Abstract
Background
This study aimed to investigate the modulatory role of prefrontal cortex (PFC) activity in older adults with mild cognitive impairment (MCI) when sensory cues were removed or presented inaccurately (i.e., increased sensory complexity) during sensory manipulation of a balance task. The research sheds light on the neural regulatory mechanisms of the brain related to balance control in individuals with MCI.
Methods
21 older adults with MCI (male/female: 9/12, age: 71.19 ± 3.36 years) were recruited as the experimental group and 19 healthy older adults (male/female: 10/9, age: 70.16 ± 4.54 years) as the control group. Participants were required to perform balance tests under four standing conditions: standing on a solid surface with eyes open, standing on a foam surface with eyes open, standing on a solid surface with eyes closed, and standing on a foam surface with eyes closed. Functional Near-Infrared Spectroscopy (fNIRS) and force measuring platform are used to collect hemodynamic signals of the PFC and center of pressure (COP) data during the balance task, respectively.
Results
Under the eyes open condition, significant Group*Surface interaction effects were found in the mean velocity of the COP (MVELO), the mean velocity in the medial-lateral (ML) direction (MVELOml) and the 95% confidence ellipse area of the COP (95%AREA-CE). Additionally, significant Group*Surface interaction effect was found in the left orbitofrontal cortex (L-OFC). The significant group effects were detected for three ROI regions, namely the left ventrolateral prefrontal cortex (L-VLPFC), the left dorsolateral prefrontal cortex (L-DLPFC), the right dorsolateral prefrontal cortex (R-DLPFC). Under the eyes closed condition, the significant Group*Surface interaction effects were found in root mean square (RMS), the RMS in the ML direction (RMSml) and the 95%AREA-CE. Additionally, significant group effects were detected for five ROI regions, namely R-VLPFC, the left frontopolar cortex (L-FPC), L-DLPFC, R-DLPFC and R-OFC.
Conclusion
Our study emphasizes the role of the PFC in maintaining standing balance control among older adults with MCI, particularly during complex sensory conditions, and provides direct evidence for the role of the PFC during balance control of a clinically relevant measure of balance.
Trial registration
ChiCTR2100044221, 12/03/2021.
Keywords: MCI, PFC, postural control, sensory orientation test, fNIRS
Background
Mild cognitive impairment (MCI) is an intermediate state between normal cognitive aging and Alzheimer’s disease [1], and is considered as a risk factor for dementia. The rate of progression to dementia in older adults with MCI is 60–100% within 5–10 years [2]. It is reported that older adults with cognitive impairment may impair their perception of the environment and their ability to identify risks, and are more likely to fall than those with normal cognitive function [3]. Cognitive and executive function decline is the main early feature of older adults with MCI, and as the disease progresses, it will seriously affect their motor function [1]. Due to significant cognitive decline, older adults with MCI experience subtle balance control and gait deficits resulting in a two-fold increase in risk of falls compared to those with normal cognition [4]. The consequences of such falls (such as fractures, head injuries, etc.) significantly affect their quality of life and independent functioning that increases financial and psychosocial burdens [5].
Balance control is a complex neuromuscular control process, that involves the accurate sensory perception [6] (visual, vestibular, and proprioceptive) of reliable information about environmental conditions, integration of sensorimotor information in the Central Nervous System, and appropriate programming and execution of neuromuscular responses [7]. Maintaining balance involves re-weighting the three sensory inputs to adapt to environmental changes [8]. Studies have shown that the sensory inputs may be used to varying degrees during different life stages [9]. Aging may affect sensory reweighting [10]. In particular, aging is accompanied by a reduced capacity to respond to external perturbations during postural control, especially when sensory cues are hindered or inaccurate [11]. This is partly due to an age-related decline in neuromuscular function [12] and reduced cognitive and visuospatial processing abilities [13].
However, compared to healthy older adults, older adults with MCI exhibit a diminished ability to allocate limited attention resources to posture control [14], This reduction leads to deficiencies in their balance and postural control abilities [15], particularly a notable decline in balance in the medial-lateral direction [16]. Previous studies have focused on exploring the effect of aging on the sensory reweighting from balance control [10] and central brain regulation [17, 18], however, there are relatively few studies on the sensory organization in older adults with MCI. Qi et al. explored the static balance ability under different sensory inputs of the MCI by manipulating the soft and hard support surfaces and vision [19]. Unfortunately, the study did not further explore how the brain is involved in processing sensory cues to maintain balance control.
Maintenance of standing balance control involves the participation of subcortical structures (brainstem, cerebellum, and spinal cord) [20] and the cerebral cortex [18]. Aging causes a shift from an automatic to a more cortical control of upright posture and locomotion [21]. Older adults show increased and more widespread involvement of cortical areas for postural control compared to young adults, notably in the prefrontal cortex (PFC) [17]. PFC is critical to selectively allocate (visual-spatial) attention [22] and to integrate visual and proprioceptive information [23] to maintain or regain balance control stability. Previous studies have indicated that PFC regions are disproportionately vulnerable to both age-related grey matter atrophy [24] and deterioration in white matter tract integrity [17]. Such changes may lead to a reduction in the available cognitive resources needed for balance control. Specifically, older adults commonly exhibit an increase in neural activation during balancing, particularly in PFC [17].
Previous studies have also reported that continual PFC activation increases with the difficulty of sensory conditions during the Neurcom Sensory Organization Test in older adults [18]. These over-activations in older adults have been interpreted as a dedifferentiation of brain activation, or as a compensation for age-related declines in brain structure and function [23]. These changes reflect a more limited information processing capacity of the aging brain [25]. However, age-related deteriorations are exaggerated when associated with cognitive decline such as in MCI or Alzheimer’s disease [6]. Previous studies found that the increased PFC activation in older adults with MCI may be considered as a compensatory strategy for maintaining dual-task gait performance [26]. Similarly, in our previous study, older adults with MCI showed higher PFC activation during maintaining a simple standing posture compared to healthy older adults [27]. At present, there is a lack of research on PFC activation during sensory manipulation of a balance task in older adults with MCI. With the increase of balance difficulty, and there are also relatively few reports on the neuromodulatory mechanisms of the PFC for balance control in older adults with MCI.
The present study aimed to investigate how PFC activity (measured via fNIRS) is modulated during sensory manipulation of a balance task in older adults with MCI. Comparing the effect on postural sway and neural activity across multiple difficulty levels of balance task will provide insight into the cortical control of standing in older adults with MCI, and also provide a theoretical basis for the formulation of targeted prevention and clinical rehabilitation intervention strategies. The following hypotheses are formulated: (1) Under both the eyes open and closed conditions, the standing balance control ability of the older adults with MCI is worse than that of the healthy older adults, and also the older adults with MCI require more PFC activation. And (2) PFC activation increases with the difficulty levels of balance tasks during sensory manipulation of a balance task in older adults with MCI.
