of Flu A. As previously reported in the literature, males have a higher risk of influenza compared to females across all age groups during influenza virus outbreaks [8, 17]. Understanding sex differences in the pathogenesis of influenza and considering them in the rational design of prophylactic and therapeutic strategies is important. In this study, the proportion of female patients has increased during the pandemic when comparing the pre- and post-pandemic periods. However, the exact reason for sex differences still remains unclear. Fever and cough were the predominant symptoms at the onset of illness for Flu A pediatric patient [18]. Our data reveals that approximately 85–90% of patients had a body temperature of 39℃ or higher. During the pandemic, hospitalized children had longer hospital stays. There was a higher percentage of them experiencing fever lasting for five days or more and encountering febrile convulsions. There could be several potential reasons for these findings, changes in healthcare practices might have had an impact. For instance, during the pandemic, hospitals may have had different admission criteria or protocols, leading to longer hospital stays. Additionally, healthcare resources might have been diverted to deal with COVID-19 cases, potentially affecting the management and duration of stay for influenza patients. Viral virulence could also play a role. It is possible that the influenza virus strains circulating during the pandemic had increased virulence, resulting in more severe symptoms and longer durations of fever. Mutations in the virus could have led to altered pathogenicity [19]. Immune responses during the pandemic could be another factor. The immune systems of individuals might have been altered due to stress, changes in lifestyle, or exposure to multiple pathogens. Moreover, the interaction between the immune response to COVID-19 and influenza could contribute to the observed phenomena. After the pandemic, we observed changes in clinical presentation, with a greater proportion of patients exhibiting respiratory symptoms such as cough, sore throat, and nasal congestion.
In the cohort of hospitalized children with confirmed Flu A, we observed that abnormalities in liver biochemistries were more frequently found in patients with Flu A after the pandemic. These abnormalities were manifested as elevations in ALT and AST, most of which were mild and gradually returned to normal during the hospital stay. Only a few cases progressed to liver injury. As a respiratory disease, liver injury typically does not occur during Flu A virus infection. Respiratory and other mucosal tissues may experience viral replication or immune damage, but this does not usually affect non-mucosal organs such as the liver or kidney [20]. However, a study finding has been pointed out that a pandemic A/H1N1 Flu A virus, which is different from seasonal influenza, can lead to liver damage [21]. The peak of winter flu in 2022 postponed until after the COVID-19 pandemic, the increased incidence of abnormal aminotransferases in patients with Flu A post-pandemic may be due to an exaggerated immune response leading to elevated enzyme levels, the underlying mechanism remains to be clarified.
Influenza is known as the most common cause of virus-associated BACM, characterized by a self-limited sudden onset of calf pain which causes difficulty walking and mainly affects pre-school and school-aged boys at a median age of 6–9 years [22]. According to our data, symptoms of lower extremity pain, myalgia, weakness, and lameness were significantly more common in children after the COVID-19 pandemic than in other periods. The characteristics such as male predominance and more common in older children are similar to the published reports [23, 24], the cause is unclear, it could be related to genetic predisposition of male gender or a greater physical activity. CK levels were significantly higher in these patients, which is consistent with what has been reported in some previous studies [25–27]. Considering the self-limiting nature of this complication and the lack of the need for complex treatments in case of early diagnosis, physicians can diagnose and treat these cases, depending on the clinical symptoms and laboratory examination.
Limitation
There were several limitations in our present study. First, we excluded other respiratory viruses that possible co-circulate in the patients. This exclusion was necessary due to specific research objectives and limitations. Additional studies should be undertaken in the future to provide more information concerning the interactions of co-infections and their implications for clinical practice. Second, limited by the original data, as some patients were not tested for SARS-CoV-2 in this study. As a consequence, the impact of co-infection of SARS-CoV-2 and Flu A virus on hospitalized children could not be analyzed. Lastly, influenza vaccination coverage data for children were not available, we were unable to evaluate the impact of Flu A vaccinations on patients.