Methods
Participants
In this study, 21 older adults over 65 years old diagnosed with MCI and 19 healthy older adults were recruited from the local community as a control group. The identification of MCI was based on the consensus criteria [28] that included the presence of subjective memory complaints from the patient and family, objective memory impairment (assessed using the Montreal Cognitive Assessment [MoCA]), preserved general intellectual function (assessed clinically), absence of significant functional impairment, and absence of clinical dementia [28]. Additionally, participants needed to score 0.5 on the Clinical Dementia Rating Scale (CDR) [14]. The inclusion and exclusion criteria of participants were as follows [14, 29]: inclusion criteria for the MCI group were (a) clinically diagnosed with MCI; (b) aged 65 years and above; (c) and able to walk independently without a gait aid; (d) MoCA scale score < 26. Inclusion criteria for the control group were 65 years and older, no cognitive impairment, no functional impairment, ability to walk independently without gait assistance, and a score of ≥ 26 on the MoCA scale. Exclusion criteria for both groups were (a) Parkinson’s disease or any neurological disease with motor deficits (e.g., stroke, epilepsy); (b) musculoskeletal disorders or a history of knee or hip replacement surgery that affected gait performance; (c) use of psychotropic medications that affected motor performance (e.g., neuroleptics, benzodiazepines), or active major depression; (d) severe uncorrected visual or auditory impairment.
Sample size calculation
The prior power analysis (G*power version 3.1) indicated that a minimum of 22 participants were needed to obtain the alpha level of 0.05 and the power level of 0.80 based a on previous study [30], which reported that a significant main effect of condition on HbO2 values, η2p = 0.289. To detect statistically significant differences, 40 participants were recruited.
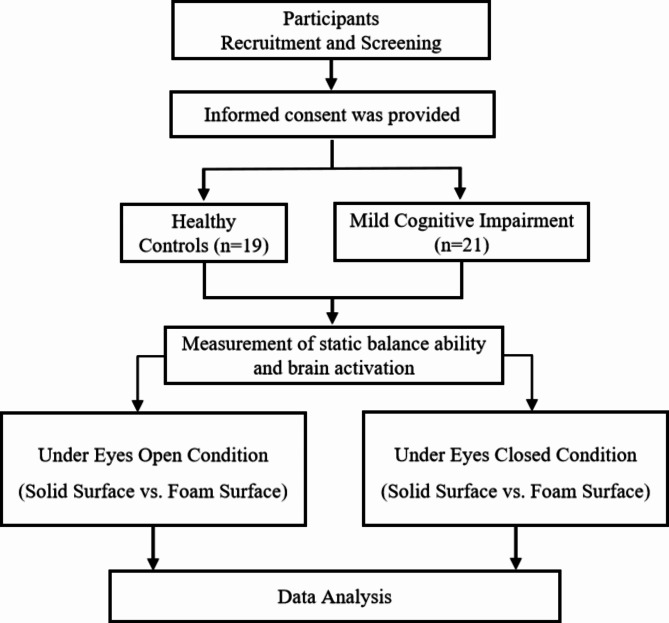
This study was approved by the Ethics Committee of Shandong Sport University (approval number: 2021006) and complied with the Declaration of Helsinki. All participants signed the informed consent form before the experiment. There were no significant differences in age, height, weight, or years of education between the MCI and control group (p > 0.05) (Table 1). A flow chart of the study is presented in Fig. 1.
Table 1.
Participants’ basic information (mean ± standard deviation)
| Control group | MCI group | p value | |
|---|---|---|---|
| Age (year) | 70.16 ± 4.54 | 71.19 ± 3.36 | 0.415 |
| Sex (male/female) | 10/9 | 9/12 | 0.536 |
| Height (cm) | 163.53 ± 6.63 | 159.71 ± 7.29 | 0.055 |
| Weight (kg) | 64.46 ± 8.07 | 63.61 ± 10.04 | 0.771 |
| Years of education (year) | 6.74 ± 3.40 | 6.05 ± 3.31 | 0.52 |
| MoCA | 26.47 ± 0.90 | 18.33 ± 2.97 | < 0.001 |
Fig. 1.
Flow diagram of study participants and allocation to group
Note. Participants were 21 older adults with MCI and 19 healthy older adults. Static balance ability was measured for 30s, and the participants were requested to complete a test of static balance control while standing on a solid surface and a foam surface, respectively, in the eyes open and eyes closed conditions. In addition, PFC activity was measured when the participants were in these static balance control conditions by fNIRS
Measures and procedures
Balance protocol
Standing balance control was performed in the Sports Biomechanics Laboratory. Participants were required to perform four balance tests: standing on a hard support surface with eyes open, standing on a soft support surface (5 cm thick foam) with eyes open, standing on a hard support surface with eyes closed, and standing on a soft support surface (5 cm thick foam) with eyes closed [19]. In addition, each participant was asked to stand barefoot with two feet, which were positioned parallel with a 20 cm distance [29]. They were positioned with arms hanging relaxed to the sides while focusing on a visual reference mark placed in front of them at a 100 cm distance with eyes open [31]. If one leg moved, then the trial failed. Each data was collected for 30 s. Participants were given three opportunities to familiarize themselves with the test procedure before the formal measurement. Three successful trials for each balance test were conducted after the procedures were familiarized. The time interval for breaks was 60 s between two consecutive tests. The research assistant was always around the participant for protection. The force measuring platform (Kistler, 9281CA, 60 cm×90 cm×10 cm) was used to collect the Center of pressure (COP) displacement data of the balance task, and the acquisition frequency was 1000 Hz. Each task was demonstrated to the participants to ensure familiarity with the experimental procedure before the experiment began. The data collection was conducted synchronously during the standing balance and fNIRS tests.
Functional near-infrared spectroscopy test
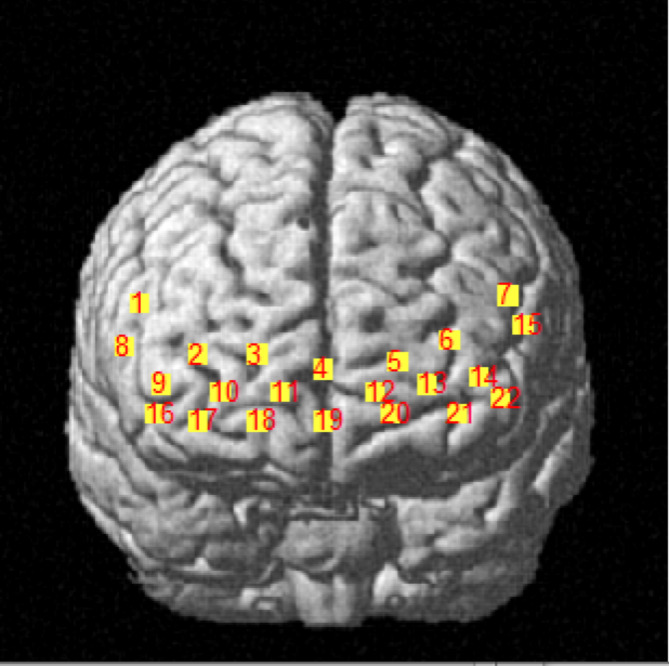
A portable near-infrared imaging system (LIGHTNIRS, Shimadzu Corp., Kyoto, Japan) was used to measure the PFC hemodynamic response via laser diodes with three wavelengths of 780 nm, 805 nm, and 830 nm (sampling frequency 13.3 Hz). The device consisted of 16 optodes with 8 light emitters and 8 light detectors (total 22 channels, as shown in Fig. 2). The distance between the emitter and detector was 30 mm. During the experiment, participants carried fNIRS while performing balance tasks and wore a whole-head fiber holder with the standard head landmarks determined according to the international 10/10 system. A 3D digitizer (FASTRAK, Polhemus, Vermont, USA) was used to determine MNI (Montreal Neurological Institute) coordinates [32]. The PFC was divided into 8 ROIs based on Brodmann areas (BA) [33].
Fig. 2.
Distribution map of 22 channels
Channels 2, 3, 4, 11, and 19 corresponded to the right frontopolar cortex (R-FPC) and belonged to the right BA10. Channels 5, 6, 12, 13, and 21 corresponded to the left frontopolar cortex (L-FPC) and belonged to the left BA10. Channels 9 and 16 corresponded to the right dorsolateral prefrontal cortex (R-DLPFC) and belonged to the right BA46. Channels 14 and 22 corresponded to the left dorsolateral prefrontal cortex (L-DLPFC) and belonged to the left BA46. Channels 1 and 8 corresponded to the right ventrolateral prefrontal cortex (R-VLPFC) and belonged to the right BA45. Channels 7 and 15 corresponded to the left ventrolateral prefrontal cortex (L-VLPFC) and belonged to the left BA45. Channels 10, 17, and 18 corresponded to the right orbitofrontal cortex (R-OFC) and belonged to the right BA11. Channel 20 corresponded to the left orbitofrontal cortex (L-OFC) and belonged to the left BA11.
Using the modified Beer-Lambert law, optical density was converted to blood oxygen concentration, including oxyhemoglobin concentration (HbO2), deoxyhemoglobin concentration (HHb), and total hemoglobin concentration (HbT). HbO2 was selected to characterize hemodynamic changes in the PFC during the balance task conditions because HbO2 is the most sensitive to locomotion-related changes in regional cerebral blood flow and individual differences in Hb are considerable in task-related changes in older adults. Furthermore, there are larger change amplitude and better signal-to-noise ratio in HbO2 [34]. In addition, using one index applicable for task-related hemodynamic changes reduced the comparison number and refrained from increasing the probability of Type I error.
Data processing
Center of pressure
The COP data were low-pass filtered (Butterworth) with a cut-off frequency of 10 Hz [35]. All output variables were calculated based on the mathematical formula in the previous study [36]. The root mean squared (RMS) distance of the COP, the RMS distance of the COP in the medial-lateral (ML) direction (RMSml), the RMS distance of the COP in the anterior-posterior (AP) direction (RMSap), the mean velocity of the COP (MVELO), the mean velocity of the COP in the ML direction (MVELOml), the mean velocity of the COP in the AP direction (MVELOap), the 95% confidence ellipse area of the COP (95%AREA-CE) were calculated.
Hemodynamics
HbO2 data preprocessing was conducted using the Homer2 toolbox, which is based on MATLAB (R2013b, MathWorks Inc., Natick, United States) [37]. The differences between each task and the baseline were calculated to determine the cerebral hemodynamic changes induced by the balance task. The raw data were first visually inspected to mark the bad channels and to remove the segments with poor signal quality. The optical intensity data were converted into optical density data. Artifact correction was performed using linear interpolation. Subsequently, the signal was band-pass filtered with a high-pass cut-off frequency (0.01 Hz) to eliminate instrumental noise and low-frequency drift, and a low-pass cut-off frequency (0.1 Hz) to remove the spontaneously generated physiological components (Mayer waves: ≈0.1 Hz, heart rate: 1.6–1.8 Hz, respiration: 0.2–0.3 Hz) [38]. Using the modified Beer–Lambert Law, optical density data were converted to the HbO2 and deoxyhemoglobin (HbR) [39]. The mean value of HbO2 within the last 5 s of each sitting was selected as a baseline for correction, resulting in the ΔHbO2. After data preprocessing, the ΔHbO2 of the three tests for each task was superimposed and averaged to obtain the time-series changes of all channels of the individual under the two tasks. The data were averaged across all channels in the ROIs and consequently across all participants. The HbO2 data were chosen, because HbO2 signals have a better signal-tonoise ratio, higher reproducibility, are more sensitive indicators of local blood flow changes and have been proven to be superior in assessing functional activity [40].
Statistical analyses
SPSS20.0 (IBMS, NY, USA) was used for statistical analysis of the data. Three successful trial data were averaged for data analysis. All variables were presented as mean ± standard deviation. The normality of all the outcomes was tested with the Shapiro-Wilk test. We compared differences in balance and PFC activation parameters with two-way multivariate analysis of variance (MANOVA), using groups (control group and MCI group), types of support surface (solid surface and foam surface) as fixed factors, and interactions between group and types of support surface. If a significant interaction was detected, the Bonferroni adjusted t-test method was conducted for post hoc comparisons. Partial eta squared (η2p) was used to represent the effect size of main effect and interaction of MANOVA. The thresholds for Partial eta squared were as follows: 0.01–0.06, small; 0.06–0.14, moderate; >0.14, large [41]. The significance level was set to P < 0.05.
Result
Balance parameters
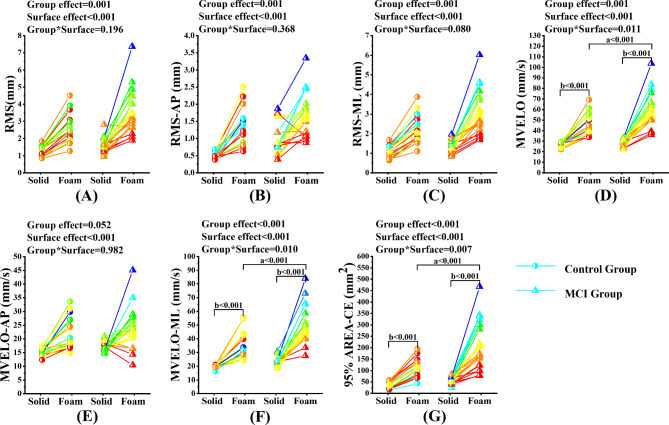
Under the eyes open condition, two-way MANOVA showed that the significant Group*Surface interaction effects were found in MVELO (p = 0.011, η2p = 0.083), MVELOml (p = 0.01, η2p = 0.084) and 95%AREA-CE (P = 0.007, η2p = 0.093). Post-hoc comparisons showed that in the MCI group, the MVELO, the MVELOml and 95%AREA-CE were significantly higher during standing on the foam surface compared to those standing on the solid surface (all p < 0.001). During standing on the foam surface, the MVELO, the MVELOml and 95%AREA-CE were significantly higher in the MCI group compared to those in the control group (all p < 0.001). No significant Group*Surface interaction effects were found in RMS (p = 0.196, η2p = 0.022), RMSap (p = 0.368, η2p = 0.011), RMSml (p = 0.08, η2p = 0.04) and MVELOap (p = 0.982, η2p = 0.000). Significant group effects were found in RMS (p = 0.001, η2p = 0.128), RMSap (p = 0.001, η2p = 0.137) and RMSml (p = 0.001, η2p = 0.129). And significant surface effects were found in RMS (p < 0.001, η2p = 0.515), RMSap (p < 0.001, η2p = 0.443), RMSml (p < 0.001, η2p = 0.515) and MVELOap (p < 0.001, η2p = 0.333). Similarly, significant group effects were found in MVELO (p = 0.001, η2p = 0.140), MVELOml (p < 0.001, η2p = 0.230) and 95%AREA-CE (p < 0.001, η2p = 0.198). Significant surface effects were also found in MVELO (p < 0.001, η2p = 0.630), MVELOml (p < 0.001, η2p = 0.628) and 95%AREA-CE (p < 0.001, η2p = 0.498). (Fig. 3).