Conclusion
After the COVID-19 epidemic, as non-pharmaceutical interventions were relaxed, the incidence of Flu A in children returned to pre-epidemic levels, and a non-seasonal outbreak occurred. The clinical manifestations also changed, warranting a deeper exploration of potential causes and implications for prevention, control strategies, and treatment approaches in childhood infectious diseases.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Not applicable.
Author contributions
LYY conceived the study and revised the manuscript. FL wrote the manuscript. ZXG performed the clinical practices and collected the data. MW analyzed the clinical data. JLL collected the data. All authors reviewed the manuscript.
Funding
This study was supported by the Scientific Research Project of Chaozhou city (No.2023ZC01), and the High-level Key Medical and Health Research Project of Yangjiang (No.2023001). The funder had no role in the study’s design, data interpretation, and manuscript writing.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
The studies involving humans were approved by the Institute Ethics Committee of Chaozhou Central Hospital (No. 2023009) and People’s Hospital of Yangjiang (No. 20230003). The studies were conducted in accordance with the local legislation and institutional requirements. The Ethics Committee/Institutional Review Board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Fen Lin and Jian-Lian Liang contributed equally to this work.
References
- 1.Taubenberger JK, Morens DM. 1918 influenza: the mother of all pandemics. Emerg Infect Dis. 2006;12:15–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kumar V. Influenza in children. Indian J Pediatr. 2017;84(2):139–43. 10.1007/s12098-016-2232-x. [DOI] [PubMed] [Google Scholar]
- 3.Chan CP, Wong NS, Leung CC, Lee SS. Positive impact of measures against COVID-19 on reducing influenza in the Northern Hemisphere. J Travel Med. 2020;27:taaa087. 10.1093/jtm/taaa087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Olsen SJ, Azziz-Baumgartner E, Budd AP, Brammer L, Sullivan S, Pineda RF, et al. Decreased influenza activity during the COVID-19 pandemic- United States, Australia, Chile, and South Africa, 2020. Am J Transpl. 2020;69:1305–9. 10.15585/mmwr.mm6937a6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pérez-López A, Mana HA, Iqbal M, Suleiman M, Mohammad Hasan R, Tang P. Resurgence of influenza A infections in children after the relaxation of COVID-19- related social distancing measures and normalization of international travel in Qatar. J Travel Med. 2022;29:taac107. 10.1093/jtm/taac107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Su Y, Guo Z, Gu X, Sun S, Wang K, Xie S et al. Influenza vaccine effectiveness against influenza A during the delayed 2022/23 epidemic in Shihezi, China. Vaccine. 2023,41:5683–6. 10.1016/j.vaccine.2023.08.039. [DOI] [PubMed]
- 7.Yang YK, Lin F, Lin JF, Lin CH, Liu LL, Ma YB, et al. Covid-19 omicron variant infection in neonates of Guangdong province-a report of 52 cases. Front Pediatr. 2023;20:111191651. 10.3389/fped.2023.1191651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin F, Chen MT, Zhang L, Wu M, Xie H, Guan ZX, et al. Resurgence of influenza A after SARS-CoV-2 omicron wave and comparative analysis of hospitalized children with COVID-19 and influenza a virus infection. Front Med (Lausanne). 2024;10:1289487. 10.3389/fmed.2023.1289487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zheng JZ, Chen FJ, Wu K, Wang JC, Li FR, Huang S, et al. Clinical and virological impact of single and dual infections with influenza A (H1N1) and SARS-CoV-2 in adult inpatients. PLoS Negl Trop Dis. 2021;29(11):e0009997. 10.1371/journal.pntd.0009997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li Y, Wang HZ, Wang F, Du H, Liu XR, Chen P, et al. Comparison of hospitalized patients with pneumonia caused by COVID-19 and influenza A in children under 5 years. Int J Infect Dis. 2020;98:80–3. 10.1016/j.ijid.2020.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.National Clinical Medical Research Center for Respiratory Diseases and Chinese Medical Association Pediatrics Branch Respiratory Group Expert Consensus on Diagnosis and Treatment of Influenza in Children. (2020 Edition). Chinese Journal of Practical Pediatric Clinical Practice, 2020,35(17):1281–1288. 10.3760/cma.j.cn101070-20200224-00240