Fig. 3.
Comparison of static balance control ability between MCI group and control group under the eyes open condition
Note. (A) ~ (G) represents the comparison of balance parameters between MCI group and control group under the eyes open condition. RMS = Root mean squared; RMSml = Root mean squared in the ML direction; RMSap = Root mean squared in the AP direction; MVELO = the average velocity of the COP; MVELOml = the average velocity of the COP in the ML direction; MVELOap = the average velocity of the COP in the AP direction; 95%AREA-CE = the 95% confidence ellipse area of the COP. a, significant difference between groups. b, significant difference between types of support surface
*p < 0.05, **p < 0.01
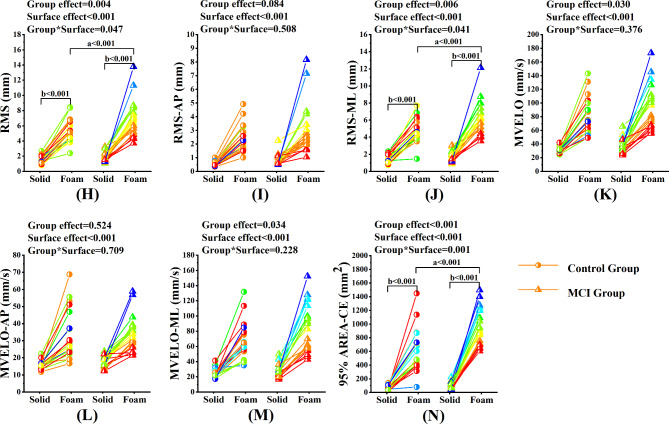
Under the eyes closed condition, the significant Group*Surface interaction effects were found in RMS (p = 0.047, η2p = 0.051), RMSml (p = 0.041, η2p = 0.054) and 95%AREA-CE (P = 0.001, η2p = 0.13). Post-hoc comparisons showed that in the MCI group, the RMS, the RMSml and the 95%AREA-CE were significantly higher during standing on the foam surface compared to those standing on the solid surface (all p < 0.001). During standing on the foam surface, the RMS, the RMSml and the 95%AREA-CE were significantly higher in the MCI group compared to those in the control group (all p = 0.001). No significant Group*Surface interaction effects were found in RMSap (p = 0.508, η2p = 0.006), MVELO (p = 0.376, η2p = 0.010), MVELOml (p = 0.228, η2p = 0.019) and MVELOap (p = 0.709, η2p = 0.002). Significant group effects were found in MVELO (p = 0.030, η2p = 0.060) and MVELOml (p = 0.034, η2p = 0.058). And significant surface effects were found in RMSap (p < 0.001, η2p = 0.467), MVELO (p < 0.001, η2p = 0.612), MVELOml (p < 0.001, η2p = 0.588) and MVELOap (p < 0.001, η2p = 0.483). Similarly, significant group effects were found in RMS (p = 0.004, η2p = 0.102), RMSml (p = 0.006, η2p = 0.096) and 95%AREA-CE (p < 0.001, η2p = 0.163). Significant surface effects were also found in RMS (p < 0.001, η2p = 0.694), RMSml (p < 0.001, η2p = 0.704) and 95%AREA-CE (p < 0.001, η2p = 0.747). (Fig. 4).
Fig. 4.
Comparison of static balance control ability between MCI group and control group under the eyes closed condition
Note. (H) ~ (N) represents the comparison of balance parameters between MCI group and control group under the eyes closed condition. RMS = Root mean squared; RMSml = Root mean squared in the ML direction; RMSap = Root mean squared in the AP direction; MVELO = the average velocity of the COP; MVELOml = the average velocity of the COP in the ML direction; MVELOap = the average velocity of the COP in the AP direction; 95%AREA-CE = the 95% confidence ellipse area of the COP. a, significant difference between groups. b, significant difference between types of support surface
*p < 0.05, **p < 0.01
PFC activation parameters
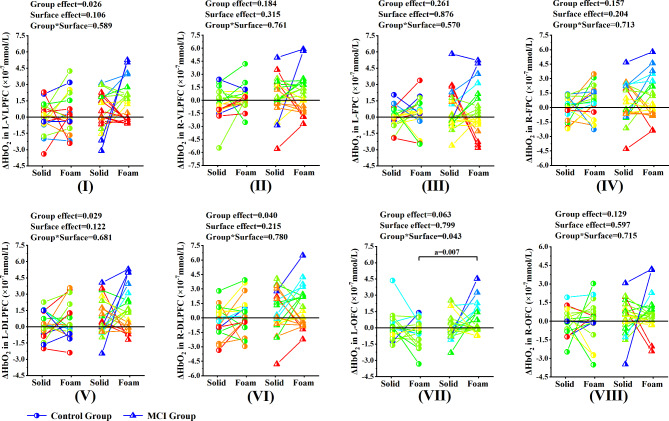
Under the eyes open condition, two-way MANOVA showed that the significant Group*Surface interaction effect was found in L-OFC (p = 0.043, η2p = 0.054). Post-hoc comparisons showed that the ΔHbO2 of L-OFC in the MCI group while standing on the foam surface were significantly higher than those in the control group (p < 0.007), while no significant difference was found between solid and foam surface in the MCI group. No significant Group*Surface interaction effect was found in the other seven ROI regions, while significant group effects were detected for three ROI regions, namely L-VLPFC (p = 0.026, η2p = 0.065), L-DLPFC (p = 0.029, η2p = 0.063), R-DLPFC (p = 0.04, η2p = 0.056). No significant surface effect was found in the seven ROI regions (Fig. 5).
Fig. 5.
Comparison of activation levels of each ROI region between MCI group and control group under the eyes open condition
Note. (I) ~ (VIII) represents the comparison of activation levels of each ROI region between MCI group and control group under the eyes open condition. (I) L-VLPFC, (II) R-VLPFC, (III) L-FPC, (IV) R-FPC, (V) L-DLPFC, (VI) R-DLPFC, (VII) L-OFC, (VIII) R-OFC. L-VLPFC = the left ventrolateral prefrontal cortex and belonged to the left BA45. R-VLPFC = the right ventrolateral prefrontal cortex and belonged to the right BA45. L-FPC = the left frontopolar cortex and belonged to the right BA10. R-FPC = the right frontopolar cortex and belonged to the right BA10. L-DLPFC = the left dorsolateral prefrontal cortex and belonged to the left BA46. L-DLPFC = the right dorsolateral prefrontal cortex and belonged to the right BA46. L-OFC = the left orbitofrontal cortex and belonged to the left BA11. R-OFC = the right orbitofrontal cortex and belonged to the right BA11. a, significant difference between groups. b, significant difference between types of support surface
*p < 0.05, **p < 0.01
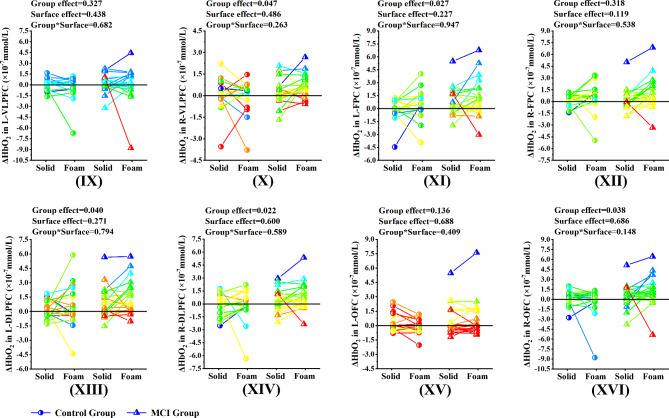
Under the eyes closed condition, no significant Group*Surface interaction effect was found in the eight ROI regions, while significant group effects were detected for five ROI regions, namely R-VLPFC (p = 0.047, η2p = 0.052), L-FPC (p = 0.027, η2p = 0.064), L-DLPFC (p = 0.04, η2p = 0.056), R-DLPFC (p = 0.022, η2p = 0.069) and R-OFC (p = 0.038, η2p = 0.057). No significant surface effect was found in the eight ROI regions (Fig. 6).
Fig. 6.
Comparison of activation levels of each ROI region between MCI group and control group under the eyes closed condition
Note. (IX) ~ (XVI) represents the comparison of activation levels of each ROI region between MCI group and control group under the eyes closed condition. (IX) L-VLPFC, (X) R-VLPFC, (XI) L-FPC, (XII) R-FPC, (XIII) L-DLPFC, (XIV) R-DLPFC, (XV) L-OFC, (XVI) R-OFC. L-VLPFC = the left ventrolateral prefrontal cortex and belonged to the left BA45. R-VLPFC = the right ventrolateral prefrontal cortex and belonged to the right BA45. L-FPC = the left frontopolar cortex and belonged to the right BA10. R-FPC = the right frontopolar cortex and belonged to the right BA10. L-DLPFC = the left dorsolateral prefrontal cortex and belonged to the left BA46. L-DLPFC = the right dorsolateral prefrontal cortex and belonged to the right BA46. L-OFC = the left orbitofrontal cortex and belonged to the left BA11. R-OFC = the right orbitofrontal cortex and belonged to the right BA11. a, significant difference between groups. b, significant difference between types of support surface
*p < 0.05, **p < 0.01
Discussion
This study investigated balance parameters and hemodynamic response in PFC brain regions during standing on solid and foam surfaces with and without visual conditions among older adults with MCI and healthy older adults. Our results partially support the hypothesis that the standing balance control ability of the older adults with MCI was worse than that of the healthy older adults, and also the older adults with MCI required more PFC activation. The above results may indicate a greater allocation of cognitive resources in older adults with MCI that is compensatory to maintain balanced performance.
The first hypothesis was demonstrated in the present study. Our results showed that under the eyes open condition, there was a significant interaction effect in terms of static balance control between the two groups of participants. Specifically, the balance control of older adults with MCI was significantly lower during standing on the foam surface compared to those standing on the solid surface. During standing on the foam surface, the balance control was significantly lower in older adults with MCI compared to those in healthy older adults (Fig. 3). This is consistent with previous studies [16]. Previous study has shown that older adults rely on proprioceptive cues more than visual cues [42], and postural sway increases when proprioceptive information is disturbed (while maintaining accurate visual cues) [43]. However, when proprioceptive information was inaccurate, such as standing on a foam surface, older adults with MCI exhibited greater postural sway compared to healthy older adults. This decreased balance control may be associated with diminished abilities in visual processing, visual search [44], and attention-related processing in MCI [45].
Only significant interaction effects in the left BA11 were found under the eyes open condition between the two groups. That is, during standing on the foam surface, the activation level of brain regions in older adults with MCI was significantly higher than that in healthy older adults (Fig. 5, VII). The BA11 area is highly susceptible to age-associated changes, including disruption of frontostriatal circuits by diffuse white matter changes and gray matter atrophy [46]. Previous studies indicate that older adults exhibited significantly greater activation in BA11 than young adults during motor imagery [47]. In this study, when proprioceptive information is inaccurate, we speculate that the increased activation of left BA11 in older adults with MCI may be a compensatory attempt to recruit additional attention resources to support task performance due to the decreased ability of visuospatial information integration [44]. However, the “compensatory attempt” seems to be “failed”. Balance in older adults with MCI was still poorer than in healthy older adults. Previous studies have shown that the BA11 is responsible for processing emotionally related sensory information [48]. Another possible explanation is that both structural and functional brain networks in MCI are disrupted which is associated with compromised cognitive performance [49], this led to poor specificity in recruiting professional brain network connections, which in turn led to widespread activation.
It is worth noting that significant group effects were only detected for the bilateral BA46 and the left BA45. Whether standing on solid or foam surfaces, older adults with MCI showed a greater level of brain region activation than healthy older adults (Fig. 5, I, V, VI). The increased activation levels of the above three regions of interest in older adults with MCI appear to be a compensatory strategy for maintaining standing posture at a certain level when proprioceptive cues are accurate. In addition, from a motor control perspective, the BA46 has been implicated in the planning of action motor sequences and allocating attentional resources [18, 50]. And BA45 area plays an important role in planning the high-level motion sequences, which not only participates in language processing but also participates in various tasks and content fields [51]. Previous studies have confirmed that the execution of various cognitive tasks and motor tasks could activate the BA45 area [52].
Interestingly, under the eyes closed condition, our results showed that the balance control of older adults with MCI was significantly lower during standing on the foam surface compared to those standing on the solid surface. During standing on the foam surface, the balance control was significantly lower in older adults with MCI compared to those in healthy older adults (Fig. 4). The PFC activation results showed that significant group effects were only detected for the bilateral BA46, the right BA45, the left BA10, and the right BA11. Whether standing on solid or foam surfaces, older adults with MCI showed a greater level of brain region activation than healthy older adults (Fig. 6, X, XI, XIII, XIV, XVI). In the absence of visual input, the balance of the human body depends mainly on proprioception [53]. However, while proprioceptive and visual cues are inaccurate, the maintenance of balance mainly relies on vestibular regulation [54]. Neuroscientific models of aging consider the brain to play a major role in determining age-related sensory and cognitive decline [55]. As a result of aging, the representation of complex sensory inputs becomes more inaccurate and manifests as temporally and spatially “noisy processing” of sensory stimuli [56]. It is assumed that noisy processing of sensory input affects cognitive processing [57]. In fact, older adults rely more on multi-sensory information to accurately perform activities of daily living [11]. Therefore, when sensory information is inaccurate, task performance in older adults will be affected, and poor balance performance may indicate deficiencies in the integration of multi-sensory input centers [43]. We suggest that increased PFC activation in older adults with MCI may be associated with actively evoked compensatory recruitment to support task performance.