- 12.Jain S, Kolber MR. A stiff-legged gait: benign acute childhood myositis. CMAJ. 2009;181:711–3. 10.1503/cmaj.090781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Georgakopoulou VE, Bali T, Adamantou M, Asimakopoulou S, Makrodimitri S, Samara S, et al. Acute hepatitis and liver injury in hospitalized patients with COVID-19 infection. Exp Ther Med. 2022;26(5):691. 10.3892/etm.2022.11627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Edwards KM. The impact of social distancing for severe acute respiratory syndrome coronavirus 2 on respiratory syncytial virus and infuenza burden. Clin Infect Dis. 2021;72(12):2076–8. 10.1093/cid/ciaa1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Achangwa C, Park H, Ryu S, Lee MS. Collateral impact of public health and social measures on respiratory virus activity during the COVID-19 pandemic 2020–2021. Viruses. 2022;14(5):1071. 10.3390/v14051071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Özgen-Top O, Aysert-Yıldız P, Özger HS, Güzel-Tunçcan O. Evaluation of hospitalized patients with Community-Acquired Influenza-Like Illness during two Influenza Seasons. Infect Dis Clin Microbiol. 2023;5(4):323–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bai Y, Tao X. Comparison of COVID-19 and influenza characteristics. J Zhejiang Univ Sci B. 2021;22(2):87–98. 10.1631/jzus.B2000479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nayak J, Hoy G, Gordon A. Influenza in children. Cold Spring Harb Perspect Med. 2021;11(1):a038430. 10.1101/cshperspect.a038430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liang Y. Pathogenicity and virulence of influenza. Virulence. 2023;14(1):2223057. 10.1080/21505594.2023.2223057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huo CY, Xiao K, Zhang SP, Tang YL, Wang M, Qi P, et al. H5N1 influenza a virus replicates productively in pancreatic cells and induces apoptosis and pro-inflammatory cytokine response. Front Cell Infect Microbiol. 2018;8:386. 10.3389/fcimb.2018.00386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang SP, Hu B, Xu JF, Ren QX, Wang LR, Wang SH. Influenza a virus infection induces liver injury in mice. Microb Pathog. 2019;137:103736. 10.1016/j.micpath.2019.103736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Magee H, Goldman RD. Viral myositis in children. Can Fam Phys. 2017;63:365–8. [PMC free article] [PubMed] [Google Scholar]
- 23.Szenborn L, Toczek-Kubicka K, Zaryczański J, Marchewka-Kowalik M, Miśkiewicz K, Kuchar E. Benign Acute Childhood Myositis during Influenza B Outbreak. Adv Exp Med Biol. 2018;1039:29–34. 10.1007/5584_2017_79. [DOI] [PubMed] [Google Scholar]
- 24.Delavar MA, Ebrahimi HK, Borhani N, Karimian P, Ehsanipour F, Jafarnejad S, et al. Evaluation of the prevalence and clinical and laboratory features of acute viral myositis in children with influenza referred to the emergency department of Ali Asghar Tehran Hospital in 2019 and 2020. J Family Med Prim Care. 2022;11(6):2744–9. 10.4103/jfmpc.jfmpc_1940_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Azevedo AC, Silva ACE, Silva CJ, Miranda SP, Costa M, Martinho I. Benign acute childhood myositis: a 5-year retrospective study. Arch Pediatr. 2022;29:490–3. 10.1016/j.arcped.2022.08.009. [DOI] [PubMed] [Google Scholar]
- 26.Öztürk B, Göktug A, Bodur I, Yaradılmı¸s RM, Güneylioglu MM, Güngör A, et al. Benign acute childhood myositis: factors associated with muscle symptoms and resolution. Pediatr Int. 2022;64:e15273. 10.1111/ped.15273. [DOI] [PubMed] [Google Scholar]
- 27.Attaianese F, Costantino A, Benucci C, Lasagni D, Trapani S. Benign acute children myositis: 5 year experiences in a tertiary care pediatric hospital. Eur J Pediatr. 2023;18:4341–9. 10.1007/s00431-023-05115-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.