However, the poor balance control and higher PFC activation shown by older adults with MCI on sensory manipulation tests may also be related to the following reasons. A recent MRI study showed that alterations in Corticocortical Vestibular Network (CVN) functional connectivity are associated with decreased balance ability in older adults with MCI [58], the CVN is a key brain region that integrates visual, auditory, and vestibular sensory information [59]. Its alterations might disrupt structural brain connectivity and interfere with neural pathways that control balance, resulting in the inability of the Central Nervous System to effectively access or integrate sensory information [58]. And previous studies have shown that a potential positive correlation exists between vestibular function and cognition, with the more severe the cognitive impairment, the more severe the impairment of vestibular function [60]. In addition, it has been reported that compared to older adults with normal cognition, older adults with MCI may have musculoskeletal disorders [61] such as muscle atrophy and hypomuscular strength, resulting in decreased control of proximal and distal muscles of their lower extremities and decreased proprioceptive function, which might affect the control of their balance function [29, 62]. These structural and functional deficiencies may also explain why older adults with MCI exhibit higher PFC activation during balance tasks compared to healthy older adults.
Our research may be of great significance in strengthening the balance control strategy of older adults with MCI. The theory of brain plasticity holds that the brain can still show plasticity in the face of cognitive decline in old age [63]. Physical exercise has been shown to stimulate the generation of new nerve cells, and aerobic exercise induces the expansion of brain tissue, leading to increased gray and white matter in crucial cognitive regions, such as the PFC [64]. Therefore, our study can be used to guide and formulate targeted task balance control therapy [65] and optimize alternative interventions such as non-invasive brain stimulation [63] combined with balance training. Regulating the excitability of specific brain regions (such as BA45 and BA10) of PFC and combining it with balance training under different sensory cue deprivation conditions may have a certain promoting effect on improving balance control and preventing falls in older adults with MCI.
The current study has some limitations. First of all, the sample size in this study was small, which limited the generalization of the findings. Secondly, due to the limitation of experimental instruments, it is impossible to obtain more information about the cerebral cortex except for PFC, such as the occipital, parietal, and temporal areas that have previously been suggested to be implicated in balance control [51], further studies could be carried out by using fNIRS systems equipped with a larger number of channels. Finally, we did not use vision as a fixed factor to explore PFC activation characteristics under visual cue manipulation. It is not clear whether the activation of PFC in older adults with MCI significantly increases with the sequential deprivation of visual cues and proprioceptive cues, further studies should focus on exploring the cerebral cortex regulation mechanism of older adults with MCI in different visual conditions.
Conclusion
Our study emphasizes the role of the PFC in maintaining standing balance control among older adults with MCI, and increased activation of PFC in older adults with MCI under different sensory conditions, indicating increased attention resources for maintaining balance. This may be a compensatory strategy for the maintenance of balance control in older adults with MCI due to the decline in cognitive function and in response to complex sensory conditions. The current findings may have positive implications for rehabilitation, and based on our research results, it remains to be seen whether targeted interventions, such as exercise or neurocognitive training, may lead to improved PFC functioning and whether such changes may elicit performance gains in balance performance in older adults with MCI.
Acknowledgements
The authors are thankful to all the participants and their power of endurance to complete the study.
Abbreviations
- MCI
Mild cognitive impairment
- PFC
Prefrontal cortex
- fNIRS
Functional near-infrared spectroscopy
- COP
Center of pressure
- MoCA
Montreal Cognitive Assessment
- ROI
Regions of interest
- BA
Brodmann areas
- L-VLPFC
The left ventrolateral prefrontal cortex
- R-VLPFC
The right ventrolateral prefrontal cortex
- L-FPC
The left frontopolar cortex
- R-FPC
The right frontopolar cortex
- L-DLPFC
The left dorsolateral prefrontal cortex
- R-DLPFC
The right dorsolateral prefrontal cortex
- L-OFC
The left orbitofrontal cortex
- R-OFC
The right orbitofrontal cortex
- RMS
Root mean squared
- AP
Anterior-posterior
- ML
Medial-lateral
Author contributions
GX is responsible for participating in the conception, study design, data collection, writing and submission of the manuscript. MZ and JW are responsible for the study design and revision of the manuscript. DM and WS are responsible for the data management and monitoring of this study.
Funding
This work was supported by the National Natural Science Foundation of China (No.12402373) and the Shandong Provincial Natural Science Foundation (No. ZR2024MH341).
Data availability
The datasets used and/ or analysed during the current study available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This study protocol was approved by the Ethics Committee of Shandong Sport University (approval number: 2021006), and all participants signed the informed consent form before the experiment.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lach HW, Harrison BE, Phongphanngam S. Falls and Fall Prevention in Older Adults With Early-Stage Dementia: An Integrative Review. Res Gerontol Nurs. 2017;10(3):139–48. [DOI] [PubMed] [Google Scholar]
- 2.Xue J, Li J, Liang J, Chen S. The Prevalence of Mild Cognitive Impairment in China: A Systematic Review. Aging Dis. 2018;9(4):706–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tyrovolas S, Koyanagi A, Lara E, Santini ZI, Haro JM. Mild cognitive impairment is associated with falls among older adults: Findings from the Irish Longitudinal Study on Ageing (TILDA). Exp Gerontol. 2016;75:42–7. [DOI] [PubMed] [Google Scholar]
- 4.Montero-Odasso M, Speechley M. Falls in Cognitively Impaired Older Adults: Implications for Risk Assessment And Prevention. J Am Geriatr Soc. 2018;66(2):367–75. [DOI] [PubMed] [Google Scholar]
- 5.Racey M, Markle-Reid M, Fitzpatrick-Lewis D, Ali MU, Gagne H, Hunter S, Ploeg J, Sztramko R, Harrison L, Lewis R, et al. Fall prevention in community-dwelling adults with mild to moderate cognitive impairment: a systematic review and meta-analysis. BMC Geriatr. 2021;21(1):689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kannan L, Bhatt T, Zhang A, Ajilore O. Association of balance control mechanisms with brain structural integrity in older adults with mild cognitive impairment. Neurosci Lett. 2022;783:136699. [DOI] [PubMed] [Google Scholar]
- 7.Jeon SY, Han SJ, Jeong JH, Fregni F. Effect of exercise on balance in persons with mild cognitive impairment. NeuroRehabilitation. 2014;35(2):271–8. [DOI] [PubMed] [Google Scholar]
- 8.O’Connor KW, Loughlin PJ, Redfern MS, Sparto PJ. Postural adaptations to repeated optic flow stimulation in older adults. Gait Posture. 2008;28(3):385–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Woollacott MH, Shumway-Cook A. Changes in posture control across the life span–a systems approach. Phys Ther. 1990;70(12):799–807. [DOI] [PubMed] [Google Scholar]
- 10.Faraldo-García A, Santos-Pérez S, Crujeiras-Casais R, Labella-Caballero T, Soto-Varela A. Influence of age and gender in the sensory analysis of balance control. Eur Arch Otorhinolaryngol. 2012;269(2):673–7. [DOI] [PubMed] [Google Scholar]
- 11.de Dieuleveult AL, Siemonsma PC, van Erp JB, Brouwer AM. Effects of Aging in Multisensory Integration: A Systematic Review. Front Aging Neurosci 2017, 9:80. [DOI] [PMC free article] [PubMed]
- 12.Takacs J, Carpenter MG, Garland SJ, Hunt MA. The role of neuromuscular changes in aging and knee osteoarthritis on dynamic postural control. Aging Dis. 2013;4(2):84–99. [PMC free article] [PubMed] [Google Scholar]
- 13.Mihara M, Miyai I, Hatakenaka M, Kubota K, Sakoda S. Role of the prefrontal cortex in human balance control. NeuroImage. 2008;43(2):329–36. [DOI] [PubMed] [Google Scholar]
- 14.Montero-Odasso M, Muir SW, Speechley M. Dual-task complexity affects gait in people with mild cognitive impairment: the interplay between gait variability, dual tasking, and risk of falls. Archives Phys Med Rehabilitation. 2012;93(2):293–9. [DOI] [PubMed] [Google Scholar]
- 15.Olivier B. Gilles, Allali, Berrut, Caroline, Hommet, Véronique, Dubost, Frédéric: Gait analysis in demented subjects: Interests and perspectives. Neuropsychiatric Disease Treatment 2008. [DOI] [PMC free article] [PubMed]
- 16.Deschamps T, Beauchet O, Annweiler C, Cornu C, Mignardot JB. Postural control and cognitive decline in older adults: Position versus velocity implicit motor strategy. Gait Posture. 2014;39(1):628–30. [DOI] [PubMed] [Google Scholar]
- 17.St George RJ, Hinder MR, Puri R, Walker E, Callisaya ML. Functional Near-infrared Spectroscopy Reveals the Compensatory Potential of Pre-frontal Cortical Activity for Standing Balance in Young and Older Adults. Neuroscience. 2021;452:208–18. [DOI] [PubMed] [Google Scholar]
- 18.Teo WP, Goodwill AM, Hendy AM, Muthalib M, Macpherson H. Sensory manipulation results in increased dorsolateral prefrontal cortex activation during static postural balance in sedentary older adults: An fNIRS study. Brain Behav. 2018;8(10):e01109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qi L, Zhou M, Mao M, Yang J. The static balance ability on soft and hard support surfaces in older adults with mild cognitive impairment. PLoS ONE. 2023;18(12):e0295569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takakusaki K. Functional Neuroanatomy for Posture and Gait Control. J Mov Disord. 2017;10(1):1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holtzer R, Epstein N, Mahoney JR, Izzetoglu M, Blumen HM. Neuroimaging of mobility in aging: a targeted review. journals Gerontol Ser Biol Sci Med Sci. 2014;69(11):1375–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holtzer R, Mahoney JR, Izzetoglu M, Wang C, England S, Verghese J. Online fronto-cortical control of simple and attention-demanding locomotion in humans. NeuroImage. 2015;112:152–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marusic U, Taube W, Morrison SA, Biasutti L, Grassi B, De Pauw K, Meeusen R, Pisot R, Ruffieux J. Aging effects on prefrontal cortex oxygenation in a posture-cognition dual-task: an fNIRS pilot study. Eur Rev Aging Phys Act. 2019;16:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pfefferbaum A, Rohlfing T, Rosenbloom MJ, Chu W, Colrain IM, Sullivan EV. Variation in longitudinal trajectories of regional brain volumes of healthy men and women (ages 10 to 85 years) measured with atlas-based parcellation of MRI. NeuroImage. 2013;65:176–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lehmann N, Kuhn YA, Keller M, Aye N, Herold F, Draganski B, Taube W, Taubert M. Brain Activation During Active Balancing and Its Behavioral Relevance in Younger and Older Adults: A Functional Near-Infrared Spectroscopy (fNIRS) Study. Front Aging Neurosci. 2022;14:828474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weng WH, Yang YR, Yeh NC, Ku PH, Wang PS, Liao YY, Wang RY. Gait performance and prefrontal cortex activation during single and dual task walking in older adults with different cognitive levels. Front Aging Neurosci. 2023;15:1177082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu G, Zhou M, Chen Y, Song Q, Sun W, Wang J. Brain activation during standing balance control in dual-task paradigm and its correlation among older adults with mild cognitive impairment: a fNIRS study. BMC Geriatr. 2024;24(1):144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Winblad B, Palmer K, Kivipelto M, Jelic V, Fratiglioni L, Wahlund LO, Nordberg A, Bäckman L, Albert M, Almkvist O, et al. Mild cognitive impairment–beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med. 2004;256(3):240–6. [DOI] [PubMed] [Google Scholar]
- 29.Shin B, Han S, Jung J, Kim J, Fregni F. Effect of mild cognitive impairment on balance. J Neurol Sci. 2011;305:121–5. [DOI] [PubMed] [Google Scholar]
- 30.Marusic U, Taube W, Morrison SA, Biasutti L, Grassi B, De Pauw K, Meeusen R, Pisot R, Ruffieux J. Aging effects on prefrontal cortex oxygenation in a posture-cognition dual-task: an fNIRS pilot study. Eur Rev Aging Phys Activity 2019, 16(1). [DOI] [PMC free article] [PubMed]
- 31.Bahureksa L, Najafi B, Saleh A, Sabbagh M, Coon D, Mohler MJ, Schwenk M. The Impact of Mild Cognitive Impairment on Gait and Balance: A Systematic Review and Meta-Analysis of Studies Using Instrumented Assessment. Gerontology. 2017;63(1):67–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cutini S, Scatturin P, Zorzi M. A new method based on ICBM152 head surface for probe placement in multichannel fNIRS. NeuroImage. 2011;54(2):919–27. [DOI] [PubMed] [Google Scholar]
- 33.Szczepanski S, Knight R. Insights into human behavior from lesions to the prefrontal cortex. Neuron. 2014;83(5):1002–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ye JC, Tak S, Jang KE, Jung J, Jang J. NIRS-SPM: statistical parametric mapping for near-infrared spectroscopy Proceedings of SPIE - The International Society for Optical Engineering 2009, 44(2):428–447. [DOI] [PubMed]
- 35.Hijmans JM, Zijlstra W, Geertzen J, Hof AL, Postema K. Foot and ankle compression improves joint position sense but not bipedal stance in older people. Gait Posture. 2009;29(2):322–5. [DOI] [PubMed] [Google Scholar]
- 36.Kodesh E, Benzoor M, Dar G. Effect of dynamic tape on postural sway in individuals with chronic ankle instability. J Bodyw Mov Ther. 2021;28:62–7. [DOI] [PubMed] [Google Scholar]
- 37.Huppert TJ, Diamond SG, Franceschini MA, Boas DA. HomER: a review of time-series analysis methods for near-infrared spectroscopy of the brain. Appl Opt. 2009;48(10):D280–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pinti P, Scholkmann F, Hamilton A, Burgess P, Tachtsidis I. Current Status and Issues Regarding Pre-processing of fNIRS Neuroimaging Data: An Investigation of Diverse Signal Filtering Methods Within a General Linear Model Framework. Front Hum Neurosci. 2018;12:505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brigadoi S, Ceccherini L, Cutini S, Scarpa F, Scatturin P, Selb J, Gagnon L, Boas DA, Cooper RJ. Motion artifacts in functional near-infrared spectroscopy: a comparison of motion correction techniques applied to real cognitive data. NeuroImage. 2014;85(0 1):181–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hoshi Y. Functional near-infrared optical imaging: utility and limitations in human brain mapping. Psychophysiology. 2003;40(4):511–20. [DOI] [PubMed] [Google Scholar]
- 41.Pierce CA. Cautionary Note on Reporting Eta-Squared Values from Multifactor ANOVA Designs. Educational Psychol Meas. 2004;64(6):916–24. [Google Scholar]
- 42.Wiesmeier IK, Dalin D, Maurer C. Elderly Use Proprioception Rather than Visual and Vestibular Cues for Postural Motor Control. Front Aging Neurosci. 2015;7:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Camicioli R, Panzer VP, Kaye J. Balance in the healthy elderly: posturography and clinical assessment. Arch Neurol. 1997;54(8):976–81. [DOI] [PubMed] [Google Scholar]
- 44.Kucharik M, Kosutzka Z, Pucik J, Hajduk M, Saling M. Processing moving visual scenes during upright stance in elderly patients with mild cognitive impairment. PeerJ. 2020;8:e10363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jungha H, Sunmin L. The effect of virtual reality program on the cognitive function and balance of the people with mild cognitive impairment. J Phys therapy Sci 2017. [DOI] [PMC free article] [PubMed]
- 46.Raz N, Gunning FM, Head D, Dupuis JH, McQuain J, Briggs SD, Loken WJ, Thornton AE, Acker JD. Selective aging of the human cerebral cortex observed in vivo: differential vulnerability of the prefrontal gray matter. Cerebral cortex (New York, NY: 1991) 1997, 7(3):268–282. [DOI] [PubMed]
- 47.Allali G, van der Meulen M, Beauchet O, Rieger SW, Vuilleumier P, Assal F. The neural basis of age-related changes in motor imagery of gait: an fMRI study. journals Gerontol Ser Biol Sci Med Sci. 2014;69(11):1389–98. [DOI] [PubMed] [Google Scholar]
- 48.Skandalakis GP, Barrios-Martinez J, Kazim SF, Rumalla K, Courville EN, Mahto N, Kalyvas A, Yeh FC, Hadjipanayis CG, Schmidt MH, et al. The anatomy of the four streams of the prefrontal cortex. Preliminary evidence from a population based high definition tractography study. Front Neuroanat. 2023;17:1214629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Holtzer R, Izzetoglu M. Mild Cognitive Impairments Attenuate Prefrontal Cortex Activations during Walking in Older Adults. Brain sciences 2020, 10(7). [DOI] [PMC free article] [PubMed]
- 50.Radel R, Brisswalter J, Perrey S. Saving mental effort to maintain physical effort: a shift of activity within the prefrontal cortex in anticipation of prolonged exercise. Cogn Affect Behav Neurosci. 2017;17(2):305–14. [DOI] [PubMed] [Google Scholar]
- 51.Lin CC, Barker JW, Sparto PJ, Furman JM, Huppert TJ. Functional near-infrared spectroscopy (fNIRS) brain imaging of multi-sensory integration during computerized dynamic posturography in middle-aged and older adults. Exp Brain Res. 2017;235(4):1247–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Clerget E, Andres M, Olivier E. Deficit in complex sequence processing after a virtual lesion of left BA45. PLoS ONE. 2013;8(6):e63722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Horak FB. Postural orientation and equilibrium: what do we need to know about neural control of balance to prevent falls? Age Ageing. 2006;35(Suppl 2):ii7–11. [DOI] [PubMed] [Google Scholar]
- 54.Nedelkou A, Hatzitaki V, Chatzinikolaou K, Grouios G. Does somatosensory feedback from the plantar foot sole contribute to verticality perception? Somatosens Motor Res. 2021;38(3):214–22. [DOI] [PubMed] [Google Scholar]
- 55.Dinse HR. Cortical reorganization in the aging brain. Prog Brain Res. 2006;157:57–80. [DOI] [PubMed] [Google Scholar]
- 56.Löffler A, Beier F, Bekrater-Bodmann R, Hausner L, Desch S, Silvoni S, Kleinböhl D, Löffler M, Nees F, Frölich L, et al. Reduced tactile sensitivity is associated with mild cognitive impairment. EBioMedicine. 2024;99:104896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Koen JD, Hauck N, Rugg MD. The Relationship between Age, Neural Differentiation, and Memory Performance. J neuroscience: official J Soc Neurosci. 2019;39(1):149–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xia R, Ren J, Li X, Liu J, Dai Y, Kuang Y, Wu Z, Chen S. Alterations in Corticocortical Vestibular Network Functional Connectivity Are Associated with Decreased Balance Ability in Elderly Individuals with Mild Cognitive Impairment. Brain Sci 2022, 13(1). [DOI] [PMC free article] [PubMed]
- 59.Noohi F, Kinnaird C, De Dios Y, Kofman IS, Wood SJ, Bloomberg J, Mulavara A, Sienko KH, Polk TA, Seidler RD. Age Differences in Vestibular Brain Connectivity Are Associated With Balance Performance. Front Aging Neurosci. 2020;12:566331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bosmans J, Gommeren H, Gilles A, Mertens G, Van Ombergen A, Cras P, Engelborghs S, Vereeck L, Lammers MJW, Van Rompaey V. Evidence of Vestibular and Balance Dysfunction in Patients With Mild Cognitive Impairment and Alzheimer’s Disease. Ear Hear. 2024;45(1):53–61. [DOI] [PubMed] [Google Scholar]
- 61.Hu Y, Peng W, Ren R, Wang Y, Wang G. Sarcopenia and mild cognitive impairment among elderly adults: The first longitudinal evidence from CHARLS. J cachexia sarcopenia muscle. 2022;13(6):2944–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lauretani F, Maggio M, Ticinesi A, Tana C, Prati B, Gionti L, Nouvenne A, Meschi T. Muscle weakness, cognitive impairment and their interaction on altered balance in elderly outpatients: results from the TRIP observational study. Clin Interv Aging. 2018;13:1437–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Beretta V, Santos P, Orcioli-Silva D, Zampier V, Vitório R, Gobbi L. Transcranial direct current stimulation for balance rehabilitation in neurological disorders: A systematic review and meta-analysis. Ageing Res Rev. 2022;81:101736. [DOI] [PubMed] [Google Scholar]
- 64.Deng J, Wang H, Fu T, Xu C, Zhu Q, Guo L, Zhu Y. Physical activity improves the visual-spatial working memory of individuals with mild cognitive impairment or Alzheimer’s disease: a systematic review and network meta-analysis. Front public health. 2024;12:1365589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ali N, Tian H, Thabane L, Ma J, Wu H, Zhong Q, Gao Y, Sun C, Zhu Y, Wang T. The Effects of Dual-Task Training on Cognitive and Physical Functions in Older Adults with Cognitive Impairment; A Systematic Review and Meta-Analysis. J Prev Alzheimer’s Disease. 2022;9(2):359–70. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/ or analysed during the current study available from the corresponding author on reasonable request.